Abstract
Survivin is one of the most important members of the inhibitors of apoptosis protein family, as it is expressed in most human cancers but is absent in normal, differentiated tissues. Lending to its importance, survivin has proven associations with apoptosis and cell cycle control, and has more recently been shown to modulate the tumor microenvironment and immune evasion as a result of its extracellular localization. Upregulation of survivin has been found in many cancers including breast, prostate, pancreatic, and hematological malignancies, and it may prove to be associated with the advanced presentation, poorer prognosis, and lower survival rates observed in ethnically diverse populations.
Keywords:
Introduction
Cancer is a major public health problem in the United States and the world. Recent epidemiological statistics indicate that cancer will develop in one in three women and one in two men in the US over their lifetime.Citation1 The three most common cancers among males are prostate, colorectal, and melanoma of the skin; and among females, breast, uterine corpus, and colorectal.Citation2 Although deaths attributed to cancer have declined among both Caucasians and African Americans, the latter continue to suffer a greater burden for each of the most common types of cancer.Citation1 This discrepancy recorded among cancer patients from different ethnicities is termed cancer health disparity. The National Cancer Institute defines cancer health disparity as an adverse difference in cancer incidence (new cases), cancer prevalence (all existing cases), cancer death (mortality), cancer survivorship, and burden of cancer or related health conditions that exist among specific population groups.Citation1 When investigating the factors that contribute to cancer health disparities, the most obvious are access to health care and socioeconomic status.Citation3–Citation5 However, evidence exists that dietary fat can influence carcinogenesis.Citation6 In 1982, the US National Academy of Sciences committee on Diet, Nutrition, and Cancer, using both epidemiological and experimental data, concluded that a causal relationship between fat intake and the occurrence of cancer exists.Citation7 However, the strongest evidence that environmental factors give rise to an etiology of cancer comes from the studies of cancer incidence in different ethnic populations and their migrations and lifestyle habits. Specifically, the adoption of a Westernized diet appears causal in the significant increase in annual deaths in native Japanese from colon,Citation8 breast,Citation9 and pancreatic cancersCitation10 upon their moving from Japan to the US. In addition, experimental animal studies agree that both specific and nonspecific evidence exists for the occurrence of cancer being strongly associated with the consumption of a diet high in fat.Citation11 Contradictory studies also exist in which lifestyle factors in cancer incidence have been described. Whereas years of smoking and number of cigarettes smoked had a correlation with an increased incidence of pancreatic cancer, there was no correlation with pancreatic cancer and body mass index, physical activity, alcohol, coffee, and green tea consumption.Citation12
Early detection is important in cancer discovery, treatment, and survival. In order to better understand cancer incidence and mortality in diverse populations, it has become imperative that we identify and then characterize markers of cancer development and progression to include both pathways and molecular mechanisms associated with these disparities. Given the strong link between cancer incidence, oxidative stress, and diets high in fat,Citation13 we must map these associations and identify the survival entities and pathways as potential targets. A long-term goal in health disparities research is to understand how an increase in oxidative stress will ultimately promote cancer cell resistance to therapy-induced death and how to overcome this resistance.
Survivin is an important member of the inhibitors of apoptosis (IAP) protein family because its tumor-specific expression is unique out of all of the human gene products.Citation14 Survivin expression is evident during embryonic and fetal development, but not in terminally differentiated tissue.Citation15 It is expressed in virtually all of the different types of human cancers (), making survivin an alluring protein in the study of carcinogenesis.Citation16 Survivin is referred to as a bifunctional protein, having essential roles in inhibiting apoptosis and controlling proper cell division.Citation17 In our most recent work, we have begun to refer to survivin as a multifunctional protein as it does much more, to include controlling diverse cellular functions, including surveillance checkpoints, suppression of cell death, the regulation of mitosis, and adaptation to unfavorable environments.Citation17,Citation18
Table 1 Influence of survivin on clinical prognosis
Localization of survivin
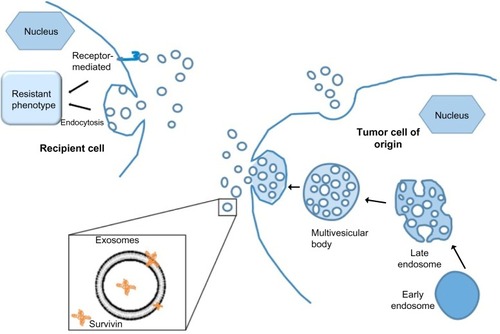
The multifaceted functionality of survivin is still being intensely scrutinized, and it appears that protein compartmentalization may be important. Survivin has been shown to localize in mitochondria, where it modulates tumor cell apoptosis similar to the Bcl-2 family.Citation19 Its localization to the nucleus and cytosol confers its role in mitosis regulation and apoptosis inhibition, respectively.Citation20 Furthermore, we have identified the existence of survivin extracellularly, contained in small membrane-bound vesicles known as exosomes (), and have shown that the exosome-bound survivin protein can be secreted by cancer cells to be taken up by surrounding cells, producing a field effect that confers a general stress-survival phenotype.Citation21–Citation23 Consistent with survivin’s association with unfavorable clinicopathological parameters, extracellular trafficking of survivin throughout the tumor microenvironment could be responsible for augmenting the aggressive status of a tumor, while prohibiting or minimizing therapeutic results.Citation21,Citation24,Citation25 This review focuses on the multifaceted roles of survivin in cancer biology, its cellular localization, and its cancer health disparity-specific upregulation, specifically in breast, prostate, pancreas, and hematological cancers.
Figure 1 Exosomes play important roles in intercellular communication.
Nuclear survivin as a cell cycle regulator
Nuclear survivin is known to be a cell-cycle-associated protein. Investigations of cell division regulation during the depletion of survivin by small interfering (si)RNA demonstrated an increase in mitotic arrest and chromosomal misalignment. Furthermore, this study confirmed that survivin is involved in microtubule assembly and centromere stabilization during mitosis.Citation26 Survivin’s role in mitosis regulation is associated with its involvement in the chromosomal packaging complex and its contribution to the formation of the mitotic spindle.Citation27,Citation28 IAP family proteins cIAP2 and survivin have been shown to dramatically increase upon exposure to hypoxia.Citation29,Citation30 Furthermore, survivin’s promoter has been shown to contain three putative HIF-1 binding or response elements.Citation31 Nuclear survivin was found to be distinctly involved in the prognosis of different cancers, as will be discussed in our specific cancers section.
Cytoplasmic/mitochondrial survivin as an apoptosis inhibitor
Survivin’s ability to interfere with cellular death pathways appears to reside in the cell’s cytoplasm. Survivin localizes to the mitochondriaCitation19 and therefore may provide, like Bcl-2, a role in mitochondrial stability. Cellular stress was shown to modulate the expression and localization of surviving, with hypoxia-induced survivin found exclusively in the mitochondria. Furthermore, upon apoptotic stimulation, mitochondrial survivin is rapidly released to the cytosol where its cytoprotective effects prevent the activation of the initiator caspase 9.Citation19
Early studies showed that survivin and XIAP protected cells from undergoing caspase-dependent apoptosis. Subsequently, in vitro binding experiments showed that survivin, like XIAP and other IAPs, bound to the terminal effector cell death proteases, caspases 3 and 7, but not to initiator caspase 8.Citation32 Controversy in the field arose when a study by Banks et alCitation33 showed that survivin did not inhibit caspase 3 activity, and where recombinant survivin failed to decrease recombinant caspase 3 activity in vitro. Current evidence suggests that survivin acts on caspases in an indirect manner by binding to the hepatitis B X-interacting protein (HBXIP) and forming a complex with procaspase 9, inhibiting the apoptosome formation.Citation34 This survivin–HBXIP complex, not individual survivin or HBXIP proteins, binds to procaspase 9 and works to prevent recruitment of apoptosis activating factor 1 (Apaf1), thus suppressing intrinsic apoptosis. In addition, survivin binds to and regulates the stability of XIAP, which is a direct caspase 3 and 9 inhibitor.Citation27 More specifically, the formation of a survivin–XIAP complex promotes increased XIAP stability, protecting XIAP from proteasomal degradation, resulting in a facilitated inhibition of caspase-dependent cell death.Citation30
Extracellular survivin as a modulator of tumor microenvironment
Survivin has recently been shown to exist in the extracellular space,Citation21 via 40–100 nm membrane vesicles called exosomes.Citation22 Various cell types, such as B- and T-lymphocytes, dendritic cells, neurons, intestinal epithelial cells, as well as tumor cells, release exosomes.Citation35–Citation38 In particular, it has been shown that both human and mouse tumor cells release tumor cell-derived exosomes (TEX) constitutively.Citation39 Additionally, specific protein content found both on and within TEX give an indication of their functional and biological roles, and their cell of origin, making TEX excellent biomarkers.Citation40–Citation43 Early detection, aggressive determination, and therapeutic efficacy may 1 day be possible through the use of these exosomes and their contents.
Our lab has shown that the extracellular pool of survivin has the ability to cause neighboring cancer cells to increase resistance to therapy, rapidly proliferate, and acquire an increased potential to become invasive in vitro,Citation21 providing a protective role to the neighboring tumor cells.Citation22 The ability of extracellular survivin to cause these effects in the surrounding cancer cells correlates with the fact that survivin overexpression is observed in virtually every human cancer type.Citation44 TEX may also be used as a tool to detect malignant conditions.Citation43 Serum taken from cancer patients has an increased level of TEX,Citation45,Citation46 which has a positive correlation with the progression of the tumor.Citation22 In addition to serum, TEX were shown to be isolated from malignant tumor fluids, urine,Citation47,Citation48 ascites fluids,Citation49,Citation50 and pleural effusions.Citation40,Citation51 We have recently shown that exosomal survivin may be a useful tool for early detection, diagnosis, and even monitoring of prostate cancer (PCa) progression.Citation52 Newly diagnosed and advanced PCa patients with high- or low-grade cancer had significantly higher levels of exosomal survivin compared to control subjects or patients with preinflammatory benign prostatic hyperplasia (BPH).Citation52
Survivin in cancer immunity evasion
Survivin has been ascribed multiple roles not only in malignancy but also in immunity and differentiation.Citation53 Survivin has been shown to be essential for T-cell maturation, homeostasis, and proliferation at various stages of development.Citation54 It has also been shown to modulate peripheral blood leukocytes when in the extracellular space by binding to leukocytes, thereby inducing molecular processes implicated in the pathogenesis of inflammation.Citation55 On the basis of the literature and our data, survivin may be said to exhibit duplicity in cancer immunity, as it can act as a tumor-associated antigen, or modulate the immune environment to permit tumor growth.Citation53–Citation62
Recently, an artificial antigen-presenting cell, developed to study anti-survivin CD4+ T-cell responses in cancer patients, was shown to elicit both Th1 and Th2 responses against survivin.Citation56 The level of avidity was appropriate to recognize tumor cells.Citation56 Previously, constructed DNA–peptide complexes (mimovirus) of survivin epitopes have been shown to stimulate strong cytotoxic T-lymphocyte-mediated long-term memory of murine immune response and exact a high antitumor effect in BALB/c mice.Citation57 Furthermore, a DNA construct encoding a secreted version of survivin, along with a plasmid coding for murine granulocyte-macrophage colony-stimulating factor as a molecular adjuvant, was observed to elicit humoral responses against survivin in sera collected from mice.Citation57 The immunoglobulin G2a antibody was the prevalent antibody subclass, thereby implicating the induction of a Th1−CD4+ cellular response.Citation58
We have recently shown that when T-cell cultures were incubated with survivin, surface binding and intracellular uptake of survivin by these T-cells occurred.Citation63 Upon further investigation, a survivin-associated decreased proliferation was observed in these T-cells. In addition, analysis of cytotoxic T-lymphocytes revealed a reduction in their functional cytotoxicity. However, T-regulatory cell function remained unaltered. Importantly, the numbers of Th1 and Tc1 cells were significantly reduced, together with the cytokines associated with them (interferon-γ and interleukin [IL]-2), while an increase in IL-4+, IL-5+, and IL-13+ T-cells was observed. These results suggest a skewing from a type 1 response, which mediates immunity with cytokines that enhance cellular cytolytic activity and can elicit an effective antitumor response to a type 2 T-cell response, which does not lead to tumor rejection and is frequently observed in cancer patients.Citation59–Citation62 The verification and molecular mechanism underlying this Th-cell plasticity is yet to be fully elucidated.
Cancer-specific upregulation of survivin: breast cancer
Breast cancer is the second most common cancer type (following lung cancer) and the most common cancer among women worldwide.Citation1 It is estimated that in the US alone there are nearly 3 million women with diagnosed breast cancer and approximately 227,000 more will be added to that number this year.Citation1 African American women are more likely than all other women to die from breast cancer, as their tumors often are discovered at a later, more advanced stage, leaving them fewer treatment options.Citation63,Citation64 There are several pathways involved in breast cancer pathogenesis with pathways of tumor cell death playing an important role in its development and maintenance. Among the proteins involved in cell death/survival pathways, survivin is one of the most studied. Using serial analysis of gene expression, survivin was found to be the fourth highest expressed transcript in a number of common cancers including breast cancers.Citation65 In a study examining the interaction of the insulin-like growth factor (IGF)-II and survivin, Kalla Singh et alCitation66 found that high IGF-II expression–regulation of survivin correlated and was significantly higher in African Americans than in Caucasians. In this study, it was shown that IFG-II regulates survivin, leading to the inhibition of mitochondrial membrane depolarization, cell survival, and chemoresistance. Furthermore, the effect of IGF-II and IGF-II siRNA on the expression of Bcl-2, Bcl-XL, and survivin in African American and Caucasian breast cancer cells was measured. IGF-II expression was shown to be causative in the upregulation of these antiapoptotic proteins, while IGF-II siRNA was prohibitive.Citation66 This intriguing observation will require further investigation.
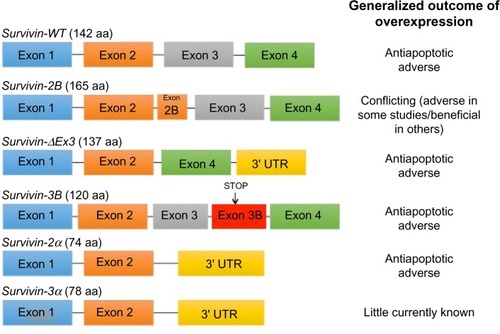
The different subcellular pools of survivin in breast cancer appear to have distinct functions. Adamkov et alCitation67 suggested that nuclear staining of the survivin antigen could be used as a marker of the degree of neoplasia, while Rexhepaj et alCitation68 suggested that increased levels of nuclear survivin are associated with a proliferative phenotype. One thing that is clear is that survivin plays a key role in the initiation and progression of breast cancer. High messenger (m)RNA expression was found to be an independent prognostic marker in breast cancer patientsCitation69 and survivin upregulation significantly correlated to lymph node involvement, tumor stage, and histological type.Citation70 By contrast, others have shown that high levels of its expression are associated with a beneficial response to chemotherapy.Citation71 This could be due to alternative splicing of survivin. Multiple studies demonstrate that alternative splicing patterns are altered during cancer progression.Citation72 Several different mechanisms contribute to changes in the regulation of alternative splicing including stress, stimulation of receptors by growth factors, cytokines, hormones, etc. Survivin, to date, has six different described variants with different apoptotic properties and intracellular localization ().Citation73 Protein and mRNA levels of the pro- and antiapoptotic isoforms of survivin correlate with cancer prognosis.Citation74
Figure 2 Splicing of the human survivin premessenger RNA produces six different splice variants.
Abbreviations: WT, wild type; UTR, untranslated region.
Early diagnosis of breast cancer is challenging due to a lack of serum biomarkers and, inadequate as it is, performed through invasive means such as needle biopsy, scanning, and invasive pathological examination. Despite the availability of numerous diagnostic and prognostic methods, there remains a need for an easy, sensitive, and noninvasive way to track tumor activity. We propose that through analysis of tumor exosomes and by specifically assaying these exosomes for tumor-specific antigens such as survivin, XIAP, cIAP1/2, and chaperone proteins such as HSP70 and HSP90, just such a biomarker discovery may 1 day be realized. We have found an extracellular survivin pool in serum exosomes in prostateCitation52 and breast cancers.Citation75 In these breast cancer patients’ sera, we found survivin levels and exosome numbers to be significantly increased over controls with a disparate expression of the survivin splice variants similar to that observed in tissues. It is important though that we recognize the possible confounding factors such as comorbidities, psychological complications, genetics, and environmental exposures that could affect these results.
Cancer-specific upregulation of survivin: prostate cancer
PCa is the most frequently diagnosed nonskin cancer in men and the second leading cause of male cancer deaths in the US, accounting for 238,590 new cases and 29,720 deaths in 2013.Citation1,Citation76 These statistics have undergone minimal changes despite advances in screening and early diagnosis, and therefore still require a significant investment if PCa is to be defeated. As has recently been described by our colleagues, African American men have a growing disparity in their PCa incidence and mortality compared to other ethnic groups,Citation77 and they present with the disease at a much younger age than do Caucasian men, which is a trait common in more aggressive cancers.Citation78,Citation79
Survivin is expressed in PCa and has been shown to be upregulated in order to protect the PCa microenvironment against apoptosis and oxidative stress-induced damage.Citation80 Survivin, therefore, directly and/or indirectly influences cell survival and death. Shariat et al, using immunohistochemistry, compared survivin protein expression in normal and malignant prostate tissue and lymph node tissue from PCa patients. There appeared to be a gradual but consistent rise of survivin expression from normal prostate specimens (36%) to PCa (71%), with the highest expression found in metastatic lymph nodes (81%).Citation81 Survivin expression therefore seemed to correlate with the degree of transition from normal prostate epithelia to a more aggressive form of PCa (metastatic PCa).
Our group recently looked at relative levels of survivin in the sera of PCa patients and compared it to that of patients with BPH and from subjects with no diagnosis of cancer or BPH.Citation52 Survivin levels proved to exhibit a stronger correlation in our hands than prostate-specific antigen when it came to distinguishing the two clinical conditions. We therefore propose that exosomal survivin evaluation should be given serious consideration as a plausible biomarker for the early detection of PCa and perhaps could be used to monitor treatment efficacy and disease recurrence. Higher levels of not only survivin but its splice variants 2B and 2α, both in vitro and in tissue, seem to correlate with PCa cell proliferation and a more aggressive phenotype.Citation82 The intracellular compartment localization of survivin has been suggested to be of prognostic value. When tissues of patients with locally advanced PCa were stained and examined for survivin, patients with higher levels of intranuclear survivin exhibited improved survival, whereas those with higher levels of cytoplasmic survivin exhibited a poorer prognosis.Citation83
In summary, survivin, in PCa has a dual role as an inhibitor of apoptosis and cell cycle mediator. Its level of expression appears to correlate with the progression from normal to indolent and to a more aggressive form of PCa. Our demonstration of exosomal survivin in the plasma of patients with newly diagnosed low-grade PCaCitation52 provides a rationale for studies to investigate the utility of exosomal survivin as an early, easily measured biomarker for PCa diagnosis, as well as a marker to monitor treatment efficacy and tumor recurrence.
Cancer-specific upregulation of survivin: pancreatic cancer
Cancer of the pancreas is the fourth most common cause of cancer death in men and women in the US. In 2013, an estimated 45,220 new cases and 38,460 deaths from pancreatic cancer occurred.Citation1 It is a highly malignant disease and lacks clear early warning signs or symptoms thus remaining silent in its victims until it is well advanced. The vast majority of patients are not diagnosed until stage III or IV, and once diagnosed, exhibit a median survival of 4–8 months with a 5-year survival rate being <5%.Citation84 Risk factors include sex, age, diabetes, chronic pancreatitis, family history, smoking, alcohol abuse, and possibly diets high in fat.Citation84 Early diagnosis continues to be the greatest obstacle and there is an urgent need for screening biomarkers.
Pancreatic cancer incidence in the US is higher in African Americans and Hispanics than in Caucasians.Citation85,Citation86 In a number of recent studies, the risk factors in men (cigarette smoking and diabetes mellitus) and women (moderate/heavy alcohol consumption and an elevated body mass index [obesity]), explain almost the entire African American/non-Hispanic White disparity in incidence.Citation85,Citation86 In the absence of these risk factors, pancreatic adenocarcinoma incidence rates among African Americans do not exceed those of Caucasians from either men or women.Citation87–Citation89 In 2003, a group at the Barbara Ann Karmanos Cancer Institute analyzed a group of pancreatic cancer patients (166 African American, 244 Caucasian) for clinicopathologic characteristics of the disease, as well as for immunohistochemical expression of commonly found pancreatic cancer biomarkers: Fas, FasL, p21/waf-1, p27, p53, and Her2. They also investigated the presence and types of K-ras mutations at codon 12.Citation90 African Americans were found to have significantly higher rates of K-ras mutations than did Caucasians, and their treatment with chemotherapy or radiation therapy was also much less effective than that recorded in Caucasians. African Americans more frequently than Caucasians were found with positive surgical margins and many clinicopathologic variables such as median survival, 5-year survival, and stage at presentation were different. African Americans were less immunoreactive to Fas expression and had a much stronger Her2 expression than did Caucasians.Citation90
As in prostate and breast cancer, as previously discussed, epidemiological evidence exists for a strong association between pancreatic cancer and a high consumption of dietary fat. Dietary fat is made up of fatty acids and lipids that are metabolized into arachidonic acid. The key enzymes for arachidonic acid metabolism are lipoxygenases (LOXs) and cyclooxygenases (COXs) which, outside of dietary fat research, have been shown to be associated with the development and progression of pancreatic cancer.Citation91 LOX and COX inhibitors prohibit the continued progression of pancreatic cancer and induce intrinsic mitochondria-associated apoptotic cell death.Citation92
There have been numerous studies performed on the prognostic implications of survivin in pancreatic cancer. A high expression of survivin was found to be related to shorter survival in patients with resected pancreatic adenocarcinoma.Citation93 In contrast, high nuclear levels of survivin predicted better prognosis than cytoplasmic survivin.Citation94 Furthermore, Sagol et alCitation95 and Sun et alCitation96 showed no significant association between survivin and long-term survival. Targeting survivin early on in the process could play an invaluable role in preventing the progression to malignancy. In addition, a screening biomarker that could potentially detect early stages of the disease is of utmost importance.
Cancer-specific upregulation of survivin: hematological malignancies
Hematological malignancies such as leukemia, lymphoma, myeloma, and myelodysplastic syndromes affect the bone marrow, the blood cells, the lymph nodes, and other parts of the lymphatic system. These pathologies are interrelated, likely the result of acquired changes to the DNA of a single stem cell. Approximately 140,000 people will be diagnosed with leukemia, lymphoma, or myeloma, accounting for approximately 9% of all new cancers diagnosed each year in the US.Citation97 Of particular interest is multiple myeloma, which accounts for ~10% of all hematologic malignancies diagnosed in the US annually.Citation98 Among the hematological malignancies, multiple myeloma is known to affect individuals from ethnically diverse populations in a disparate manner.
In accordance with reports for many types of solid tumors,Citation27,Citation99 cancer-specific upregulation of survivin also occurs in hematological malignancies,Citation100,Citation101 though to date, there have been no published reports taking ethnicity into account. In hematological cancers, expression of survivin is associated with poor clinical outcomes and resistance to chemotherapy.Citation102–Citation105 Survivin expression levels are linked to risk of early relapse in pediatric B-cell acute lymphoblastic leukemia,Citation106–Citation108 and to tumor aggressivenessCitation109 and chemoresistance in adult acute lymphoblastic leukemia.Citation110
High levels of survivin expression have also been linked to cell proliferation and antiapoptotic characteristics in chronic myelogenous leukemiaCitation111 and chronic lymphocytic leukemia.Citation112 In acute myeloid leukemia, levels of survivin expression were found to be significantly predictive of shorter overall and event-free survival.Citation113 In addition, the highest survivin expression levels are detected in the CD34(+)CD38(−) acute myeloid leukemia stem/progenitor cell populations,Citation113 further validating survivin’s potential as a prognostic biomarker and therapeutic target. Overexpression of survivin in CD34+ hematopoietic cells has been found to induce hematological malignancies in vivo, suggesting that it has a role in the development of these diseases.Citation114
Localization of survivin to the nucleus versus the cytoplasm is very important because the functional dynamics of survivin are dependent on the site of survivin expression.Citation115 Using chemotherapeutic drugs in hematologic cancer, Bernardo et alCitation116 reported that cytoplasmic survivin was more relevant to the apoptotic index than that associated with nuclear survivin. Investigating survivin’s cellular locations, alternative splice variant profiles within the context of cancer health disparities and novel therapeutic modalities will continue to be important areas of study.
Liquid biopsy
The tumor microenvironment is being increasingly recognized as providing many key factors necessary for many of the stages of disease progression including local resistance, immune escape, and distant metastasis. Understanding this tumor microenvironment, including the cells involved and the communications ongoing between them, will continue to prove instrumental in our understanding of cancer and eventually our ability to control it, if not terminate it. In order to fully “learn the language”, there is a need for new biomarker discovery. Specifically, biomarkers that are easily isolated and identified from blood, urine, saliva, cerebral spinal fluid, ascites, etc, as well as from tissue biopsies, will need to be identified. The term “liquid biopsy” has been used recently to describe the source of these biomarkers and could be defined as broadly as circulating tumor cells, circulating tumor DNA, exosomes, and secretomes.Citation48 Differential expression of exosomal survivin may serve as a diagnostic and or prognostic marker in early cancer patients, and it may soon lead to the development of potential therapeutics for the treatment of these diseases.
Conclusion
Most efforts on the identification of candidate cancer biomarkers, and on analyzing differences in the cancer biology that exists between African Americans and Caucasian patients, have focused on gene expression differentials in tumor tissues, epigenetic issues (such as methylation patterns), and on single nucleotide polymorphisms (SNPs). While these efforts have been necessary in providing important clues for understanding the biochemical mechanisms associated with cancer health disparities, it is also imperative to develop noninvasive approaches that analyze, indirectly and early in the disease process, the molecular profiles of tumors. One recent study has investigated the −31G>C survivin promoter polymorphism across approximately 7,500 cancer cases and 9,000 controls.Citation117 This polymorphism was significantly associated with an increased cancer risk in colorectal, gastric, and urothelial cancers. In contrast, this SNP was remarkably decreased in patients with hepatocellular carcinoma. With regard to ethnic diversity, this SNP was shown to increase cancer risk in Asian populations,Citation117 as well as a higher Wilms’ tumor risk in Serbian children.Citation118 Findings such as these encourage us to not only look at the overall abundance of gene or protein products in racial disparities and cancer but also look deeper into the minutia that may have been historically overlooked and may provide important insights not before recognized as factors in cancer development and resistance.
Recent studies have shown that small membrane-bound vesicles called exosomes constitute the latest mode of intercellular information transfer or communication. This exchange of molecular information is facilitated by their unique composition, which is enriched with enzymes, structural proteins, adhesion molecules, lipid rafts, microRNA, and RNA. Importantly, cancer cells have been shown to secrete more exosomes than do their normal counterparts, indicating that exosomes can be used as diagnostic markers and their active secretion has functional implications. In addition, recent studies revealed that genes involved in inflammation and autoimmune responses are differentially upregulated in cancer patients compared to controls. This could imply that differences in antitumor immune responses may exist between racial groups in tumors.
It is very important to specifically target survivin in a defined location for therapeutic purposes. Survivin is a unique inhibitor of apoptosis with triple functionality: in cell cycle regulation when it is present in the nucleus; inhibition of apoptosis when it is in the mitochondria; and resistance to chemotherapy when it exists in the tumor microenvironment packaged in exosomes. Survivin’s upregulation in specific cancers, in addition to its presence in serum exosomes, makes it an important molecule as a diagnostic as well as prognostic marker. Unfortunately, controversy exists as to whether survivin expression is favorable or unfavorable in the outcome of cancer. Survivin expression is an unfavorable prognostic indicator in esophageal, hepatocellular, and ovarian cancers, cholangiocarcinoma, and endometrial cancers, but it has associated favorable outcomes in gastric, bladder, breast, ependymoma osteosarcoma, and pancreatic ductal adenocarcinomas.Citation113,Citation119 To validate its role, a large number of case–control studies need to be adapted. Subsequent studies exploiting the exosomal packaging of survivin may also 1 day be used in cancer therapeutics.
In conclusion, this review addresses an urgent need in the fight against cancer health disparities: the need to identify and evaluate novel serum biomarkers such as survivin and its alternative splice variants for the noninvasive, early detection of cancer in interventions that can be tailored to Americans of different ethnicities, ultimately paving the way for future studies focused on analyzing these biomarkers in larger cohorts of ethnically diverse cancer patients.
Informed consent and animal studies
No animal or human studies were carried out by the authors for this article.
Acknowledgments
Funding for our laboratory comes from grants for health disparity research: NIH-NCMHD Project EXPORT Program 5P20MD001631/Project 3 (NRW); and NIH-NIMHD P20-MD006988 subproject 2. Funding also comes from a National Merit Test Bed (NMTB) award sponsored by the Department of the Army under Cooperative Agreement Number DAMD17-97-2-7016 (NRW). The funders had no role in the study design, data collection and analysis, the decision to publish, or preparation of the manuscript. We would like to thank the entire NRW lab for careful review of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRNaishadhamDJemalACancer statistics, 2013CA Cancer J Clin2013631113023335087
- SharpLDeadySGallagherPThe magnitude and characteristics of the population of cancer survivors: using population-based estimates of cancer prevalence to inform service planning for survivorship careBMC Cancer20141476725319534
- JadavSRajanSSAbughoshSSansgirySSThe role of socioeconomic status and health care access in breast cancer screening compliance among HispanicsJ Public Health Manag Pract Epub392015
- BeydounHABeydounMAPredictors of colorectal cancer screening behaviors among average-risk older adults in the United StatesCancer Causes Control200819433935918085415
- GuessousIDashCLapinPDoroshenkMSmithRAKlabundeCNNational Colorectal Cancer Roundtable Screening Among the 65 Plus Task GroupColorectal cancer screening barriers and facilitators in older personsPrev Med2010501–231020006644
- TsaiCJGiovannucciELHyperinsulinemia, insulin resistance, vitamin D, and colorectal cancer among whites and African AmericansDig Dis Sci201257102497250322562539
- PalmerSDiet, nutrition and cancerProg Food Nutr Sci198593–42833413010379
- TakachiRTsubonoYBabaKRed meat intake may increase the risk of colon cancer in Japanese, a population with relatively low red meat consumptionAsia Pac J Clin Nutr201120460361222094846
- KonoSHost and environmental factors predisposing to cancer developmentGan To Kagaku Ryoho2010374571576 Japanese20414009
- KasugaMUekiKTajimaNReport of the Japan Diabetes Society/Japanese Cancer Association Joint Committee on Diabetes and CancerCancer Sci2013104796597623879470
- GuthrieNCarrollKKSpecific versus non-specific effects of dietary fat on carcinogenesisProg Lipid Res199938326127110664796
- NakamuraKNagataCWadaKCigarette smoking and other lifestyle factors in relation to the risk of pancreatic cancer death: a prospective cohort study in JapanJpn J Clin Oncol201141222523121075833
- De PergolaGSilvestrisFObesity as a major risk factor for cancerJ Obes2013201329154624073332
- ReedJCThe Survivin saga goes in vivoJ Clin Invest2001108796596911581297
- LiFAmbrosiniGChuEYControl of apoptosis and mitotic spindle checkpoint by survivinNature199839667115805849859993
- AndersenMHSvaneIMBeckerJCStratenPTThe universal character of the tumor-associated antigen survivinClin Cancer Res200713205991599417947459
- AltieriDCSurvivin, versatile modulation of cell division and apoptosis in cancerOncogene200322538581858914634620
- AltieriDCThe case for survivin as a regulator of microtubule dynamics and cell death decisionsCurr Opin Cell Biol200618660961516934447
- DohiTBeltramiEWallNRPlesciaJAltieriDCMitochondrial survivin inhibits apoptosis and promotes tumorigenesisJ Clin Invest200411481117112715489959
- FortugnoPWallNRGiodiniASurvivin exists in immunochemically distinct subcellular pools and is involved in spindle microtubule functionJ Cell Sci2002115Pt 357558511861764
- KhanSAspeJRAsumenMGExtracellular, cell-permeable survivin inhibits apoptosis while promoting proliferative and metastatic potentialBr J Cancer200910071073108619293795
- KhanSJutzyJMAspeJRMcGregorDWNeidighJWWallNRSurvivin is released from cancer cells via exosomesApoptosis201116111220717727
- WebberJYeungVClaytonAExtracellular vesicles as modulators of the cancer microenvironmentSemin Cell Dev Biol201540273425662446
- LiFAckermannEJBennettCFPleiotropic cell-division defects and apoptosis induced by interference with survivin functionNat Cell Biol19991846146610587640
- LiFLingXSurvivin study: an update of “what is the next wave”?J Cell Physiol2006208347648616557517
- YangDWelmABishopJMCell division and cell survival in the absence of survivinProc Natl Acad Sci U S A200410142151001510515477601
- ChurchDNTalbotDCSurvivin in solid tumors: rationale for development of inhibitorsCurr Oncol Rep201214212012822234703
- AltieriDCSurvivin, cancer networks and pathway-directed drug discoveryNat Rev Cancer200881617018075512
- DongZVenkatachalamMAWangJUp-regulation of apoptosis inhibitory protein IAP-2 by hypoxia. Hif-1-independent mechanismsJ Biol Chem200127622187021870911278985
- DohiTOkadaKXiaFAn IAP-IAP complex inhibits apoptosisJ Biol Chem200427933340873409015218035
- ZagórskaADulakJHIF-1: the knowns and unknowns of hypoxia sensingActa Biochim Pol200451356358515448722
- TammIWangYSausvilleEIAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugsCancer Res19985823531553209850056
- BanksDPPlesciaJAltieriDCSurvivin does not inhibit caspase-3 activityBlood200096124002400311186274
- MarusawaHMatsuzawaSWelshKHBXIP functions as a cofactor of survivin in apoptosis suppressionEMBO J200322112729274012773388
- DenzerKKleijmeerMJHeijnenHFStoorvogelWGeuzeHJExosome: from internal vesicle of the multivesicular body to intercellular signaling deviceJ Cell Sci2000113Pt 193365337410984428
- KellerSSandersonMPStoeckAAltevogtPExosomes: from biogenesis and secretion to biological functionImmunol Lett2006107210210817067686
- SimpsonRJLimJWMoritzRLMathivananSExosomes: proteomic insights and diagnostic potentialExpert Rev Proteomics20096326728319489699
- GreeningDWGopalSKXuRSimpsonRJChenWExosomes and their roles in immune regulation and cancerSemin Cell Dev Biol201540728125724562
- WolfersJLozierARaposoGTumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-primingNat Med20017329730311231627
- AndreFSchartzNEMovassaghMMalignant effusions and immunogenic tumour-derived exosomesLancet2002360932929530512147373
- WieckowskiEWhitesideTLHuman tumor-derived vs dendritic cell-derived exosomes have distinct biologic roles and molecular profilesImmunol Res2006361–324725417337785
- ZitvogelLRegnaultALozierAEradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomesNat Med1998455946009585234
- AlečkovićMKangYRegulation of cancer metastasis by cell-free miRNAsBiochim Biophys Acta201518551244225450578
- AltieriDCValidating survivin as a cancer therapeutic targetNat Rev Cancer200331465412509766
- GinestraALa PlacaMDSaladinoFCassaràDNagaseHVittorelliMLThe amount and proteolytic content of vesicles shed by human cancer cell lines correlates with their in vitro invasivenessAnticancer Res1998185A343334379858920
- GinestraAMiceliDDoloVRomanoFMVittorelliMLMembrane vesicles in ovarian cancer fluids: a new potential markerAnticancer Res1999194C3439344510629632
- NilssonJSkogJNordstrandAProstate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancerBr J Cancer2009100101603160719401683
- RolfoCCastigliaMHongDLiquid biopsies in lung cancer: the new ambrosia of researchersBiochim Biophys Acta20141846253954625444714
- AdamsMNavabiHCrostonDThe rationale for combined chemo/immunotherapy using a Toll-like receptor 3 (TLR3) agonist and tumour-derived exosomes in advanced ovarian cancerVaccine20052317–182374237815755631
- ShenderVOPavlyukovMSZiganshinRHProteome-metabolome profiling of ovarian cancer ascites reveals novel components involved in intercellular communicationMol Cell Proteomics201413123558357125271300
- ParkJOChoiDYChoiDSIdentification and characterization of proteins isolated from microvesicles derived from human lung cancer pleural effusionsProteomics201313142125213423585444
- KhanSJutzyJMValenzuelaMMPlasma-derived exosomal survivin, a plausible biomarker for early detection of prostate cancerPLoS One2012710e4673723091600
- Zangemeister-WittkeUSimonHUAn IAP in action: the multiple roles of survivin in differentiation, immunity and malignancyCell Cycle2004391121112315326382
- XingZConwayEMKangCWinotoAEssential role of survivin, an inhibitor of apoptosis protein, in T cell development, maturation, and homeostasisJ Exp Med20041991698014699085
- MeraSMagnussonMTarkowskiABokarewaMExtracellular survivin up-regulates adhesion molecules on the surface of leukocytes changing their reactivity patternJ Leukoc Biol200883114915517938276
- TanakaMButlerMOAnsénSInduction of HLA-DP4-restricted anti-survivin Th1 and Th2 responses using an artificial antigen-presenting cellClin Cancer Res201117165392540121705450
- YangZWangLWangHA novel mimovirus vaccine containing survivin epitope with adjuvant IL-15 induces long-lasting cellular immunity and high antitumor efficiencyMol Immunol20084561674168118035418
- LladserAPárragaMQuevedoLNaked DNA immunization as an approach to target the generic tumor antigen survivin induces humoral and cellular immune responses in miceImmunobiology20062111–2112716446167
- WanYYMulti-tasking of helper T cellsImmunology2010130216617120557575
- PietraGManziniCRivaraSMelanoma cells inhibit natural killer cell function by modulating the expression of activating receptors and cytolytic activityCancer Res20127261407141522258454
- AmarnathSMaqusCWWangJCThe PDL1-PD1 axis converts human TH1 cells into regulatory T cellsSci Transl Med20113111111ra120
- JutzyJMKhanSAsuncion-ValenzuelaMMMilfordTAPayneKJWallNRTumor-released survivin induces a type-2 t cell response and decreases cytotoxic T cell function, in vitroCancer Microenviron201361576822322461
- DehalAAbbasAJohnaSRacial disparities in clinical presentation, surgical treatment and in-hospital outcomes of women with breast cancer: analysis of nationwide inpatient sample databaseBreast Cancer Res Treat2013139256156923690143
- JohnsonRHChienFLBleyerAIncidence of breast cancer with distant involvement among women in the United States, 1976 to 2009JAMA2013309880080523443443
- VelculescuVEMaddenSLZhangLAnalysis of human transcriptomesNat Genet199923438738810581018
- Kalla SinghSTanQWBritoCDe LeónMGarberoglioCDe LeónDDifferential insulin-like growth factor II (IGF-II) expression: A potential role for breast cancer survival disparityGrowth Horm IGF Res201020216217020089431
- AdamkovMKajoKVybohovaDKrajcovicJStullerFRajcaniJCorrelations of survivin expression with clinicomorphological parameters and hormonal receptor status in breast ductal carcinomaNeoplasma2012591303722103896
- RexhepajEJirstromKO’ConnorDPValidation of cytoplasmic-to-nuclear ratio of survivin as an indicator of improved prognosis in breast cancerBMC Cancer20101063921092276
- XuCYamamoto-IbusukiMYamamotoYHigh survivin mRNA expression is a predictor of poor prognosis in breast cancer: a comparative study at the mRNA and protein levelBreast Cancer201421448249022968628
- Dedić PlavetićNJakić-RazumovićJKulićAVrbanecDPrognostic value of proliferation markers expression in breast cancerMed Oncol201330252323468220
- SpanPNTjan-HeijnenVCMandersPvan TienovenDLehrJSweepFCHigh survivin predicts a poor response to endocrine therapy, but a good response to chemotherapy in advanced breast cancerBreast Cancer Res Treat200698222323016541327
- LiFRole of survivin and its splice variants in tumorigenesisBr J Cancer200592221221615611788
- Necochea-CampionRdChenCSMirshahidiSHowardFDWallNRClinico-pathologic relevance of Survivin splice variant expression in cancerCancer Lett2013339216717423791888
- BoidotRVegranFLizard-NacolSPredictive value of survivin alternative transcript expression in locally advanced breast cancer patients treated with neoadjuvant chemotherapyInt J Mol Med200923228529119148555
- KhanSBennitHFTurayDEarly diagnostic value of survivin and its alternative splice variants in breast cancerBMC Cancer20141417624620748
- BrawleyOWProstate cancer epidemiology in the United StatesWorld J Urol201230219520022476558
- BasuABanerjeeHRojasHDifferential expression of peroxiredoxins in prostate cancer: consistent upregulation of PRDX3 and PRDX4Prostate201171775576521031435
- HoffmanRMGillilandFDEleyJWRacial and ethnic differences in advanced-stage prostate cancer: the Prostate Cancer Outcomes StudyJ Natl Cancer Inst200193538839511238701
- KaramiSYoungHAHensonDEEarlier age at diagnosis: another dimension in cancer disparity?Cancer Detect Prev2007311293417303347
- ZaffaroniNPennatiMDaidoneMGSurvivin as a target for new anticancer interventionsJ Cell Mol Med20059236037215963255
- ShariatSFLotanYSaboorianHSurvivin expression is associated with features of biologically aggressive prostate carcinomaCancer2004100475175714770431
- KoikeHSekineYKamiyaMNakazatoHSuzukiKGene expression of survivin and its spliced isoforms associated with proliferation and aggressive phenotypes of prostate cancerUrology20087261229123318336887
- ZhangMHoAHammondEHPrognostic value of survivin in locally advanced prostate cancer: study based on RTOG 8610Int J Radiat Oncol Biol Phys20097341033104218977097
- LowenfelsABMaisonneuvePEpidemiology and risk factors for pancreatic cancerBest Pract Res Clin Gastroenterol200620219720916549324
- GordisLGoldEBEpidemiology and Etiology of Pancreatic CancerGoPancreas, Biology, and Pathobiology of Disease Second EditionNew YorkRaven Press1993837855
- WoutersenRAAppelMJvan Garderen-HoetmerAWijnandsMVDietary fat and carcinogenesisMutat Res19994431–211112710415435
- SingalVSingalAKKuoYFRacial disparities in treatment for pancreatic cancer and impact on survival: a population-based analysisJ Cancer Res Clin Oncol2012138471572222246279
- HayangaAJRisk of pancreatic adenocarcinoma: disparity between African Americans and other race/ethnic groupsCancer20051041125302531 author reply 253116240447
- ChangKJParasherGChristieCLargentJAnton-CulverHRisk of pancreatic adenocarcinoma: disparity between African Americans and other race/ethnic groupsCancer2005103234935715593353
- PernickNLSarkarFHPhilipPAClinicopathologic analysis of pancreatic adenocarcinoma in African Americans and CaucasiansPancreas2003261283212499914
- DingXZTongWGAdrianTECyclooxygenases and lipoxygenases as potential targets for treatment of pancreatic cancerPancreatology20011429129912120207
- TongWGDingXZWittRCAdrianTELipoxygenase inhibitors attenuate growth of human pancreatic cancer xenografts and induce apoptosis through the mitochondrial pathwayMol Cancer Ther200211192993512481414
- XieHJiangWXiaoSYLiuXHigh expression of survivin is prognostic of shorter survival but not predictive of adjuvant gemcitabine benefit in patients with resected pancreatic adenocarcinomaJ Histochem Cytochem201361214815523124118
- ToniniGVincenziBSantiniDNuclear and cytoplasmic expression of survivin in 67 surgically resected pancreatic cancer patientsBr J Cancer200592122225223215928668
- SagolOYavuzsenTOztopIThe effect of apoptotic activity, survivin, Ki-67, and P-glycoprotein expression on prognosis in pancreatic carcinomaPancreas200530434334815841045
- SunHCQiuZJLiuJExpression of hypoxia-inducible factor-1 alpha and associated proteins in pancreatic ductal adenocarcinoma and their impact on prognosisInt J Oncol20073061359136717487356
- The Leukemia and Lymphoma Society [webpage on the Internet]Facts and statisticsWhite Plains, NYThe Leukemia and Lymphoma Society2015 Available from: http://www.lls.org/diseaseinformation/getinformationsupport/factsstatistics/Accessed February 15, 2015
- PulteDRedanielMTBrennerHJeffreysMChanges in survival by ethnicity of patients with cancer between 1992–1996 and 2002–2006: is the discrepancy decreasing?Ann Oncol20122392428243422396445
- Waligórska-StachuraJJankowskaAWaśkoRSurvivin – prognostic tumor biomarker in human neoplasms – reviewGinekol Pol201283753754022880480
- FuldaSInhibitor of apoptosis proteins in hematological malignanciesLeukemia200923346747619039324
- FuldaSExploiting inhibitor of apoptosis proteins as therapeutic targets in hematological malignanciesLeukemia20122661155116522230799
- KamihiraSYamadaYHirakataYAberrant expression of caspase cascade regulatory genes in adult T-cell leukaemia: survivin is an important determinant for prognosisBr J Haematol20011141636911472346
- AdidaCRecherCRaffouxEExpression and prognostic significance of survivin in de novo acute myeloid leukaemiaBr J Haematol2000111119620311091201
- ParkEGangEJHsiehYTTargeting survivin overcomes drug resistance in acute lymphoblastic leukemiaBlood201111882191219921715311
- KellyRJLopez-ChavezACitrinDJanikJEMorrisJCImpacting tumor cell-fate by targeting the inhibitor of apoptosis protein survivinMol Cancer2011103521470426
- TroegerASiepermannMEscherichGSurvivin and its prognostic significance in pediatric acute B-cell precursor lymphoblastic leukemiaHaematologica20079281043105017640858
- EshAMAtfyMAziziNAEl NaggarMMKhalilEESheriefLPrognostic significance of survivin in pediatric acute lymphoblastic leukemiaIndian J Hematol Blood Transfus2011271182522379290
- TynerJWJemalAMThayerMDrukerBJChangBHTargeting survivin and p53 in pediatric acute lymphoblastic leukemiaLeukemia201226462363221960246
- AhmedMBShehataHHMoussaMIbrahimTMPrognostic significance of survivin and tumor necrosis factor-alpha in adult acute lymphoblastic leukemiaClin Biochem2012451–211211621933669
- MorrisonDJHoganLECondosGEndogenous knockdown of survivin improves chemotherapeutic response in ALL modelsLeukemia201226227127921844871
- WangZSampathJFukudaSPelusLMDisruption of the inhibitor of apoptosis protein survivin sensitizes Bcr-abl-positive cells to STI571-induced apoptosisCancer Res200565188224823216166298
- Grzybowska-IzydorczykOCebulaBRobakTSmolewskiPExpression and prognostic significance of the inhibitor of apoptosis protein (IAP) family and its antagonists in chronic lymphocytic leukaemiaEur J Cancer201046480081020045309
- CarterBZQiuYHuangXSurvivin is highly expressed in CD34(+)38(−) leukemic stem/progenitor cells and predicts poor clinical outcomes in AMLBlood2012120117318022645176
- SmallSKeerthivasanGHuangZGurbuxaniSCrispinoJDOverexpression of survivin initiates hematologic malignancies in vivoLeukemia201024111920192620882051
- KumarBYadavALangJCYM155 reverses cisplatin resistance in head and neck cancer by decreasing cytoplasmic survivin levelsMol Cancer Ther20121191988199822723337
- BernardoPSReisFRMaiaRCImatinib increases apoptosis index through modulation of survivin subcellular localization in the blast phase of CML cellsLeuk Res201236121510151622975581
- QinQZhangCZhuHAssociation between survivin -31G>C polymorphism and cancer risk: meta-analysis of 29 studiesJ Cancer Res Clin Oncol2014140217918824077840
- Radojevic-SkodricSBasta-JovanovicGBrasanacDSurvivin gene promoter −31 G/C polymorphism is associated with Wilms tumor susceptibility in Serbian childrenJ Pediatr Hematol Oncol2012348e310e31422858571
- LiFYangJRamnathNJavleMMTanDNuclear or cytoplasmic expression of survivin: what is the significance?Int J Cancer2005114450951215578717
