Abstract
Hepatocellular carcinoma (HCC) is a major cause of cancer death worldwide. HCC is usually asymptomatic at potential curative stages, and it has very poor prognosis if detected later. Thus, the identification of early biomarkers and novel therapies is essential to improve HCC patient survival. Ion channels have been proposed as potential tumor markers and therapeutic targets for several cancers including HCC. Especially, the ether à-go-go-1 (Eag1) voltage-gated potassium channel has been suggested as an early marker for HCC. Eag1 is overexpressed during HCC development from the cirrhotic and the preneoplastic lesions preceding HCC in a rat model. The channel is also overexpressed in human HCC. Astemizole has gained great interest as a potential anticancer drug because it targets several proteins involved in cancer including Eag1. Actually, in vivo studies have shown that astemizole may have clinical utility for HCC prevention and treatment. Here, we will review first some general aspects of HCC including the current biomarkers and therapies, and then we will focus on Eag1 channels as promising tools in the early diagnosis of HCC.
Introduction
Primary liver cancer is a major health problem,Citation1 representing the second leading cause of cancer-related deaths in the world.Citation2 Hepatocellular carcinoma (HCC) accounts for up to 90% of primary liver cancers.Citation1 Other liver cancer types include childhood hepatoblastoma, adult cholangiocarcinoma (originating from the intrahepatic biliary ducts), and angiosarcoma (from the intrahepatic blood vessels).Citation3
HCC affects men three times more frequently than women worldwide. It is the fifth most frequently diagnosed cancer in adult men, and the seventh most commonly diagnosed cancer in adult women.Citation4 Interestingly, significant differences have been noted between the races. Asians are affected two times more than blacks, and Hispanics are affected two times more than whites. The ethnic variability reflects the contribution of specific causal factor differences among populations.Citation4 Because of its poor prognosis, it is compulsory to find novel early markers of the disease as well as new therapeutic approaches for HCC prevention and treatment.
HCC pathophysiology
Liver cirrhosis is the strongest HCC predisposing factor. Actually, 80% of HCC cases formerly developed cirrhosis. Other well-defined risk factors comprise viral infections (chronic hepatitis B and C), toxics (alcohol and aflatoxin B1), and altered metabolic conditions (diabetes, nonalcoholic fatty liver disease, hereditary hemochromatosis).Citation5–Citation7 Recently, tobacco and obesity have also been proposed as potential risk factors for the development of HCC.Citation8 The major risk factors for HCC are described in .
Table 1 Main HCC risk factors
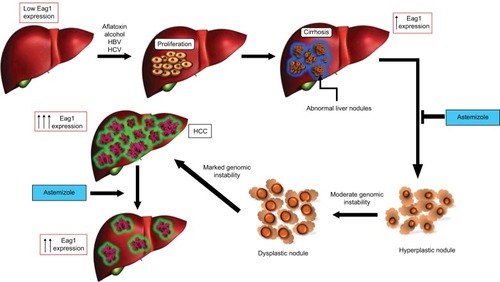
Hepatocarcinogenesis is a multistep process in which a number of genetic alterations accumulate in the cells. After hepatic injury due to major predisposing risk factors for HCC, necrosis arises followed by hepatocyte proliferation. This continuous process of destructive–regenerative cycles in the hepatocyte promotes chronic liver injury and progressive liver fibrosis resulting in cirrhosis. If the process continues, the next step is the progressive malignant transformation of cirrhotic nodules and premalignant lesions, which will finally lead to HCCCitation9,Citation10 ().
Figure 1 Eag1 expression in the progression of HCC.
Abbreviations: Eag1, ether à-go-go-1; HCC, hepatocellular carcinoma.
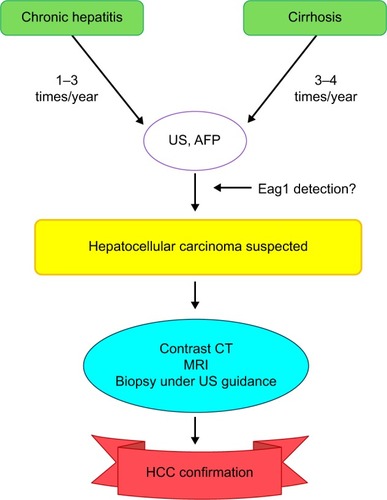
Because most HCC cases are associated with chronic viral hepatitis, prevention of virus infection should lead to the prevention of HCC.Citation11–Citation13 Some preventive strategies already used include vaccination (the universal vaccination was introduced in all newborns and high-risk groups as routine immunizationCitation13–Citation15), antiviral treatment (it has been shown that antiviral treatment of chronic hepatitis B virus and hepatitis C virus infections may reduce the risk of HCCCitation13,Citation16,Citation17), and periodical surveillance in patients at risk of developing a disease ().Citation11,Citation13,Citation18
Figure 2 Follow-up for patients at risk of HCC.
Abbreviations: AFP, alfa-fetoprotein; CT, computed tomography; Eag1, ether à-go-go-1; HCC, hepatocellular carcinoma; MRI, magnetic resonance imaging; US, ultrasound.
HCC diagnosis and treatment
Diagnosis
HCC is frequently diagnosed at an asymptomatic stage while the patients are being evaluated for liver transplantation or as part of routine screening in cirrhotic patients. The classic clinical features of HCC include right upper quadrant pain, weight loss, and worsening of liver function. Patients with chronic liver diseases belong to a high-risk group for HCC and a follow-up based on imaging and tumor marker levelsCitation11,Citation19 should be regularly made for early diagnosis.
Current and emerging potential HCC biomarkers
Alfa-fetoprotein (AFP) is commonly used as an HCC marker; serum AFP levels may be helpful in the diagnosis and management of HCC. AFP is higher than 20 ng/mL in more than 70% of HCC patients. However, AFP levels from 10 to 500 ng/mL and even up to 1,000 ng/mL may be found in patients with other liver diseases who do not have HCC. AFP is useful mainly in monitoring the response to treatment and detecting recurrence after treatment of HCC, if the AFP levels were elevated before treatment.Citation11,Citation12,Citation19,Citation20
Despite that an ideal biomarker should have high sensitivity and specificityCitation21 and be detectable in any of different samples such as blood, urine, or tissues,Citation22 the coexistence of inflammation and cirrhosis in HCC makes the early diagnosis and prognostic assessment much more difficult. This complication highlights the need to identify valuable biomarkers for the diagnosis and treatment of HCC.Citation22
Recent advances in genomics and proteomics have facilitated the identification of many biomarkers, including new HCC molecular markers. However, only a few biomarkers are acceptable for clinical utility because the rest have low predictive accuracy and/or high cost.Citation23,Citation24 The most relevant biomarkers in liver cancer are described in .
Table 2 Principal and potential HCC biomarkers
Most of these markers have been studied in a retrospective manner, and only a few prospective trials have evaluated their clinical significance. However, as HCC is a complex disease with multiple underlying pathogenic mechanisms caused by a variety of risk factors, it is difficult to characterize HCC with a single biomarker. In addition, most of the used biomarkers are associated with liver damage and not HCC itself. Therefore, novel HCC markers and diagnostic approaches are needed. The combination of biomarkers in a detection kit may be more valuable for the diagnosis and prognosis of HCC. It is also important to identify noninvasive and cost-effective biomarkers for early diagnosis.
Imaging
Ultrasound (US) studies still play an important role in detecting HCC because US is able to detect small liver lesions and has relatively high sensitivity and specificity. Its use in diagnosis is being replaced by computed tomography and magnetic resonance imaging, especially for those patients whose liver cannot be fully imaged by US because the tumors are very small or whose echograms do not show lesions clearly.Citation11,Citation12,Citation19
Biopsy
If a tumor is detected by imaging, a biopsy can be obtained typically with a needle under US radiologic guidance. The material obtained by fine-needle aspiration is evaluated to determine HCC staging.Citation11,Citation12,Citation19 Because HCC patients are diagnosed at advanced stages of the disease, it is advisable to screen patients who are at risk of developing the disease like those with chronic hepatitis and cirrhosis for early diagnosis (). The most common classification of HCC depends on its stage (from early to terminal) and its differentiation grade (well, moderately, or poorly differentiated).Citation13
Treatment
HCC treatment depends on the tumor stage, patient performance status, and liver function and requires a multidisciplinary approach. The treatment strategies of HCC are summarized in .
Table 3 Therapies according to the HCC stage
Unfortunately, HCC is among the most chemoresistant tumors. Chemotherapy with cytotoxic agents, such as doxorubicin, gemcitabine, cisplatin, and 5-fluorouracil, or combined regimen for palliative care is associated with low response rates. Interestingly, targeted chemotherapy with multikinase inhibitors () seems to be an attractive alternative to conventional systemic chemotherapy.
Table 4 Molecular targeted therapies for HCC
Electrochemotherapy is a treatment used in cutaneous and subcutaneous tumors with positive results. Its use in deep tumors has been studied. The study showed favorable results in the treatment of liver metastases. Therefore this treatment was proposed to be used in other types of liver tumors including HCC.Citation25
Due to the very poor prognosis of HCC and its late detection, it is essential to find early tumor markers as well as new therapeutic targets and potential anticancer drugs. Ion channels seem to be promising alternative tools in oncology to reach these goals.
Eag1 potassium channels as potential early biomarkers and alternative tools for HCC prevention and treatment
Several reports have shown the abnormal expression of potassium channels in many tumors. Especially, the voltage-gated potassium channel ether à-go-go-1 (Eag1, KCNH1, Kv10.1) has gained enormous interest in cancer research because of its oncogenic properties.Citation26–Citation28 Eag1 is overexpressed in most human tumors, including liver, cervical, lung, breast, colon, and prostate cancer.Citation26–Citation32 Eag1 channels have been also proposed as early tumor biomarkers and therapeutic targets for different types of cancers.Citation30–Citation36 In accordance with its role in cell proliferation, inhibition of Eag1 reduces tumor cell proliferation in vitro and in vivo Citation26,Citation28,Citation32,Citation33,Citation35,Citation37–Citation40 Astemizole is an antihistamine that has gained great interest as a potential anticancer drug because it targets several proteins involved in cancer, including Eag1 channels, histamine receptors, and proteins involved in drug resistanceCitation41 Cells expressing Eag1 and treated with astemizole display lower cell proliferation; it is hypothesized that this cell proliferation inhibition may be due to astemizole’s blockage of the Eag1 channel.Citation32,Citation33,Citation37,Citation42 In vivo studies showed that astemizole administration reduced the growth rate of xenograft tumors in mice implanted with Eag1-expressing cells.Citation42 Thus, Eag1 channels are inhibited by astemizole and may be potential targets for cancer therapy.Citation26,Citation27,Citation32,Citation41 Because Eag1 expression has been reported in human biopsies from liver tumors,Citation30 astemizole may be used as an anticancer therapy in patients with liver cancer.
Considering the poor prognosis of HCC, our research group investigated if Eag1 channels could serve as early HCC markers by studying Eag1 expression during tumor development in vivo. We used the already established animal model of HCC development, where chronicle injection with diethylnitrosamine (DEN) induces liver injury in rats. This model recapitulates the sequential change from cirrhosis to HCC, as it occurs in humans. Interestingly, we observed high Eag1 mRNA expression in most of the DEN-treated groups, and strong Eag1 protein expression was observed at early stages of HCC developmentCitation32 (). Eag1 expression at the mRNA and protein level was clearly higher in cirrhotic tissues and preneoplastic lesions, in comparison to normal livers.Citation32 Thus, our results showed the potential of Eag1 channels as HCC early biomarkers.
We also observed that astemizole clearly prevented the development of HCC; the antihistamine prevented tumor formation when administered either from the start of DEN treatment or from week 12 of DEN treatment, when cirrhosis is already present.Citation32 These results suggest that astemizole might be used as a chemopreventive agent in patients at risk of developing liver cancer. Additionally, astemizole could be used as an antineoplastic because when administered at the end of the DEN treatment, the animals did not develop big tumors as those receiving astemizole,Citation32 suggesting that this compound may induce tumor regression.
Eag1 mRNA and protein are expressed in the human HCC cell lines HepG2 and HuH7; accordingly, astemizole decreased their cell proliferation and induced apoptosis.Citation32 Taken together, all these results suggest that astemizole may be a novel therapeutic approach for HCC patients.
Other types of channels and transporters also displayed a differential expression during HCC development, suggesting other channels as potential early markers and/or therapeutic targets of HCC.Citation43
Future research on Eag1 channels as HCC biomarkers and challenges
The discovery of cancer biomarkers in recent years has become a major focus of cancer research. The development of new technologies, especially genomics and proteomics, has made possible the identification and discovery of potential biomarkers. In the recent years, the study of microRNAs and exosomes has gained great interest to diagnose HCC in at-risk patients.Citation44–Citation48 An ideal tumor marker would be DNA-, RNA-, protein-, or antibody-based measurable in serum, urine, or sputum.Citation49,Citation50
Because Eag1 may have clinical potential as an early biomarker for HCC,Citation32 it would be very interesting to track Eag1 protein expression using sensitive imaging techniques in human livers. Actually, when Eag1-expressing cells were injected into mice, the channel was detected in nonpalpable tumorsCitation51 using dye-tagged antibodies and imaging techniques. This type of approach might also be used to screen Eag1 expression in the liver of patients at risk of developing HCC and in HCC animal models. It would be also very important to study exosomes containing Eag1 at different stages of HCC in liquid biopsies. Astemizole may be a very promising preventive option for patients at risk of developing HCC as well as a hopeful therapeutic option for HCC patients.Citation32 Therefore, clinical trials testing the effect of astemizole alone or in combination with other antineoplastic drugs in patients either at risk of developing or with HCC should be performed. In addition, detection of Eag1 could be included in the surveillance scheme for patients who are at risk of developing HCC for timely diagnosis ().
The precise role of Eag1 channels in HCC development remains to be elucidated. This might be accomplished in different manners, such as the use of mouse models like Eag1 knockout mice.Citation52 It would be very interesting to investigate whether these knockout mice are resistant to the development of liver cirrhosis and HCC.Citation32
Another very important issue to investigate is the mechanism of the oncogenic potential of the channel. Eag1 expression is regulated by the p53-mir-34-E2F1pathway.Citation53 It has been suggested that mutated or inactive p53 increases Eag1 expression; p53 is mutated in most tumors including HCC.Citation54 The increased Eag1 expression could lead to the activation of different proteins such as HIF-1, cyclins D/E, ERK1/2, and FAK complex as well as increase the intracellular calcium concentration and induce vascular endothelial growth factor release. All these changes may lead to angiogenesis, therapy resistance, cell proliferation, migration, and survival, contributing to carcinogenesis.Citation55 However, the precise molecular mechanism of how Eag1 may contribute to HCC development remains to be elucidated.
Liquid biopsies studying HCC circulating cells may provide novel insights into HCC diagnosis and prognosis.Citation56,Citation57 Because Eag1 channels might serve as early markers for different types of cancer, it may be very useful to detect Eag1 mRNA/protein expression in such liquid biopsies. This approach may help to find the urgently needed HCC markers, which are hardly available. However, one of the biggest challenges to overcome would be the sensitivity and specificity of the HCC detection method based on Eag1 expression. In addition, prospective studies should be made to know if cirrhotic patients expressing Eag1 channels would develop HCC.
Conclusion
Because of the poor prognosis of HCC, it is very important to find novel preventive and therapeutic alternatives. Recent studies have suggested that astemizole may be a very promising preventive option for patients at risk of developing HCC as well as a hopeful therapeutic option for HCC patients. It has also been recently proposed that Eag1 may be a potential early biomarker of HCC. Astemizole-based prevention and therapy and early Eag1 detection in the liver should help to reduce mortality from this disease.
Disclosure
The authors report no conflicts of interest in this work.
References
- MittalSEl-SeragHBEpidemiology of hepatocellular carcinoma: consider the populationJ Clin Gastroenterol201347SupplS2S623632345
- JemalASiegelRWardECancer statistics, 2008CA Cancer J Clin2008582719618287387
- ChuangSCLa VecchiaCBoffettaPLiver cancer: descriptive epidemiology and risk factors other than HBV and HCV infectionCancer Lett2009286191419091458
- VenookAPPapandreouCFuruseJde GuevaraLLThe incidence and epidemiology of hepatocellular carcinoma: a global and regional perspectiveOncologist201015Suppl 451321115576
- HerbstDAReddyKRRisk factors for hepatocellular carcinomaClin Liver Dis201216180182
- ShermanMLlovetJMSmoking, hepatitis B virus infection, and development of hepatocellular carcinomaJ Natl Cancer Inst2011103221642164322021668
- BruixJLlovetJMHepatitis B virus and hepatocellular carcinomaJ Hepatol200339Suppl 1S59S6314708679
- GaoJXieLYangWSRisk factors of hepatocellular carcinoma – current status and perspectivesAsian Pac J Cancer Prev201213374375222631642
- HongMLiSTanHYWangNTsaoSWFengYCurrent status of herbal medicines in chronic liver disease therapy: the biological effects, molecular targets and future prospectsInt J Mol Sci20151612287052874526633388
- FaraziPADePinhoRAHepatocellular carcinoma pathogenesis: from genes to environmentNat Rev Cancer20066967468716929323
- ImawariMLiver cancer, prevention and early diagnosisJMAJ200253130133
- WangCHWeyKCMoLRChangKKLinRCKuoJJCurrent trends and recent advances in diagnosis, therapy, and prevention of hepatocellular carcinomaAsian Pac J Cancer Prev20151693595360425987009
- InglePVSamsudinSZChanPQDevelopment and novel therapeutics in hepatocellular carcinoma: a reviewTher Clin Risk Manag20161244545527042086
- GiacominACazzagonNSergioAVaninVFarinatiFHepatitis B virus-related hepatocellular carcinoma: primary, secondary, and tertiary preventionEur J Cancer Prev201120538138821540746
- ChanCYLeeSDLoKJLegend of hepatitis B vaccination: the Taiwan experienceJ Gastroenterol Hepatol200419212112614731119
- DhanasekaranRLimayeACabreraRHepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis, and therapeuticsHepat Med20124193724367230
- ChanHLWongGLTseCHChanHYWongVWViral determinants of hepatitis B surface antigen seroclearance in hepatitis B e antigen-negative chronic hepatitis B patientsJ Infect Dis2011204340841421742839
- LlovetJMBruixJNovel advancements in the management of hepatocellular carcinoma in 2008J Hepatol200848suppl 1S20S3718304676
- BefelerASDi BisceglieAMHepatocellular carcinoma: diagnosis and treatmentGastroenterology200212261609161912016426
- JohnsonPJThe role of serum alpha-fetoprotein estimation in the diagnosis and management of hepatocellular carcinomaClin Liver Dis20015114515911218912
- ParikhNIVasanRSAssessing the clinical utility of biomarkers in medicineBiomark Med20071341943620477384
- HartwellLMankoffDPaulovichARamseySSwisherECancer biomarkers: a systems approachNat Biotechnol200624890590816900126
- GonzalezSANovel biomarkers for hepatocellular carcinoma surveillance: has the future arrived?Hepatobiliary surgery and nutrition20143641041425568864
- ZhuKDaiZZhouJBiomarkers for hepatocellular carcinoma: progression in early diagnosis, prognosis, and personalized therapyBiomark Res2013111824252729
- EdhemovicIBreceljEGasljevicGIntraoperative electrochemotherapy of colorectal liver metastasesJ Surg Ocol20141103320327
- PardoLAStühmerWEag1: an emerging oncological targetCancer Res20086861611161318339837
- WulffHCastleNAPardoLAVoltage-gated potassium channels as therapeutic targetsNat Rev Drug Discov2009812982100119949402
- PardoLAdel CaminoDSanchezAOncogenic potential of EAG K(+) channelsEMBO J199918205540554710523298
- Rodríguez-RasgadoJAAcuña-MacíasICamachoJEag1 channels as potential cancer biomarkersSensors (Basel)20121255986599522778627
- HemmerleinBWeselohRMMello de QueirozFOverexpression of Eag1 potassium channels in clinical tumoursMol Cancer200654117022810
- OusingsawatJSpitznerMPuntheeranurakSExpression of voltage-gated potassium channels in human and mouse colonic carcinomaClin Cancer Res200713382483117289873
- de Guadalupe Chávez-LópezMPérez-CarreónJIZuñiga-GarcíaVAstemizole-based anticancer therapy for hepatocellular carcinoma (HCC), and Eag1 channels as potential early-stage markers of HCCTumor Biol201536861496158
- DiazLCeja-OchoaIRestrepo-AnguloIEstrogens and human papilloma virus oncogenes regulate human ether-a-go-go-1 potassium channel expressionCancer Res20096983300330719351862
- FariasLMOcanaDBDiazLEther a go-go potassium channels as human cervical cancer markersCancer Res200464196996700115466192
- Gómez-VarelaDZwick-WallaschEKnötgenHMonoclonal antibody blockade of the human Eag1 potassium channel function exerts antitumor activityCancer Res200767157343734917671204
- OrtizCSMontante-MontesDSaqui-SalcesMEag1 potassium channels as markers of cervical dysplasiaOncol Rep20112661377138321887469
- Ouadid-AhidouchHLe BourhisXRoudbarakiMToillonRADelcourtPPrevarskayaNChanges in the K+ current-density of MCF-7 cells during progression through the cell cycle: possible involvement of a h-ether.a-gogo K+ channelReceptors Channels20017534535611697078
- WeberCMello de QueirozFDownieBRSuckowAStuhmerWPardoLASilencing the activity and proliferative properties of the human EagI potassium channel by RNA InterferenceJ Biol Chem200628119130301303716537547
- Garcia-BecerraRDiazLCamachoJCalcitriol inhibits ether-a go-go potassium channel expression and cell proliferation in human breast cancer cellsExp Cell Res2010316343344219932096
- Gavrilova-RuchOSchonherrKGessnerGEffects of imipramine on ion channels and proliferation of IGR1 melanoma cellsJ Membr Biol2002188213714912172639
- Garcia-QuirozJCamachoJAstemizole: an old anti-histamine as a new promising anti-cancer drugAnticancer Agents Med Chem201111330731421443504
- DownieBRSanchezAKnötgenHEag1 expression interferes with hypoxia homeostasis and induces angiogenesis in tumorsJ Biol Chem200828352362343624018927085
- Zúñiga-GarcíaVChávez-López MdeGQuintanar-JuradoVDifferential expression of ion channels and transporters during hepatocellular carcinoma developmentDig Dis Sci20156082373238325842354
- HayesCNChayamaKMicroRNAs as biomarkers for liver disease and hepatocellular carcinomaInt J Mol Sci201617328026927063
- LinXJChongYGuoZWA serum microRNA classifier for early detection of hepatocellular carcinoma: a multicentre, retrospective, longitudinal biomarker identification study with a nested case-control studyLancet Oncol201516780481526088272
- KaiserJMalignant messengersScience2016352628216416627124448
- MinciacchiVRFreemanMRDi VizioDExtracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomesSemin Cell Dev Biol201540415125721812
- FeoFPascaleRMMultifocal hepatocellular carcinoma: intrahepatic metastasis or multicentric carcinogenesis?Ann Transl Med201531425705636
- VermaMKaganJSidranskyDSrivastavaSProteomic analysis of cancer-cell mitochondriaNat Rev Cancer200331078979514570046
- KumarSMohanAGuleriaRBiomarkers in cancer screening, research and detection: present and future: a reviewBiomarkers200611538540516966157
- PardoLAContreras-JuradoCZientkowskaMAlvesFStühmerWRole of voltage-gated potassium channels in cancerJ Membr Biol2005205311512416362499
- UfartesRSchneiderTMortensenLSBehavioural and functional characterization of Kv10.1 (Eag1) knockout miceHum Mol Genet201322112247226223424202
- LinHLiZChenCTranscriptional and post-transcriptional mechanisms for oncogenic overexpression of ether a go-go K+ channelPLoS One201165e2036221655246
- De BenedettiVMWelshJAYuMCBennettWPp53 mutations in hepatocellular carcinoma related to oral contraceptive useCarcinogenesis19961711451498565124
- Ouadid-AhidouchHAhidouchAPardoLAKv10.1 K(+) channel: from physiology to cancerPflugers Arch2016468575176226743871
- XueRLiRGuoHVariable intra-tumor genomic heterogeneity of multiple lesions in patients with hepatocellular carcinomaGastroenterology20161504998100826752112
- ZakiMYWReevesHLThe genetic heterogeneity of hepatocellular carcinoma and the implications for personalised medicineTransl Cancer Res20165S1S1S4
- StarrSPRainesDCirrhosis: diagnosis, management, and preventionAm Family Physician2011841213531359
- TsochatzisEABoschJBurroughsAKLiver cirrhosisLancet201438399301749176124480518
- KewMCHepatitis B virus x protein in the pathogenesis of hepatitis B virus-induced hepatocellular carcinomaJ Gastroenterol Hepatol201126suppl 114415221199526
- GengMXinXBiLQZhouLTLiuXHMolecular mechanism of hepatitis B virus X protein function in hepatocarcinogenesisWorld J Gastroenterol20152138107321073826478665
- TarocchiMPolvaniSMarronciniGGalliAMolecular mechanism of hepatitis B virus-induced hepatocarcinogenesisWorld J Gastroenterol20142033116301164025206269
- WirthTCMannsMPThe impact of the revolution in hepatitis C treatment on hepatocellular carcinomaAnn Oncol20162781467147427226385
- JahanSAshfaqUAQasimMKhaliqSSaleemMJAfzalNHepatitis C virus to hepatocellular carcinomaInfect Agent Cancer201271222289144
- KewMCAflatoxins as a cause of hepatocellular carcinomaJ Gastrointestin Liver Dis9201322330531024078988
- TestinoGLeoneSBorroPAlcohol and hepatocellular carcinoma: a review and a point of viewWorld J Gastroenterol20142043159431595425473148
- Ali KamkarMMAhmadRAlsmadiOBehbehaniKInsight into the impact of diabetes mellitus on the increased risk of hepatocellular carcinoma: mini-reviewJ Diabet Metab Disord20141357
- StrebaLAVereCCRogoveanuIStrebaCTNonalcoholic fatty liver disease, metabolic risk factors, and hepatocellular carcinoma: an open questionWorld J Gastroenterol201521144103411025892859
- KikuchiLOliveiraCPCarrilhoFJNonalcoholic fatty liver disease and hepatocellular carcinomaBiomed Res Int201420146
- KewMCHepatic iron overload and hepatocellular carcinomaLiver Cancer201431314024804175
- PurohitVRapakaRKwonOSSongBJRoles of alcohol and tobacco exposure in the development of hepatocellular carcinomaLife Sci20139213923123447
- KewMCObesity as a cause of hepatocellular carcinomaAnn Hepatol201514329930325864208
- ArrietaOCachoBMorales-EspinosaDRuelas-VillavicencioAFlores-EstradaDHernandez-PedroNThe progressive elevation of alpha fetoprotein for the diagnosis of hepatocellular carcinoma in patients with liver cirrhosisBMC Cancer200772817288606
- PrietoPAChaCHDKK1 as a serum biomarker for hepatocellular carcinomaHepatobiliary Surge Nutr201223127128
- TungEKNgIOSignificance of serum DKK1 as a diagnostic biomarker in hepatocellular carcinomaFuture Oncol20128121525152823231514
- ShenQFanJYangXRSerum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre studyLancet Oncol201213881782622738799
- XuZLiuLPanXSerum Golgi protein 73 (GP73) is a diagnostic and prognostic marker of chronic HBV liver diseaseMedicine (Baltimore)20159412e65925816035
- ZakharyNIKhodeerSMShafikHEAbdel MalakCAImpact of PIVKA-II in diagnosis of hepatocellular carcinomaJ Adv Res20134653954625685463
- YuRDingSTanWPerformance of protein induced by vitamin k absence or antagonist-II (PIVKA-II) for hepatocellular carcinoma screening in Chinese populationHepat Mon2015157e2880626300931
- BeneduceLCastaldiFMarinoMSquamous cell carcinoma antigen-immunoglobulin M complexes as novel biomarkers for hepatocellular carcinomaCancer2005103122558256515887222
- SchütteKSchulzCLinkAMalfertheinerPCurrent biomarkers for hepatocellular carcinoma: Surveillance, diagnosis and prediction of prognosisWorld J Hepatol20157213914925729470
- WangKGuoWLiNAlpha-1-fucosidase as a prognostic indicator for hepatocellular carcinoma following hepatectomy: a large-scale, long-term studyBr J Cancer201411071811181924569461
- ZhangSYLinBDLiBREvaluation of the diagnostic value of alpha-1-fucosidase, alpha-fetoprotein and thymidine kinase 1 with ROC and logistic regression for hepatocellular carcinomaFEBS Open Bio20155240244
- FilmusJCapurroMGlypican-3: a marker and a therapeutic target in hepatocellular carcinomaFEBS J2013280102471247623305321
- WangHLAnatelliFZhaiQJAdleyBChuangSTYangXJGlypican-3 as a useful diagnostic marker that distinguishes hepatocellular carcinoma from benign hepatocellular mass lesionsArch Pathol Lab Med2008132111723172818976006
- WangZRuanYBGuanYLiuSHExpression of IGF-II in early experimental hepatocellular carcinomas and its significance in early diagnosisWorld J Gastroenterol20039226727012532445
- WangLYaoMDongZZhangYYaoDCirculating specific biomarkers in diagnosis of hepatocellular carcinoma and its metastasis monitoringTumour Biol201435192024006223
- SimãoAMadalenoJSilvaNPlasma osteopontin is a biomarker for the severity of alcoholic liver cirrhosis, not for hepatocellular carcinoma screeningBMC Gastroenterol2015157326122937
- NovellinoLRossiRLBoninoFCirculating hepatitis B surface antigen particles carry hepatocellular microRNAsPLoS One201273e3195222470417
- JiangLChengQZhangBHZhangMZCirculating microRNAs as biomarkers in hepatocellular carcinoma screening: a validation set from ChinaMedicine (Baltimore)20159410e60325761179
- LiuAMYaoTJWangWCirculating miR-15b and miR-130b in serum as potential markers for detecting hepatocellular carcinoma: a retrospective cohort studyBMJ Open201222e000825
- MotawiTKShakerOGEl-MaraghySASenousyMASerum microRNAs as potential biomarkers for early diagnosis of hepatitis C virus-related hepatocellular carcinoma in Egyptian patientsPLoS One2015109e013770626352740
- WenYHanJChenJPlasma miRNAs as early biomarkers for detecting hepatocellular carcinomaInt J Cancer201513771679169025845839
- KudoMImanakaKChidaNPhase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinomaEur J Cancer201147142117212721664811
- European Association for Study of LiverEuropean Organisation for Research and TreatmentEASL-EORTC clinical practice guidelines: management of hepatocellular carcinomaEur J Cancer201248559964122424278
- ChengALKangYKLinDYSunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trialJ Clin Oncol201331324067407524081937
- KudoMHanGFinnRSBrivanib as adjuvant therapy to trans-arterial chemoembolization in patients with hepatocellular carcinoma: a randomized phase III trialHepatology20146051697170724996197
