Abstract
Despite the availability of multiple disease-modifying therapies for relapsing multiple sclerosis (MS), there remains a need for highly efficacious targeted therapy with a favorable benefit–risk profile and attributes that encourage a high level of treatment adherence. Daclizumab is a humanized monoclonal antibody directed against CD25, the α subunit of the high-affinity interleukin 2 (IL-2) receptor, that reversibly modulates IL-2 signaling. Daclizumab treatment leads to antagonism of proinflammatory, activated T lymphocyte function and expansion of immunoregulatory CD56bright natural killer cells, and has the potential to, at least in part, rectify the imbalance between immune tolerance and autoimmunity in relapsing MS. The clinical pharmacology, efficacy, and safety of subcutaneous daclizumab have been evaluated extensively in a large clinical study program. In pivotal studies, daclizumab demonstrated superior efficacy in reducing clinical and radiologic measures of MS disease activity compared with placebo or intramuscular interferon beta-1a, a standard-of-care therapy for relapsing MS. The risk of hepatic disorders, cutaneous events, and infections was modestly increased. The monthly subcutaneous self-injection dosing regimen of daclizumab may be advantageous in maintaining patient adherence to treatment, which is important for optimal outcomes with MS disease-modifying therapy. Daclizumab has been approved in the US and in the European Union and represents an effective new treatment option for patients with relapsing forms of MS, and is currently under review by other regulatory agencies.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Multiple sclerosis (MS) is a chronic, autoimmune, inflammatory, and degenerative disorder of the brain, optic nerves, and spinal cord.Citation1 MS affects an estimated 2.3 million people worldwide, with a global median prevalence of 33 cases per 100,000 people.Citation2 The prevalence is highest in North America and Europe.Citation2 MS is most commonly diagnosed between the ages of 20 and 40 years, affects approximately two to three times as many women as men, and is the most frequent chronic disabling neurologic disease in young adults in the industrialized world.Citation2,Citation3
In the relapsing form of MS (RMS), autoreactive lymphoid cells directed against central nervous system (CNS) antigens are activated in the periphery and subsequently migrate into the CNS, producing multifocal inflammatory lesions that result in demyelination and axonal transection, thereby giving rise to the characteristic acute or subacute neurologic symptoms of a relapse.Citation1 Relapses are transient and followed by complete or incomplete clinical recovery, which may result from resolution of inflammatory activity and a variable degree of remyelination.Citation1,Citation4 The use of corticosteroids during relapses may shorten the duration and/or the intensity of inflammation.Citation3,Citation5 If left untreated, recurring relapses and residual damage can result in worsening sustained neurological disability as a consequence of irreversible axonal damage and neurodegeneration, as well as significant reduction in health-related quality of lifeCitation3,Citation6,Citation7 and shortened life expectancy.Citation8
The interferon beta products (eg, Betaseron®/Betaferon® [Bayer HealthCare Pharmaceuticals Inc., Montville, NJ, USA/Bayer Pharma AG, Berlin, Germany], Avonex® [Biogen, Cambridge, MA, USA], Rebif® [EMD Serono, Rockland, MA, USA], Extavia® [Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA]) and glatiramer acetate (Copaxone® [Teva Pharmaceuticals, North Wales, PA, USA]), which were approved by the US Food and Drug Administration (US FDA) or the European Medicines Agency (EMA) between 1993 and 2002, reduced the frequency of relapses in clinical studies by ~30% compared with placebo over a 2- to 3-year period of observation.Citation9–Citation12 Interferon beta-1a (Avonex and Rebif) also was shown to reduce the risk of confirmed disability worsening.Citation9,Citation11 These products, which appear to have broad-spectrum immune-modulating properties,Citation13 were the first proven disease-modifying therapies (DMTs) for the chronic treatment of RMS. Mitoxantrone (Novantrone®; EMD Serono), an antineoplastic agent that damages and prevents the repair of DNA and interferes with RNA function, was approved for the treatment of aggressive relapsing–remitting, secondary-progressive, and progressive-relapsing MS in the early 2000s, but it has largely fallen out of use because of limitations in dosage and duration of use due to its potential for serious cardiac toxicity and a heightened risk of acute myeloid leukemia.Citation14
The use of more targeted therapies that regulate immune pathways mediating CNS damage in RMS was first established with the approval of natalizumab (Tysabri®; Biogen), a humanized monoclonal antibody that selectively inhibits inflammatory cell migration into the CNS.Citation15 In clinical studies, natalizumab resulted in a robust therapeutic response, significantly reducing relapse risk, magnetic resonance imaging (MRI) measures of disease activity, and risk of confirmed disability worsening.Citation16,Citation17 Natalizumab also was effective on composite measures of disease activity; in a retrospective analysis of the AFFIRM study, more than 30% of natalizumab-treated patients showed no evidence of radiologic or clinical disease activity (no evidence of disease activity [NEDA]) during 2 years of treatment.Citation18
Three oral agents were subsequently approved by the FDA and EMA as MS DMTs. The first oral agent approved was fingolimod (Gilenya®; Novartis AG, Basel, Switzerland), a sphingosine-1-phosphate receptor modulator. A putative mechanism of action in MS is retention of reactive memory lymphocytes in lymphoid organs.Citation19 This was followed by delayed-release dimethyl fumarate (Tecfidera®; Biogen), which in RMS may increase tolerance to the oxidative stress of inflammation by promoting de novo synthesis of oxidative quenching species.Citation20 Teriflunomide (Aubagio®; Genzyme Corporation, Cambridge, MA, USA) is reported to reduce the population of activated lymphocytes by blocking mitochondrial pyrimidine synthesis.Citation21 Each of these oral agents reduces risk of relapse, disease activity as observed on MRI, and risk of confirmed disability worsening.Citation22–Citation28
A more recently approved MS DMT is alemtuzumab (Lemtrada®; Genzyme Corporation), an anti-CD52 monoclonal antibody that results in the sustained depletion of circulating T and B lymphocytes.Citation29 Alemtuzumab results in reduced risk of relapse activity, disease activity on MRI, and risk of confirmed disability worsening.Citation30,Citation31 Ocrelizumab (Roche, Basel, Switzerland) is unique among the MS DMTs because it has shown significant treatment benefits in Phase III clinical studies in both RMS versus an active comparator and in primary-progressive MS versus placebo.Citation32 Ocrelizumab is an anti-CD20 monoclonal antibody that causes selective and sustained depletion of CD20 B lymphocytes and has been submitted for regulatory approval as an MS DMT.Citation32,Citation33
While many of the recent therapeutic developments for MS were driven by a greater understanding of immune system function, such as the regulatory role of the α 4-integrin receptor subunit (blocked by natalizumab) on the ingress of activated peripheral lymphocytes and monocytes into the CNSCitation34 and the sequestration of activated lymphocytes in peripheral lymphoid organs (enhanced by fingolimod),Citation19 these agents also are nonselective in the sense that they exploit the inhibition of aspects of overall general immune responsivity as a mechanism to block autoimmunity. One potential consequence of broad inhibition of immune system response is increased risk of infection, including opportunistic infection, which has been encountered with some MS DMTs.Citation35–Citation38
An alternative therapeutic approach is to attempt to reestablish equilibrium between autoimmunity and self-tolerance as a means of controlling the abnormal autoreactive state driving MS. Daclizumab, a humanized monoclonal antibody that blocks the α subunit (CD25) of the interleukin 2 (IL-2) receptor (IL-2R), has the potential to restore the homeostasis between autoimmunity and autotolerance that is characteristic of the nondisease state.Citation39,Citation40 Daclizumab has been shown to reduce the expansion of proinflammatory, actived T lymphocytes, and it may simultaneously promote autotolerance through the expansion of CD56bright natural killer (NK) cells, and at least partially preserve the regulatory T cell (TREG) population and function.Citation40 Daclizumab high-yield process (daclizumab [Zinbryta™ [Biogen, Cambridge, MA, USA and AbbVie Inc., North Chicago, IL, USA]]) was approved for noninvestigational use in the US and in the European Union as a once-monthly subcutaneous (SC) injection for the treatment of RMS in May and July 2016, respectively. As described in more detail in the following section, daclizumab reduced relapse risk, disease activity as monitored by MRI, and risk of confirmed disability worsening in pivotal controlled clinical studies.Citation41,Citation42
Proof of concept and the clinical development program
A previous form of daclizumab was developed as an intravenous (IV) therapy (Zenapax®; Hoffmann-La Roche Ltd, Nutley, NJ, USA) and was used as part of a prophylactic drug regimen in patients with a renal transplant to prevent acute organ rejection and for prevention of severe sight-threatening intraocular inflammation in patients with uveitis.Citation39 Because RMS and solid-organ graft rejection are both associated with pathologically heightened effector T lymphocyte activity, it was hypothesized that daclizumab could be of clinical benefit in patients with active RMS.Citation39,Citation43 Subsequent small-scale open-label clinical studies showed promising results.Citation43–Citation46 Daclizumab was reformulated as an SC injection and tested as an adjunct to interferon beta in the Phase II, multicenter, randomized, double-blind CHOICE study, which assessed its efficacy and safety in patients with active RMS.Citation47 CHOICE demonstrated that daclizumab as an add-on therapy with interferon beta reduced disease activity on MRI compared with interferon beta alone.Citation47
Based on these positive results, daclizumab high-yield process was subsequently developed. Daclizumab high-yield process has an identical amino acid sequence and the same antigen-binding domain as earlier forms of daclizumab, but differs in its glycosylation profile, leading to altered binding at the Fc receptor. This new form of daclizumab has been associated with less antibody-dependent cell-mediated cytotoxicity compared with earlier forms of daclizumab.Citation48 In the multicenter, randomized, double-blind, placebo-controlled (SELECT) and active-comparator (DECIDE) clinical studies, daclizumab monotherapy, administered SC every 4 weeks, resulted in statistically significant reductions in both clinical and MRI indicators of disease activity in patients with RMS.Citation41,Citation42 These studies are described in detail in a later section of this review.
The clinical pharmacology, efficacy, and safety of SC daclizumab has been evaluated extensively in Phase I pharmacokinetic studies;Citation48 an open-label safety, immunogenicity, pharmacokinetic, and pharmacodynamic study;Citation49,Citation50 and randomized, double-blind, placebo- and active-controlled studies, and their extension phases ( and ).Citation41,Citation42,Citation51–Citation53
Figure 1 The SELECT and DECIDE studies and their extension studies.Citation41,Citation42,Citation51–Citation53,Citation129
Abbreviations: IM, intramuscular; IFN, interferon; RMS, relapsing multiple sclerosis.
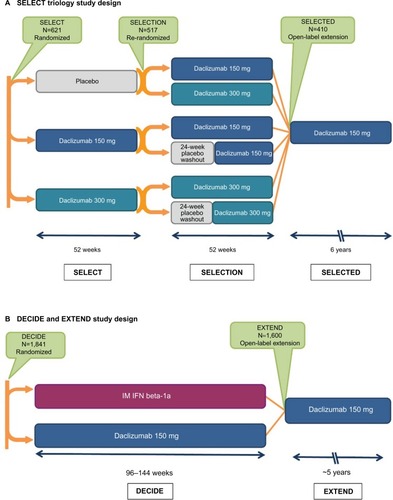
Table 1 Overview of completed and ongoing daclizumab clinical studies
Pharmacology
Mechanism of action
The putative mechanism of action for daclizumab is unique compared with other approved DMTs for the treatment of RMS. As described earlier, daclizumab is a monoclonal antibody that binds to the α subunit (CD25) of the IL-2R expressed on activated T lymphocytes, thereby preventing interaction of IL-2 with its high-affinity receptor and inhibiting the activation of CNS antigen-experienced, proinflammatory, activated T lymphocytes,Citation39,Citation54–Citation57 one of the main drivers of MS pathogenesis.Citation1
The IL-2/IL-2R pathway also has an important role in maintaining the homeostatic balance between proinflammatory autoimmunity and anti-inflammatory autotolerance.Citation40 The cytokine IL-2 is a lymphocyte growth factor produced primarily by activated CD4+ T lymphocytes, and to a lesser degree by CD8+ T lymphocytes, dendritic cells, and NK cells.Citation58 Antigen presentation to T lymphocytes promotes both IL-2 production and secretion by these antigen-activated T lymphocytes and also upregulates IL-2R expression on T lymphocytes.Citation59–Citation61 The IL-2/IL-2R interaction drives T lymphocyte clonal expansion when an immune response is mounted, as is the case in autoimmune conditions such as MS.Citation62 Production of IL-2 is generally short lived, and IL-2 levels are regulated by an autoinhibitory feedback loop such that IL-2 binding to IL-2R activates secondary intracellular messenger systems to repress the IL2 gene.Citation63–Citation65 This process serves to reduce or prevent prolonged T lymphocyte–driven inflammatory responses. IL-2 signaling through IL-2R also is necessary for the support and function of TREG cells, which are required for the maintenance of autotolerance.Citation66–Citation72 Thus, the IL-2/IL-2R system provides a mechanism for both upregulation of proinflammatory responses and maintenance of immune homeostasis.
Evidence suggests that MS may result, in part, from a breakdown in immune self-tolerance due to abnormalities in the IL-2/IL-2R signaling pathway, resulting in an excess of peripherally activated CNS antigen-primed T lymphocytes.Citation40 For example, polymorphisms in the CD25 gene are associated with MS susceptibility, directly implicating IL-2 signaling in MS pathogenesis.Citation73,Citation74 These polymorphisms also are associated with increased CD25 expression on naive CD4+ T lymphocytes.Citation75 Levels of soluble CD25, a marker of IL- 2-driven T lymphocyte proliferation,Citation76 also are elevated in MS,Citation77 and have been linked with MS severity.Citation78
IL-2 signaling is mediated through two IL-2R isoforms, which differ in their affinity for IL-2 and expression across different immune cell types.Citation79 On the surface of activated T lymphocytes and TREG cells, IL-2 signaling is mediated via the high-affinity isoform that is composed of the IL-2-capturing α subunit (CD25), which is expressed transiently following antigen activation in T lymphocytes and constitutively in TREG cells, and two signaling subunits, CD132 and CD122 (the γ and β chains, respectively; ).Citation58,Citation70,Citation79–Citation81 IL-2 binding to CD25 promotes the association with CD122 and CD132, resulting in the formation of the quaternary high-affinity receptor complex.Citation79,Citation82 CD25 functions solely to increase the affinity of IL-2R for IL-2 and has no known signaling function.Citation58,Citation79 Thus, the intracellular transmission of the IL-2 signal is dependent on the cytoplasmic tails of CD122 and CD132.Citation83 In contrast, the intermediate-affinity IL-2R, composed only of CD122 and CD132, is found on resting T and B lymphocytes and cytotoxic immunoregulatory CD56bright NK cells, and binds IL-2 with an affinity significantly lower than the high-affinity CD25-containing IL-2R ().Citation79
Figure 2 Proposed mechanism of action of daclizumab and effects on key immune cell populations.
Abbreviations: IL-2R, interleukin 2 receptor; IL-2, interleukin 2; MS, multiple sclerosis; Tact, activated T cell; NK, natural killer; TREG, regulatory T cell.
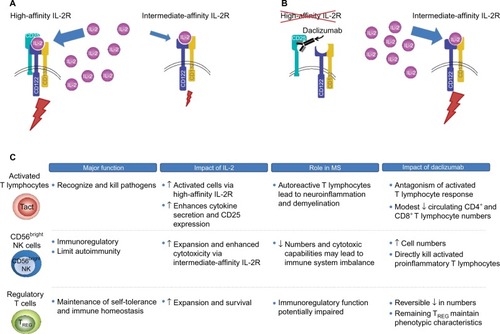
Daclizumab binds selectively and with greater affinity than IL-2 to CD25 expressed on effector T lymphocytes (dissociation constants, 0.27 and 10 nM, respectively), thus blocking the assembly of the high-affinity IL-2R and preventing IL-2 signal transmission via this route ().Citation84,Citation85 Daclizumab does not block IL-2 signaling via the intermediate-affinity IL-2R on resting T lymphocytes and other immune cells.Citation84,Citation86 The primary overall effects of CD25 blockade by daclizumab in patients with RMS are antagonism of proinflammatory, activated T lymphocytes, expansion of CD56bright NK cells, and a reversible reduction in TREG cell numbers ().Citation54,Citation86–Citation89
Daclizumab, by inhibiting the formation of the high-affinity IL-2R, results in an increase in the amount of IL-2 available for interaction with the intermediate-affinity IL-2R expressed on CD56bright NK cells,Citation55,Citation79 to which daclizumab cannot bind due to the absence of CD25.Citation39,Citation79 Unimpeded activation of the intermediate-affinity IL-2R activates and induces the expansion of CD56bright NK cells,Citation41,Citation44,Citation54–Citation56,Citation90 which can penetrate the blood–brain barrier where they recognize, establish direct contact with, and cause lysis of proinflammatory, activated T lymphocytes, while leaving resting T lymphocytes intact.Citation54,Citation90,Citation91 CD25 blockade also may have indirect effects on proinflammatory T lymphocyte responses by inhibiting trans-presentation of CD25 by dendritic cells to the IL-2R on resting T lymphocytes, further preventing the formation of the high-affinity IL-2R.Citation57 The effects of daclizumab on activated T lymphocytes and CD56bright NK cells are fully reversible after treatment is stopped.Citation54,Citation87,Citation89
As noted earlier, TREG cells express the high-affinity CD25-containing IL-2R, and the function of TREG cells is believed to be dependent upon IL-2/IL-2R interaction.Citation66–Citation69,Citation71 If this is the case, one might anticipate potential worsening of autoimmunity upon CD25 blockade, but just the opposite is observed.Citation40 Daclizumab has demonstrated efficacy in reducing MS disease activity, even though treatment with daclizumab results in a reversible reduction in TREG cell numbers of ~50%.Citation86,Citation89 Several lines of evidence may provide at least partial answers to this seeming paradox. Expression of the TREG cell phenotype is largely defined by the presence of FOXP3+ expression in CD4+ T lymphocytes.Citation92,Citation93 The signal that activates the FOXP3 gene is transmitted via the γ (CD132) chain of IL-2R.Citation68 Thus, FOXP3 gene activation by IL-2 via the intermediate-affinity receptor may serve to maintain and support the TREG population.Citation94,Citation95 In addition, the CD132 γ chain, which is found in multiple cytokine receptors, also may be stimulated by other cytokines, such as IL-4, IL-7, IL-9, and IL-21.Citation94 Daclizumab does not result in TREG cell depletion, and evidence suggests that the TREG cells that remain preserve the normal TREG lineage and phenotype.Citation86 In this scenario, daclizumab reduces activated T lymphocyte support by IL-2 by blocking CD25 bioavailability, while increasing NK cell activation and maintaining TREG cell function and phenotype via the still-available intermediate- and low-affinity receptor subunits of IL-2R and other cytokine receptors.Citation55,Citation86
Lymphoid tissue inducer (LTi) cells are a proinflammatory subset of lymphocytes that appear to originate from hematopoetic CD34+ precursor cells.Citation96,Citation97 Among the proposed actions of LTi cells is the promotion of ectopic lymphoid follicles, as are found in the meninges of patients with MS.Citation97–Citation99 The findings of two studies suggest that daclizumab therapy in patients with RMS was associated with a decrease in circulating LTi cells and a decrease in the cerebrospinal fluid content of CXCL13, a chemokine produced by CNS lymphoid aggregates.Citation97,Citation100 The reported decrease in LTi cells was hypothesized to be a consequence of increased stimulation of the intermediate-affinity IL-2R due to CD25 blockade by daclizumab, driving its progenitor cells toward the formation of CD56bright NK cells at the expense of the LTi lineage.Citation97,Citation100 In the past few years, studies have shown that innate lymphoid cells are more diverse than previously appreciated.Citation101 More recently published data do not confirm the previously reported reduction in LTi cells by daclizumab, and may be a result of different analytic methods used in these studies to identify innate lymphoid cell subsets.Citation102 The findings from this recent study confirmed that circulating CD56bright NK cells were increased in patients with RMS treated with daclizumab, but there was no apparent effect of daclizumab on circulating innate lymphoid cell subpopulations, including LTi cells.Citation102
Pharmacokinetics
Four studies evaluated the pharmacokinetics of IV daclizumab and SC daclizumab in healthy individuals and patients with RMS.Citation48,Citation50 In general, there were no clinically meaningful differences between the two populations in the pharmacokinetic profile of daclizumab, which was characterized by slow clearance, linear pharmacokinetics (doses ≥100 mg), high SC bioavailability (>80%), and an elimination half-life suitable for monthly administration (~3 weeks).Citation48,Citation50 Daclizumab had a low volume of distribution of 6.4 L in healthy volunteers, which is to be expected for an immunoglobulin G1 monoclonal antibody that is primarily confined to the vascular and interstitial spaces.Citation48
Daclizumab best fits a two-compartment pharmacokinetic model with first-order absorption and elimination, and this finding was used to predict its steady-state pharmacokinetic profile in Phase III studies.Citation48 Steady-state serum concentrations of daclizumab were attained by week 16 in 26 patients with RMS who received daclizumab 150 mg SC every 4 weeks for 20 weeks (total of six doses) in the Phase III OBSERVE study (NCT01462318).Citation50 Maximum observed concentration (Cmax) was achieved after a median of 7 and 5 days for doses 1 and 6, respectively, with a mean steady-state serum peak-to-trough concentration ratio of ~2 ().Citation50 There are no known adverse drug–drug interactions in patients treated with daclizumab. In a recently reported study of the effects of daclizumab on the pharmacokinetics of drugs metabolized by a range of hepatic cytochrome P450 isoenzymes, no interaction was seen, co-administration neither inhibiting nor inducing enzymatic activities, and the serum kinetics of medications characteristically metabolized by these isoenzymes were not affected.Citation103
Table 2 Pharmacokinetic parameters of daclizumab after administration of daclizumab 150 mg subcutaneously every 4 weeks in patients with RMSCitation50
Pharmacodynamics
Daclizumab has a well-defined pharmacodynamic profile, demonstrating rapid saturation of CD25 on peripheral blood T lymphocytes within a few hours after SC administration that persisted throughout the 4-week dosing interval, and more slowly reversed following treatment discontinuation.Citation104 Unbound CD25 returned to pretreatment levels within 4–6 months after the last daclizumab dose due to its long half-life of elimination.Citation104 The longevity of this effect suggests that delayed or isolated missed doses of daclizumab due to patient nonadherence may have minimal to no adverse impact upon clinical efficacy.
Total CD4+ and CD8+ T lymphocyte counts were modestly reduced in daclizumab-treated patients in the SELECT (mean reductions of 7%–10% over 52 weeks) and DECIDE (median reductions of 15%–18% over 96 weeks) studies.Citation41,Citation105 Despite reductions in each of these T lymphocyte populations, the ratio of the number of CD4+ T lymphocytes to CD8+ T lymphocytes remained stable during daclizumab treatment in both studies.Citation41,Citation105 Furthermore, no association was observed between CD4+ or CD8+ T lymphocyte counts and the occurrence of infections in daclizumab-treated patients in DECIDE.Citation105 In contrast, activated effector CD4+ T lymphocytes that express HLA-DR2, a haplotype associated with susceptibility to MS,Citation106 were reduced by ~25% during the treatment period in daclizumab-treated patients with active RMS in CHOICE,Citation107 suggesting a selective reduction in activated CD4+ T lymphocytes.
Serum IL-2 levels increased approximately twofold within 4 weeks of daclizumab administration and stabilized thereafter with continued treatment.Citation89 Accompanying the increased IL-2 levels was a rapid, up to fivefold, increase in the number of CD56bright NK cells, reaching a plateau within 52 weeks that was sustained with continued treatmentCitation54,Citation87,Citation89 and subsequently decreased during a placebo washout period.Citation89 A significant increase in the number of CD56bright NK cells also was observed in cerebrospinal fluid after IV daclizumab administration.Citation90 Notably, higher CD56bright NK cell counts at week 4 were associated with lower numbers of new or newly enlarging T2 hyperintense lesions over 52 weeks (P<0.001).Citation87 During the first year of treatment, both the lowest and highest quartiles of peripheral blood CD56bright NK counts at week 8 were associated with fewer new or newly enlarging T2 hyperintense lesions between weeks 24 and 52 (63% and 86%, respectively) compared with placebo (P<0.0001 for each quartile comparison).Citation87 The observed reduction in MRI measures of disease activity may be the direct result of the cytotoxic effects of CD56bright NK cells upon activated T lymphocytes.Citation108 However, even in daclizumab-treated patients who experienced no expansion of CD56bright NK cell numbers, there was a 54% reduction in new or newly enlarging T2 hyperintense lesions over 52 weeks versus the placebo group, emphasizing that mechanisms other than the expansion of CD56bright NK cells also contribute to the reduction in MS disease activity.Citation87 The predictive value of CD56bright NK cell counts measured during the first 4 or 8 weeks after daclizumab initiation upon disease activity during the first 52 weeks of observation was not seen in the second year of daclizumab therapy.Citation87
In addition, TREG cells express CD25, and reductions in TREG cell counts of ~50% that persisted throughout the duration of treatment have been observed in daclizumab-treated patients.Citation86,Citation88 The effect of daclizumab on TREG cell counts is reversible on treatment discontinuation and nondepleting because remaining TREG cells maintain the TREG function and phenotype (ie, do not acquire effector CD4+ T lymphocyte characteristics).Citation86,Citation89 No association has been found to date between daclizumab-mediated declines in TREG cell numbers and clinical efficacy during treatment.Citation86 Daclizumab shows continued efficacy in RMS despite reducing the number of TREG cells.Citation86 This reduction in TREG cell numbers may in part be compensated for by daclizumab-induced expansion of CD56bright NK cells, the latter playing a significant role in restoring immune tolerance through their destruction of proinflammatory T cells,Citation54,Citation91 and thus may be a major mediator of the effects of daclizumab on RMS disease activity.
Efficacy
Pivotal studies
Two large double-blind controlled studies compared daclizumab 150 mg SC every 4 weeks with placebo over 52 weeks (SELECT)Citation41 and with intramuscular (IM) interferon beta-1a 30 mcg once weekly for a minimum of 96 weeks and up to a maximum of 144 weeks (DECIDE; and ).Citation42 Eligible patients were adults (18–55 years of age) with documented relapsing–remitting MS according to the 2005 McDonald criteria.Citation109 The primary endpoint in both SELECT and DECIDE was the annualized relapse rate (ARR), where relapse was defined as new or recurrent neurological symptoms not associated with fever or infection, lasting 24 hours or more, and accompanied by new or worsened objective neurological findings at assessment.Citation41,Citation42 Secondary and tertiary outcomes included the impact of daclizumab on confirmed disability worsening, MRI lesion burden and activity, patient-reported outcomes, safety, and immune system activity markers. SELECT (and its extension study, SELECTION; ) also included a daclizumab 300 mg treatment group, but because efficacy outcomes were similar between the 150 and 300 mg dose groups, only the 150 mg dose was evaluated in subsequent pivotal studies in MS.Citation110 Thus, this review focuses on results from treatment with daclizumab 150 mg.
The primary endpoint was met in both SELECT and DECIDE; ARR was reduced significantly by daclizumab 150 mg compared with placebo over 52 weeks in SELECT (54% reduction; P<0.0001) and compared with IM interferon beta-1a over 144 weeks in DECIDE (45% reduction; P<0.001; ).Citation41,Citation42 In DECIDE, daclizumab also significantly reduced the annualized rate of severe/serious relapses (38% reduction; P=0.002).Citation42 In SELECT, there was a 57% reduction in 12-week confirmed disability worsening with daclizumab (P=0.021).Citation41 In DECIDE, there was a 16% difference in the percentage of patients with 12-week confirmed disability worsening that favored daclizumab, but the effect was not statistically significant (P=0.16).Citation42 However, there was an imbalance between treatment arms in the number of patients censored who experienced disability worsening, but left the study before disability worsening could be confirmed 12 weeks later, which was considered to have impacted the test for statistical significance. Specifically, the primary analysis assumed that none of the censored patients had 12-week confirmed disability worsening. However, when all censored patients were instead assumed to have 12-week confirmed disability worsening, a 24% reduction in 12-week confirmed disability worsening was observed for daclizumab versus IM interferon beta-1a (P=0.016).Citation42 When censored patients were assumed as either confirmed or not confirmed based on an imputation of the observed rate, a 21% reduction in 12-week confirmed disability worsening was observed that favored daclizumab (P=0.047).Citation42 DECIDE also included assessment of 24-week confirmed disability worsening – considered by the EMA to be a more robust measure than 12-week confirmed disability worseningCitation111 – as a preplanned tertiary endpoint using the imputed methodological approach.Citation42 Daclizumab significantly reduced 24-week confirmed disability worsening by 27% compared with IM interferon beta-1a (P=0.03).Citation42
Table 3 Clinical efficacy of daclizumab 150 mg given subcutaneously every 4 weeks in multicenter, randomized, double-blind, comparative studies of patients with RMSCitation41,Citation42
Consistent with the effects of daclizumab on clinical endpoints, statistically significant effects were observed on MRI measures of disease activity in both SELECT and DECIDE ().Citation41,Citation42 At week 52 in SELECT and week 96 in DECIDE, daclizumab 150 mg significantly reduced the number of gadolinium-enhancing (Gd+) lesions (new Gd+ lesions in SELECT, P<0.0001, and all Gd+ lesions in DECIDE, P<0.001, respectively) and new or newly enlarging T2 hyperintense lesions (P<0.0001 and P<0.001, respectively).Citation41,Citation42 In DECIDE, the effects on both of these MRI lesion parameters were observed as early as the first MRI assessment at week 24 (P<0.001 for both measures),Citation42 while in SELECT, the effect on new Gd+ lesions was seen as early as 4 weeks after initiating treatment, which was the time of the first post-baseline MRI.Citation41 The mean percentage of brain volume loss, which may be indicative of neurodegeneration and tissue damage,Citation112 was not significantly different between treatment groups after 1 year of treatment in SELECT, but was significantly different between the daclizumab and IM interferon beta-1a arms after 2 years of treatment in DECIDE (P<0.001; ).Citation41,Citation42 A benefit for daclizumab over IM interferon beta-1a on brain volume loss also was observed during the intervals of baseline to week 24 (P=0.03) and weeks 24–96 (P<0.001).Citation42 The effects of daclizumab across all MRI lesion outcomes were maintained in the subgroup of patients treated for 144 weeks.Citation113,Citation114
Table 4 Neuroradiological efficacy of daclizumab 150 mg given subcutaneously every 4 weeks in multicenter, randomized, double-blind, comparative studies of patients with RMSCitation41,Citation42
A post hoc subgroup analysis of SELECT revealed statistically significant reductions in ARR, new or newly enlarging T2 hyperintense lesions, and new Gd+ lesions with daclizumab compared with placebo in patients with highly active RMS, defined as at least two relapses in the year before randomization and at least one Gd+ lesion at baseline.Citation115 A benefit for daclizumab versus IM interferon beta-1a on relapse and MRI lesion activity in patients with highly active RMS also has been reported in DECIDE.Citation116 In DECIDE, the clinical and MRI benefits of daclizumab were consistent across a number of predefined patient subgroups stratified by demographics and baseline MS disease characteristics, including patients with or without prior use of interferon beta.Citation117
Additional post hoc analyses examined the proportion of patients with NEDA, defined as no relapses, no confirmed disability worsening, no new Gd+ lesions (SELECT) or no Gd+ lesions (DECIDE), and no new or newly enlarging T2 hyperintense lesions on brain MRI.Citation18,Citation118 In SELECT, a significantly higher percentage of patients receiving dacli-zumab 150 mg compared with placebo exhibited NEDA at week 52 (36% versus 11%; adjusted odds ratio 6.31; 95% confidence interval [CI] 3.59–11.11; P<0.0001).Citation119 Similarly, in DECIDE, a significantly higher percentage of patients receiving daclizumab 150 mg compared with IM interferon beta-1a exhibited NEDA at week 96 (25% versus 14%; odds ratio 2.06; 95% CI 1.59–2.66; P<0.0001).Citation118
Extension studies
Both SELECT and DECIDE are being followed up with extension phases, enabling evaluation of the long-term safety profile and persistence of daclizumab efficacy ().Citation51–Citation53,Citation120 Final results are available for SELECTION, a 1-year extension of SELECT,Citation52 and interim 3-year results for SELECTED, an ongoing, long-term, open-label extension of SELECTION.Citation53 In SELECTION, half of the patients who received daclizumab 150 mg SC in SELECT were randomized to continue daclizumab 150 mg SC for an additional year ( and ). Patients who completed SELECTION were then eligible to enroll in the SELECTED long-term extension study ().Citation53 For patients who received daclizumab 150 mg for 1 year in SELECT and continued to receive daclizumab 150 mg for an additional year in SELECTION, the ARR (0.148 and 0.165), the proportion of patients with relapse (14.7% and 13.6%), and the proportion of patients with 12-week confirmed disability worsening (6% and 5%) were similar in years 1 and 2 of treatment, respectively.Citation52 In the subset of patients (n=94) in SELECTED who had received continuous daclizumab 150 mg since their initial enrollment in SELECT, efficacy outcomes were sustained in the third year of treatment.Citation53 EXTEND is an ongoing open-label extension study of DECIDE, from which no interim data have yet been reported, that will provide additional information on the long-term safety and efficacy of daclizumab for up to 6 years of treatment.Citation51
Patient-centered outcomes
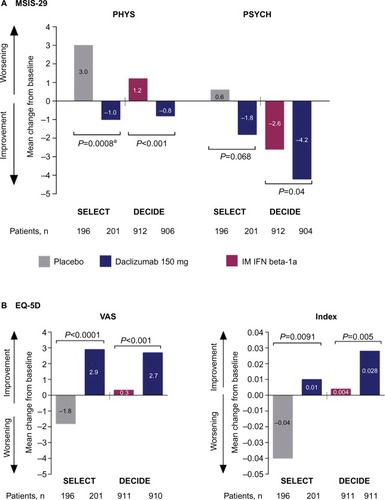
Consistent with the effects of daclizumab on primary and secondary clinical and MRI endpoints in SELECT and DECIDE, significant benefits of daclizumab were observed on health-related quality-of-life measures.Citation41,Citation42 Patient-reported outcomes in both studies included the 29-item Multiple Sclerosis Impact Scale (MSIS-29), which is used to assess the impact of MS on physical (physical impact subscale [PHYS]) and psychological (psychological impact subscale) functioning,Citation121 as well as the EuroQol 5-Dimensions (EQ-5D), which is used to assess general health status.Citation122 The EQ-5D includes a descriptive component in which patients self-report their current level of difficulty on five dimensions of health (index score) and a valuation component in which patients self-rate their general health status on a visual analog scale (VAS).Citation122
In SELECT, a statistically significant benefit for daclizumab 150 mg compared with placebo was observed on MSIS-29 PHYS subscale (P=0.00082), ED-5D index (P=0.0091), and EQ-5D VAS (P<0.0001) scores ().Citation41 In DECIDE, significantly greater mean improvements from baseline were observed at week 96 with daclizumab 150 mg compared with IM interferon beta-1a on MSIS-29 PHYS subscale (P<0.001), MSIS-29 psychological impact subscale (P=0.04), EQ-5D index (P=0.005), and EQ-5D VAS (P<0.001) scores ().Citation42 Clinically meaningful worsening on the MSIS PHYS subscale has been defined as a ≥7.5-point change from baseline,Citation110 and this was evaluated post hoc in SELECT and as a secondary outcome in DECIDE.Citation42,Citation110 In SELECT, significantly fewer patients treated with daclizumab 150 mg compared with placebo exhibited clinically meaningful worsening on the MSIS-29 PHYS subscale at week 52 (20% versus 28%, respectively; P<0.01).Citation110 In DECIDE, significantly fewer patients treated with daclizumab 150 mg compared with IM interferon beta-1a exhibited clinically meaningful worsening on the MSIS-29 PHYS subscale at week 96 (19% versus 23%, respectively), representing a 24% relative reduction in the odds for worsening between treatment groups (95% CI 5–40; P=0.0176).Citation42,Citation123
Figure 3 Changes from baseline to weeks 52 and 96 in health-related quality-of-life endpoints in SELECT and DECIDE.Citation41,Citation42
Abbreviations: MSIS-29, 29-item Multiple Sclerosis Impact Scale; PHYS, physical impact subscale; PSYCH, psychological impact subscale; IM, intramuscular; IFN, interferon; EQ-5D, EuroQol 5-Dimensions; VAS, visual analog scale.
The Multiple Sclerosis Functional Composite (MSFC) was administered in DECIDE. The MSFC is a quantitative measure with three components assessing lower limb function/walking ability (Timed 25-Foot Walk [T25FW]), upper limb function (9-Hole Peg Test [9HPT]), and cognitive function (3-second Paced Auditory Serial Addition Test [PASAT-3]).Citation124 At week 96 of DECIDE, daclizumab 150 mg was associated with a greater improvement baseline in MSFC composite score compared with IM interferon beta-1a (0.091 versus 0.055, respectively; P<0.001).Citation42 Significant benefits of daclizumab treatment versus IM interferon beta-1a also were seen on all three separate components that comprise the MSFC at week 96 (T25FW [P=0.006], 9HPT [P=0.002], and PASAT-3 [P=0.04]).Citation42 A benefit for daclizumab on cognitive outcomes also was observed on the Symbol Digit Modalities Test at week 96 with greater mean improvements from baseline observed for daclizumab (4.1±12.4) versus IM interferon beta-1a (2.9±12.7; P=0.03).Citation42
Safety and tolerability
Two decades of clinical experience with interferon beta and glatiramer acetate have shown that these DMTs are relatively safe, although serious adverse events (AEs) do infrequently occur.Citation125,Citation126 Newer DMTs, including natalizumab, fingolimod, delayed-release dimethyl fumarate, teriflunomide, and alemtuzumab, have improved on the modest efficacy outcomes achieved with interferon beta and glatiramer acetate; however, each is associated with a greater degree of concern regarding safety or tolerability.Citation37,Citation125,Citation127,Citation128 It is important to have a clear understanding of the safety profile of each DMT in order to weigh potential risks against potential benefits.
Safety data have been reported for SELECT, SELECTION, and DECIDE (),Citation41,Citation42,Citation52 and for the 3-year interim analysis of SELECTED.Citation129 The overall incidence of AEs was similar between daclizumab and placebo in SELECT and between daclizumab and IM interferon beta-1a in DECIDE ().Citation41,Citation42 The incidence of serious AEs, excluding MS relapse, was similar in the daclizumab and placebo groups after 1 year of treatment in SELECT,Citation41 but was higher in daclizumab-treated patients than in IM interferon beta-1a-treated patients after 2–3 years of treatment in DECIDECitation42 (). Most treatment-emergent AEs in patients receiving daclizumab were mild or moderate in severity, and the incidence of these AEs did not increase with increasing duration of treatment.Citation42,Citation129 Daclizumab treatment was associated with a higher incidence of AEs leading to treatment discontinuation compared with both placebo (3% versus <1%) and IM interferon beta-1a (15% versus 12%), respectively.Citation42,Citation129 There was no evidence of an increased risk of malignancies in either SELECT or DECIDE, and rates were similar between comparator arms ().Citation42,Citation129 Five deaths were reported in patients treated with daclizumab during the clinical studies; in two of them (ischemic colitis as a complication of psoas abscess and autoimmune hepatitis in a patient treated with daclizumab 300 mg), a contributory role for daclizumab could not be ruled out, while the other three deaths (acute exacerbation of MS [n=2]; subarachnoid hemorrhage due to a fall [n=1]) were considered by the investigators to be unrelated to treatment.Citation41,Citation42,Citation52,Citation120 Daclizumab does not appear to be associated with tolerability issues that are common among the interferon betas, such as influenza-like symptoms and injection site reactions ().Citation41,Citation42,Citation130 The safety profile for patients in SELECTION in year 2 of daclizumab 150 mg treatment was similar to that observed in patients in SELECT and SELECTION after 1 year of treatment ().Citation52 The incidence of AEs in daclizumab-treated patients did not increase over time in a 3-year interim analysis of SELECTED; the safety profile of daclizumab after extended treatment was similar to that observed in SELECT and SELECTION.Citation129
Table 5 Incidence of AEs in daclizumab 150 mg-treated patients in SELECT, SELECTION, and DECIDECitation41,Citation42,Citation52
Hepatic AEs, including liver enzyme elevations, have been observed in daclizumab treatment groups, but the incidence of treatment-limiting events is low ().Citation41,Citation42,Citation52 Similar percentages of patients had elevations of alanine aminotransferase (ALT) or aspartate aminotransferase (AST) 1–3× or 3–5× the upper limit of normal (ULN) in the daclizumab and placebo groups after 1 year of treatment in SELECT ().Citation41 The incidence of elevations of ALT or AST 1–3× ULN was slightly higher in patients in year 2 of treatment than in year 1 of treatment in SELECTION; however, the incidence of ALT or AST elevations >5× ULN was similar in years 1 and 2 of treatment ().Citation52 Elevations of ALT or AST >5× ULN were more common with daclizumab 150 mg than with placebo (4% versus <1%) or IM interferon beta-1a (6% versus 3%), respectively ().Citation41,Citation42 Elevated serum transaminases were observed throughout the treatment period in the daclizumab group, but occurred predominantly in the early phases of treatment in the IM interferon beta-1a group.Citation42 In DECIDE, drug-related hepatic events, as defined by a Medical Dictionary of Regulatory Activities query, were observed in 16% of daclizumab-treated patients and 14% of IM interferon beta-1a-treated patients, with serious hepatic events occurring in 1% and <1% of patients, respectively.Citation42 Seven patients receiving daclizumab and one patient receiving IM interferon beta-1a experienced ALT or AST ≥3× ULN concurrent with total bilirubin >2× ULN.Citation42 However, the independent hepatic safety assessment concluded that only one patient in each treatment group that met the criteria for Hy’s lawCitation131 had a causality score of probable or higherCitation132 ().
Daclizumab has been associated with an increased risk of cutaneous AEs when compared with placebo or IM interferon beta-1a ().Citation41,Citation42 In DECIDE, cutaneous AEs occurred in 37% of daclizumab-treated patients and 19% of IM interferon beta-1a-treated patients over 2–3 years of treatment and resulted in treatment discontinuation in 5% and 1% of patients, respectively.Citation42 The most common cutaneous events were rash (7% versus 3% of patients) and eczema (4% versus 1%) in the daclizumab and IM interferon beta-1a groups, respectively.Citation42 Most patients with cutaneous AEs had mild (55%) or moderate (38%) events, and these events were predominantly treated with topical corticosteroids or did not require any topical or systemic corticosteroid treatment (ie, 81% of mild and 73% of moderate events).Citation133 Severe or serious cutaneous AEs were each reported in 2% of daclizumab-treated patients and most commonly were treated with systemic corticosteroids.Citation133 Among patients with serious cutaneous events in the daclizumab group, dermatitis was reported in three patients and angioedema in two patients; all other serious cutaneous events were reported in one patient each.Citation42 There were no cases of Stevens–Johnson syndrome or toxic epidermal necrolysis reported in DECIDE.Citation42 In SELECTED, one case was reported by the investigator as Stevens–Johnson syndrome, but the diagnosis was not supported by the study’s central independent dermatologist or by the local site dermatologist upon their review of the case.Citation129
Both infections and serious infections have been observed at a higher incidence in daclizumab-treated patients compared with those treated with placebo or IM interferon beta-1a ().Citation41,Citation42 Most patients with infections remained on daclizumab treatment.Citation42 The most common (incidence ≥10%) infections in DECIDE were nasopharyngitis (25% versus 21%), upper respiratory tract infection (16% versus 13%), and urinary tract infection (10% versus 11%) in the daclizumab and IM interferon beta-1a groups, respectively.Citation42 In SELECT and SELECTION, most patients stayed on daclizumab treatment or restarted daclizumab treatment after resolution of their serious infection.Citation41,Citation52 No cases of progressive multifocal leukoencephalopathy have been reported to date in the daclizumab clinical study program.Citation41,Citation42,Citation52,Citation129 Interim analyses of safety extension studies showed that the risk of infections did not increase with longer duration of treatment with daclizumab (up to 6 years).Citation120,Citation129
Antidrug antibodies (ADAs) and neutralizing antibodies (NAbs) have been detected in relatively low proportions of daclizumab-treated patients. In patients who initiated daclizumab treatment in SELECTION, 4% of patients were ADA positive and 2% of patients were NAb positive during the 52-week treatment period.Citation52 In these patients, NAbs were not persistent, because patients who tested positive at week 72 were negative for NAbs at week 104.Citation52 In patients continuing daclizumab treatment in SELECTION, one patient was ADA positive, and there were no new cases of NAb positivity in the second year of treatment.Citation52 In DECIDE, ADAs were observed in 19% of evaluable patients treated with daclizumab; in 7% of patients, ADAs were persistent (defined as more than one consecutive positive evaluation ≥74 days apart or a positive evaluation at the final assessment with no further samples available).Citation134 NAbs were observed in 8% of evaluable patients treated with daclizumab; in 2% of patients, NAbs were persistent.Citation134 The majority of ADA reactivity to daclizumab occurred early during treatment (during the first year), and this reactivity was transient.Citation134 ADA titers were generally low in DECIDE, and ADAs and NAbs had no discernible impact on efficacy or safety outcomes associated with daclizumab.Citation134
There was no evidence of increased risk of adverse pregnancy outcomes or fetal abnormalities following gestational exposure to daclizumab during the first trimester.Citation135 Because the number of pregnancies occurring during the daclizumab clinical studies was small (45 pregnancies in 40 patients), no definitive conclusions can be drawn regarding safety during pregnancy.Citation135 There is no information available on the safety of daclizumab in breastfeeding women.
A substudy of daclizumab-treated patients enrolled in SELECTED who received seasonal influenza vaccination achieved acceptable levels of serologic protection against the seasonal influenza and experienced no unique or worsened safety issues associated with vaccination.Citation136
Administration/acceptability
Poor adherence to self-injectable DMT regimens may lead to suboptimal treatment outcomes for patients with MS.Citation137,Citation138 Furthermore, higher frequency of injection has been associated with reduced adherence.Citation139 Daclizumab has several properties that may improve use on a long-term basis. First, self-administration of daclizumab on a once-monthly schedule is likely to be better adhered to by more patients than the daily to twice-monthly administration schedules of other DMTs, because reduced patient adherence to MS medications is clearly related to the frequency of administration.Citation138–Citation141 Furthermore, reducing the dosing frequency of drugs in indications other than MS has been shown to be associated with better treatment adherence, health-related quality of life, patient satisfaction, and reduced costs.Citation142,Citation143 The relatively simple and infrequent procedure of SC administration is unlikely to be a barrier to routine use,Citation141,Citation144 and the favorable pharmacokinetic and pharmacodynamic profile of daclizumab should minimize the risk of breakthrough disease activity resulting from delayed dosing. Second, the superior therapeutic profile of daclizumab compared with platform MS medications such as interferon beta may encourage patients to adhere to the dosing regimen of daclizumab, because some patients report perceived lack of efficacy as contributing to nonadherence.Citation138,Citation140 Finally, the overall tolerability of daclizumab may improve adherence, because some patients report tolerability issues as a reason for nonadherence.Citation138,Citation140 Better adherence to DMTs would be expected to lead to improved clinical outcomes, in addition to lower medical resource utilization and costs.Citation138
Because daclizumab received its first approval for use outside of approved investigational clinical studies in May 2016, patient adherence to daclizumab and its clinical efficacy have not been studied in the overall “real-world” community clinic-based RMS patient population. Additional data, including its use in the noninvestigational clinical practice setting, are needed to confirm the validity of these hypotheses.
Conclusion
Management of RMS can be challenging for clinicians because of highly variable intra- and inter-individual therapeutic responses regarding efficacy and safety.Citation145,Citation146 Only a minority of patients attain the goal of NEDA with current DMTs,Citation18,Citation147,Citation148 while the majority of patients achieve a partial reduction in the frequency of relapses and inflammatory brain lesion activity on MRI, and delayed accumulation of disability.Citation145,Citation149 It is important to note that although patients with residual clinical or radiologic disease activity despite treatment with a first-line DMT may be experiencing some therapeutic benefit, they are commonly at increased risk of disability worsening in the short termCitation150,Citation151 and a lower health-related quality of life.Citation152,Citation153 Apart from a high incidence of breakthrough disease activity associated with first-line DMTs,Citation145,Citation146 many patients experience treatment-related AEs,Citation154 as well as reduced quality of therapeutic response to the extent that clinical and pharmacoeconomic outcomes are compromised because of patient nonadherence to therapy.Citation137,Citation140,Citation155–Citation157 Hence, new therapies are required that have a favorable benefit–risk profile, few tolerability issues, and different attributes to encourage improved levels of adherence. In this regard, the superior efficacy of daclizumab over IM interferon beta-1a, a standard of care in MS, has been demonstrated. The risks associated with daclizumab, including hepatic disorders, cutaneous events, and infections, were typically mild or moderate in severity, manageable with routine medical care, and did not appear to increase with extended treatment. Daclizumab has been approved by the US FDA and EMA for relapsing MS.
Acknowledgments
The daclizumab clinical development program was funded by Biogen and AbbVie Biotherapeutics Inc. Biogen and AbbVie Biotherapeutics Inc. provided funding for medical writing support in the development of this paper; Malcolm Darkes from Excel Scientific Solutions wrote the first draft of the manuscript based on input from Dr Cohan, and Kristen DeYoung from Excel Scientific Solutions copyedited and styled the manuscript per journal requirements. Biogen and AbbVie Biotherapeutics Inc. reviewed and provided feedback on the paper to Dr Cohan. Dr Cohan had full editorial control of the paper and provided his final approval of all content.
Disclosure
Stanley Cohan serves on advisory boards for Biogen, Mallinckrodt, Novartis, and Sanofi-Genzyme; has received research support from Biogen, Mallinckrodt, Novartis, Opexa, Roche-Genentech, Sanofi-Genzyme, and Teva; has received speaker honoraria from Acorda, Biogen, Novartis, Roche-Genentech and Sanofi-Genzyme; and has received funds for transportation, meals, and lodging from Acorda, Biogen, Mallinckrodt, Novartis, Roche-Genentech, and Sanofi-Genzyme. The author reports no other conflicts of interest in this work.
References
- CompstonAColesAMultiple sclerosisLancet200837296481502151718970977
- Multiple Sclerosis International FederationAtlas of MS2013 Available from: http://www.msif.org/about-us/advocacy/atlas/Accessed August 26, 2015
- FreedmanMSDisease-modifying drugs for multiple sclerosis: current and future aspectsExpert Opin Pharmacother20067Suppl 1S1S917020427
- LublinFDThe incomplete nature of multiple sclerosis relapse resolutionJ Neurol Sci2007256Suppl 1S14S1817337274
- MillerDMWeinstock-GuttmanBBéthouxFA meta-analysis of methylprednisolone in recovery from multiple sclerosis exacerbationsMult Scler20006426727310962547
- BuchananRJHuangCKaufmanMHealth-related quality of life among young adults with multiple sclerosisInt J MS Care2010124190199
- ForbesAWhileAMathesLGriffithsPHealth problems and health-related quality of life in people with multiple sclerosisClin Rehabil2006201677816502752
- CapkunGDahlkeFLahozRMortality and comorbidities in patients with multiple sclerosis compared with a population without multiple sclerosis: an observational study using the US Department of Defense administrative claims databaseMult Scler Relat Disord20154654655426590661
- JacobsLDCookfairDLRudickRAIntramuscular interferon beta-1a for disease progression in relapsing multiple sclerosisAnn Neurol19963932852948602746
- JohnsonKPBrooksBRCohenJAThe Copolymer 1 Multiple Sclerosis Study GroupCopolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trialNeurology1995457126812767617181
- PRISMS (Prevention of Relapses and Disability by Interferon β-1a Subcutaneously in Multiple Sclerosis) Study GroupRandomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosisLancet19983529139149815049820297
- The IFNB Multiple Sclerosis Study GroupInterferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trialNeurology19934346556618469318
- LiuZPelfreyCMCotleurALeeJCRudickRAImmunomodulatory effects of interferon beta-1a in multiple sclerosisJ Neuroimmunol20011121–215316211108944
- CoccoEMarrosuMGThe current role of mitoxantrone in the treatment of multiple sclerosisExpert Rev Neurother201414660761624834466
- AktasOKieseierBHartungHPNeuroprotection, regeneration and immunomodulation: broadening the therapeutic repertoire in multiple sclerosisTrends Neurosci201033314015220045200
- PolmanCHO’ConnorPWHavrdovaEAFFIRM InvestigatorsA randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosisN Engl J Med2006354989991016510744
- RudickRAStuartWHCalabresiPASENTINEL InvestigatorsNatalizumab plus interferon beta-1a for relapsing multiple sclerosisN Engl J Med2006354991192316510745
- HavrdovaEGalettaSHutchinsonMEffect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) studyLancet Neurol20098325426019201654
- MehlingMKapposLDerfussTFingolimod for multiple sclerosis: mechanism of action, clinical outcomes, and future directionsCurr Neurol Neurosci Rep201111549249721789537
- FoxRJKitaMCohanSLBG-12 (dimethyl fumarate): a review of mechanism of action, efficacy, and safetyCurr Med Res Opin201430225126224131282
- Bar-OrAPachnerAMenguy-VacheronFKaplanJWiendlHTeriflunomide and its mechanism of action in multiple sclerosisDrugs201474665967424740824
- CalabresiPARadueEWGoodinDSafety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trialLancet Neurol201413654555624685276
- CohenJABarkhofFComiGTRANSFORMS Study GroupOral fingolimod or intramuscular interferon for relapsing multiple sclerosisN Engl J Med2010362540241520089954
- ConfavreuxCO’ConnorPComiGTOWER Trial GroupOral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trialLancet Neurol201413324725624461574
- FoxRJMillerDHPhillipsJTCONFIRM Study InvestigatorsPlacebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosisN Engl J Med2012367121087109722992072
- GoldRKapposLArnoldDLDEFINE Study InvestigatorsPlacebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosisN Engl J Med2012367121098110722992073
- KapposLRadueEWO’ConnorPFREEDOMS Study GroupA placebo-controlled trial of oral fingolimod in relapsing multiple sclerosisN Engl J Med2010362538740120089952
- O’ConnorPWolinskyJSConfavreuxCTEMSO Trial GroupRandomized trial of oral teriflunomide for relapsing multiple sclerosisN Engl J Med2011365141293130321991951
- HartungHPAktasOBoykoANAlemtuzumab: a new therapy for active relapsing-remitting multiple sclerosisMult Scler2015211223425344374
- CohenJAColesAJArnoldDLCARE-MS I investigatorsAlemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trialLancet201238098561819182823122652
- ColesAJTwymanCLArnoldDLCARE-MS II investigatorsAlemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trialLancet201238098561829183923122650
- RocheRoche’s ocrelizumab first investigational medicine to show positive pivotal study results in both relapsing and primary progressive forms of multiple sclerosis [news release]BaselSwitzerlandRoche1082015 Available from: http://www.roche.com/media/store/releases/med-cor-2015-10-08.htmAccessed January 7, 2016
- KapposLLiDCalabresiPAOcrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trialLancet201137898051779178722047971
- YednockTACannonCFritzLCSanchez-MadridFSteinmanLKarinNPrevention of experimental autoimmune encephalomyelitis by antibodies against α4ß1 integrinNature1992356636463661538783
- ArvinAMWolinskyJSKapposLVaricella-zoster virus infections in patients treated with fingolimod: risk assessment and consensus recommendations for managementJAMA Neurol2015721313925419615
- BloomgrenGRichmanSHotermansCRisk of natalizumab-associated progressive multifocal leukoencephalopathyN Engl J Med2012366201870188022591293
- RosenkranzTNovasMTerborgCPML in a patient with lymphocytopenia treated with dimethyl fumarateN Engl J Med2015372151476147825853765
- Novartis Pharmaceuticals CorporationGilenya [prescribing information]East Hanover, NJNovartis Pharmaceuticals Corporation2015
- WaldmannTAAnti-Tac (daclizumab, Zenapax) in the treatment of leukemia, autoimmune diseases, and in the prevention of allograft rejection: a 25-year personal odysseyJ Clin Immunol200727111817216565
- WiendlHGrossCCModulation of IL-2Rα with daclizumab for treatment of multiple sclerosisNat Rev Neurol20139739440423732529
- GoldRGiovannoniGSelmajKSELECT study investigatorsDaclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): a randomised, double-blind, placebo-controlled trialLancet201338198842167217523562009
- KapposLWiendlHSelmajKDaclizumab HYP versus interferon beta-1a in relapsing multiple sclerosisN Engl J Med2015373151418142826444729
- BielekovaBRichertNHowardTHumanized anti-CD25 (daclizumab) inhibits disease activity in multiple sclerosis patients failing to respond to interferon ßProc Natl Acad Sci U S A2004101238705870815161974
- BielekovaBHowardTPackerANEffect of anti-CD25 antibody daclizumab in the inhibition of inflammation and stabilization of disease progression in multiple sclerosisArch Neurol200966448348919364933
- RoseJWWattHEWhiteATCarlsonNGTreatment of multiple sclerosis with an anti–interleukin-2 receptor monoclonal antibodyAnn Neurol200456686486715499632
- RoseJWBurnsJBBjorklundJKleinJWattHECarlsonNGDaclizumab phase II trial in relapsing and remitting multiple sclerosis: MRI and clinical resultsNeurology200769878578917709711
- WynnDKaufmanMMontalbanXCHOICE investigatorsDaclizumab in active relapsing multiple sclerosis (CHOICE study): a phase 2, randomised, double-blind, placebo-controlled, add-on trial with interferon betaLancet Neurol20109438139020163990
- OthmanAATranJQTangMTDuttaSPopulation pharmacokinetics of daclizumab high-yield process in healthy volunteers: integrated analysis of intravenous and subcutaneous, single- and multiple-dose administrationClin Pharmacokinet2014531090791825212703
- ClinicalTrials.gov [homepage on the Internet]An immunogenicity and pharmacokinetics (PK) study of BIIB019 (daclizumab high yield process (DAC HYP)) prefilled syringe in relapsing remitting multiple sclerosis (RRMS) (OBSERVE) Available from: https://clinicaltrials.gov/ct2/show/NCT01462318Accessed December 7, 2015
- TranJQOthmanAMikulskisAWolstencroftPElkinsJPharmacokinetics of daclizumab high-yield process with repeated administration of the clinical subcutaneous regimen in patients with relapsing-remitting multiple sclerosisClin Pharmacol2016891326929672
- ClinicalTrials.gov [homepage on the Internet]Long-term extension study in participants with multiple sclerosis who have completed study 205MS301 (NCT01064401) to evaluate the safety and efficacy of BIIB019 (EXTEND) Available from: https://clinicaltrials.gov/ct2/show/NCT01797965Accessed December 7, 2015
- GiovannoniGGoldRSelmajKSELECTION Study InvestigatorsDaclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECTION): a multicentre, randomised, double-blind extension trialLancet Neurol201413547248124656609
- RadueE-WGiovannoniGGoldRLong-term efficacy of daclizumab HYP in relapsing-remitting multiple sclerosis: 3 year results from the SELECTED extension studyNeurology201584Suppl 14P7.226
- BielekovaBCatalfamoMReichert-ScrivnerSRegulatory CD56bright natural killer cells mediate immunomodulatory effects of IL-2Rα-targeted therapy (daclizumab) in multiple sclerosisProc Natl Acad Sci U S A2006103155941594616585503
- MartinJFPerryJSJakheteNRWangXBielekovaBAn IL-2 paradox: blocking CD25 on T cells induces IL-2–driven activation of CD56bright NK cellsJ Immunol201018521311132020543101
- SheridanJPZhangYRiesterKIntermediate-affinity interleukin-2 receptor expression predicts CD56bright natural killer cell expansion after daclizumab treatment in the CHOICE study of patients with multiple sclerosisMult Scler201117121441144821807759
- WuestSCEdwanJHMartinJFA role for interleukin-2 trans- presentation in dendritic cell-mediated T cell activation in humans, as revealed by daclizumab therapyNat Med201117560460921532597
- MalekTRThe biology of interleukin-2Annu Rev Immunol20082645347918062768
- DepperJMLeonardWJDrogulaCKrönkeMWaldmannTAGreeneWCInterleukin 2 (IL-2) augments transcription of the IL-2 receptor geneProc Natl Acad Sci U S A19858212423042342987968
- MalekTRAshwellJDInterleukin 2 upregulates expression of its receptor on a T cell cloneJ Exp Med19851616157515803925066
- WaldmannTAThe IL-2/IL-2 receptor system: a target for rational immune interventionImmunol Today19931462642708397768
- SmithKAInterleukin-2: inception, impact, and implicationsScience19882404856116911763131876
- GongDMalekTRCytokine-dependent Blimp-1 expression in activated T cells inhibits IL-2 productionJ Immunol2007178124225217182561
- ShawJMeerovitchKBleackleyRCPaetkauVMechanisms regulating the level of IL-2 mRNA in T lymphocytesJ Immunol19881407224322483258332
- VillarinoAVTatoCMStumhoferJSHelper T cell IL-2 production is limited by negative feedback and STAT-dependent cytokine signalsJ Exp Med20072041657117227909
- AlmeidaARLegrandNPapiernikMFreitasAAHomeostasis of peripheral CD4+ T cells: IL-2Rα and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbersJ Immunol200216994850486012391195
- de la RosaMRutzSDorningerHScheffoldAInterleukin-2 is essential for CD4+CD25+ regulatory T cell functionEur J Immunol20043492480240815307180
- FontenotJDRasmussenJPGavinMARudenskyAYA function for interleukin 2 in Foxp3-expressing regulatory T cellsNat Immunol20056111142115116227984
- MalekTRYuAVincekVScibelliPKongLCD4 regulatory T cells prevent lethal autoimmunity in IL-2Rß-deficient mice. Implications for the nonredundant function of IL-2Immunity200217216717812196288
- SakaguchiSSakaguchiNAsanoMItohMTodaMImmunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseasesJ Immunol19951553115111647636184
- SetoguchiRHoriSTakahashiTSakaguchiSHomeostatic maintenance of natural Foxp3+ CD25+ CD4+ regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralizationJ Exp Med2005201572373515753206
- TakahashiTKuniyasuYTodaMImmunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive stateInt Immunol19981012196919809885918
- CavanillasMLAlcinaANuñezCPolymorphisms in the IL2, IL2RA and IL2RB genes in multiple sclerosis riskEur J Hum Genet201018779479920179739
- HaflerDACompstonASawcerSInternational Multiple Sclerosis Genetics ConsortiumRisk alleles for multiple sclerosis identified by a genomewide studyN Engl J Med2007357985186217660530
- DendrouCAPlagnolVFungECell-specific protein phenotypes for the autoimmune locus IL2RA using a genotype-selectable human bioresourceNat Genet20094191011101519701192
- RubinLAKurmanCCFritzMESoluble interleukin 2 receptors are released from activated human lymphoid cells in vitroJ Immunol19851355317231773930598
- GreenbergSJMarconLHurwitzBJWaldmannTANelsonDLElevated levels of soluble interleukin-2 receptors in multiple sclerosisN Engl J Med198831915101910203138540
- MaierLMAndersonDESeversonCASoluble IL-2RA levels in multiple sclerosis subjects and the effect of soluble IL-2RA on immune responsesJ Immunol200918231541154719155502
- NelsonBHWillerfordDMBiology of the interleukin-2 receptorAdv Immunol1998701819755337
- Baecher-AllanCBrownJAFreemanGJHaflerDACD4+CD25high regulatory cells in human peripheral bloodJ Immunol200116731245125311466340
- RogersWOWeaverCTKrausLALiJLiLBucyRPVisualization of antigen-specific T cell activation and cytokine expression in vivoJ Immunol199715826496578992980
- WangXRickertMGarciaKCStructure of the quaternary complex of interleukin-2 with its α, ß, and γc receptorsScience200531057511159116316293754
- NelsonBHLordJDGreenbergPDCytoplasmic domains of the interleukin-2 receptor ß and γ chains mediate the signal for T-cell proliferationNature199436964783333367514277
- GoebelJStevensEForrestKRoszmanTLDaclizumab (Zenapax) inhibits early interleukin-2 receptor signal transduction eventsTranspl Immunol20008315315911147695
- YangHWangJDuJStructural basis of immunosuppression by the therapeutic antibody daclizumabCell Res201020121361137120820193
- HussDJMehtaDSSharmaAIn vivo maintenance of human regulatory T cells during CD25 blockadeJ Immunol20151941849225416807
- ElkinsJSheridanJAmaravadiLCD56bright natural killer cells and response to daclizumab HYP in relapsing-remitting MSNeurol Neuroimmunol Neuroinflamm201522e6525635261
- OhUBlevinsGGriffithCRegulatory T cells are reduced during anti-CD25 antibody treatment of multiple sclerosisArch Neurol200966447147919364932
- MehtaDRiesterKSheridanJElkinsJAmaravadiLRapid, sustained and reversible pharmacodynamics of DAC HYP in MS patients supports mechanism of action via modulation of the IL-2 pathwayMult Scler201420Suppl 1491492
- BielekovaBRichertNHermanMLIntrathecal effects of daclizumab treatment of multiple sclerosisNeurology201177211877188622076546
- JiangWChaiNRMaricDBielekovaBUnexpected role for granzyme K in CD56bright NK cell-mediated immunoregulation of multiple sclerosisJ Immunol2011187278179021666061
- FontenotJDGavinMARudenskyAYFoxp3 programs the development and function of CD4+CD25+ regulatory T cellsNat Immunol20034433033612612578
- HoriSNomuraTSakaguchiSControl of regulatory T cell development by the transcription factor Foxp3Science200329956091057106112522256
- BurchillMAYangJVogtenhuberCBlazarBRFarrarMAIL-2 receptor ß-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cellsJ Immunol2007178128029017182565
- PasseriniLAllanSEBattagliaMSTAT5-signaling cytokines regulate the expression of FOXP3 in CD4+CD25+ regulatory T cells and CD4+CD25− effector T cellsInt Immunol200820342143118270368
- SpitsHArtisDColonnaMInnate lymphoid cells – a proposal for uniform nomenclatureNat Rev Immunol201313214514923348417
- PerryJSHanSXuQInhibition of LTi cell development by CD25 blockade is associated with decreased intrathecal inflammation in multiple sclerosisSci Transl Med20124145145ra106
- MagliozziRHowellOVoraAMeningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathologyBrain2007130Pt 41089110417438020
- SerafiniBRosicarelliBMagliozziRStiglianoEAloisiFDetection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosisBrain Pathol200414216417415193029
- LinYCWinokurPBlakeAWuTRommEBielekovaBDaclizumab reverses intrathecal immune cell abnormalities in multiple sclerosisAnn Clin Transl Neurol20152544545526000318
- DiefenbachAColonnaMKoyasuSDevelopment, differentiation, and diversity of innate lymphoid cellsImmunity20144133546525238093
- GillardGOSaenzSAHussDJFontenotJDCirculating innate lymphoid cells are unchanged in response to DAC HYP therapyJ Neuroimmunol2016294414527138097
- TranJQOthmanAAWolstencroftPElkinsJTherapeutic protein-drug interaction assessment for daclizumab high-yield process in patients with multiple sclerosis using a cocktail approachBr J Clin Pharmacol201652116016726991517
- MinochaMTranJQSheridanJPOthmanAABlockade of the high-affinity interleukin-2 receptors with daclizumab high-yield process: pharmacokinetic/pharmacodynamic analysis of single- and multiple-dose phase I trialsClin Pharmacokinet201555112113026242380
- WiendlHGiovannoniGCastro-BorreroWLymphocyte counts in patients receiving daclizumab HYP in DECIDEPresented at: 31st Congress of the European Committee for Treatment and Research in Multiple SclerosisOctober 7–10, 2015Barcelona, Spain
- HainesJLTerwedowHABurgessKThe Multiple Sclerosis Genetics GroupLinkage of the MHC to familial multiple sclerosis suggests genetic heterogeneityHum Mol Genet199878122912349668163
- ZhangYMcClellanMEfrosLDaclizumab reduces CD25 levels on T cells through monocyte-mediated trogocytosisMult Scler201420215616423846354
- XuWFazekasGHaraHTabiraTMechanism of natural killer (NK) cell regulatory role in experimental autoimmune encephalomyelitisJ Neuroimmunol20051631–2243015885305
- PolmanCHReingoldSCEdanGDiagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”Ann Neurol200558684084616283615
- PhillipsGAWyrwichKWGuoSResponder definition of the Multiple Sclerosis Impact Scale physical impact subscale for patients with physical worseningMult Scler201420131753176024740371
- European Medicines AgencyGuideline on clinical investigation of medicinal products for the treatment of multiple sclerosis Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/03/WC500185161.pdfAccessed November 3, 2015
- MillerDHBarkhofFFrankJAParkerGJThompsonAJMeasurement of atrophy in multiple sclerosis: pathological basis, methodological aspects and clinical relevanceBrain2002125Pt 81676169512135961
- ArnoldDLKapposLKhanOReduction in brain volume loss in patients receiving daclizumab HYP versus intramuscular interferon beta-1a: results of the DECIDE studyMult Scler201521Suppl 1125725825186208
- ArnoldDLKapposLWiendlHBenefits on brain MRI lesion activity with daclizumab HYP compared with intramuscular interferon beta-1a are maintained through 144 weeks’ treatment: results from the DECIDE studyMult Scler201521Suppl 1125525625070677
- GiovannoniGRadueEWHavrdovaEEffect of daclizumab high-yield process in patients with highly active relapsing-remitting multiple sclerosisJ Neurol2014261231632324375015
- WiendlHKapposLSelmajKDaclizumab high-yield process (DAC HYP) vs. interferon beta-1a in patients with highly active disease: DECIDE study resultsEur J Neurol201522Suppl 150
- WiendlHHavrdovaERoseJRiesterKTsaoLCGreenbergSDaclizumab HYP versus interferon ß-1a across patient demographic and disease activity subgroups in the DECIDE phase 3 studyNeurology201584Suppl 14P4.007
- KapposLHavrdovaEGiovannoniGEffect of daclizumab HYP versus intramuscular interferon beta-1a on no evidence of disease activity in patients with relapsing-remitting multiple sclerosis: analysis of the DECIDE studyPresented at: 31st Congress of the European Committee for Treatment and Research in Multiple SclerosisOctober 7–10, 2015Barcelona, Spain
- HavrdovaEGiovannoniGStefoskiDDisease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with daclizumab high-yield process in the SELECT studyMult Scler201420446447024022270
- GiovannoniGKapposLGoldRSafety and tolerability of daclizumab HYP in patients with relapsing-remitting multiple sclerosis: an integrated analysis of six clinical studiesPresented at: 31st Congress of the European Committee for Treatment and Research in Multiple SclerosisOctober 7–10, 2015Barcelona, Spain
- HobartJLampingDFitzpatrickRRiaziAThompsonAThe Multiple Sclerosis Impact Scale (MSIS-29): a new patient-based outcome measureBrain2001124Pt 596297311335698
- RabinRde CharroFEQ-5D: a measure of health status from the EuroQol GroupAnn Med200133533734311491192
- KapposLSelmajKArnoldDDaclizumab HYP versus interferon beta-1a in relapsing-remitting multiple sclerosis: primary results of the DECIDE studyNeurology20158414 SupplS4.003
- FischerJSRudickRACutterGRReingoldSCNational MS Society Clinical Outcomes Assessment Task ForceThe Multiple Sclerosis Functional Composite Measure (MSFC): an integrated approach to MS clinical outcome assessmentMult Scler19995424425010467383
- YadavVBourdetteDNew disease-modifying therapies and new challenges for MSCurr Neurol Neurosci Rep201212548949122760478
- LublinFDCofieldSSCutterGRRandomized study combining interferon and glatiramer acetate in multiple sclerosisAnn Neurol201373332734023424159
- MillerAETeriflunomide: a once-daily oral medication for the treatment of relapsing forms of multiple sclerosisClin Ther201537102366238026365096
- WillisMDHardingKEPickersgillTPAlemtuzumab for multiple sclerosis: long term follow-up in a multi-centre cohortMult Scler Epub2015102910.1177/1352458515614092
- GoldRGiovannoniGSelmajKLong-term safety of daclizumab HYP in patients with relapsing-remitting multiple sclerosis: 3–4 year results from the SELECTED extension studyPresented at: 67th Annual Meeting of the American Academy of NeurologyApril 18–25, 2015Washington, DC
- TremlettHLOgerJInterrupted therapy: stopping and switching of the ß-interferons prescribed for MSNeurology200361455155412939437
- TempleRHy’s law: predicting serious hepatotoxicityPharmacoepidemiol Drug Saf200615424124316552790
- RockeyDCSeeffLBRochonJCausality assessment in drug-induced liver injury using a structured expert opinion process: comparison to the Roussel-Uclaf causality assessment methodHepatology20105162117212620512999
- KircikLKruegerJLebwohlMIncidence, severity, duration, and treatment of cutaneous adverse events in the DECIDE study of daclizumab HYP versus intramuscular interferon beta-1a in patients with relapsing-remitting multiple sclerosisMult Scler201521Suppl 11251252
- AmaravadiLMikulskisALucasNRiesterKSweetserMElkinsJEvaluation of immunogenicity in patients with multiple sclerosis treated with daclizumab HYPPresented at: 67th Annual Meeting of the American Academy of NeurologyApril 18–25, 2015Washington, DC
- GoldRStefoskiDSelmajKPregnancy experience: preclinical data and pregnancy outcomes in the daclizumab high-yield process clinical programPresented at: 31st Congress of the European Committee for Treatment and Research in Multiple SclerosisOctober 7–10, 2015Barcelona, Spain
- MehtaLPriestleyRUmansKImmune response to seasonal influenza vaccine in patients with relapsing-remitting multiple sclerosis on long-term daclizumab HYP treatmentMult Scler201521Suppl 11234235
- SteinbergSCFarisRJChangCFChanATankersleyMAImpact of adherence to interferons in the treatment of multiple sclerosis: a non-experimental, retrospective, cohort studyClin Drug Investig201030289100
- MenzinJCaonCNicholsCWhiteLAFriedmanMPillMWNarrative review of the literature on adherence to disease-modifying therapies among patients with multiple sclerosisJ Manag Care Pharm2013191 Suppl AS24S4023383731
- ChenCBarabanEStuchinerTKimESuhKCohanSEvaluating medication adherence to disease-modifying therapy (DMT) and associated factors using data from the Pacific Northwest Multiple Sclerosis RegistryMult Scler201319Suppl 11479
- DevonshireVLapierreYMacdonellRGAP Study GroupThe Global Adherence Project (GAP): a multicenter observational study on adherence to disease-modifying therapies in patients with relapsing-remitting multiple sclerosisEur J Neurol2011181697720561039
- PoulosCKinterEYangJ-CBridgesJFPPosnerJRederATPatient preferences for injectable treatments for multiple sclerosis in the United States: a discrete-choice experimentPatient20169217118026259849
- RichterAAntonSFKochPDennettSLThe impact of reducing dose frequency on health outcomesClin Ther200325823072335 discussion 230614512137
- CottéFEFardellonePMercierFGaudinAFRouxCAdherence to monthly and weekly oral bisphosphonates in women with osteoporosisOsteoporos Int201021114515519459025
- StonerKLHarderHFallowfieldLJJenkinsVAIntravenous versus subcutaneous drug administration. Which do patients prefer? A systematic reviewPatient201582145153
- RudickRAPolmanCHCurrent approaches to the identification and management of breakthrough disease in patients with multiple sclerosisLancet Neurol20098654555919446274
- FreedmanMSCohenBDhib-JalbutSRecognizing and treating suboptimally controlled multiple sclerosis: steps toward regaining commandCurr Med Res Opin200925102459247019678753
- ArnoldDLCalabresiPAKieseierBCEffect of peginterferon beta-1a on MRI measures and achieving no evidence of disease activity: results from a randomized controlled trial in relapsing-remitting multiple sclerosisBMC Neurol20141424025551571
- KhatriBBarkhofFComiGJinJFrancisGCohenJFingolimod treatment increases the proportion of patients who are free from disease activity in multiple sclerosis compared to IFN-ß1a: results from a phase 3, active-controlled study (TRANSFORMS)Neurology201278Meeting Abstracts 1PD5.006
- MillerAERhoadesRWTreatment of relapsing-remitting multiple sclerosis: current approaches and unmet needsCurr Opin Neurol201225SupplS4S1022398662
- ProsperiniLGalloVPetsasNBorrielloGPozzilliCOne-year MRI scan predicts clinical response to interferon beta in multiple sclerosisEur J Neurol200916111202120919538207
- RioJRoviraATintoréMRelationship between MRI lesion activity and response to IFN-ß in relapsing–remitting multiple sclerosis patientsMult Scler200814447948418562504
- Benito-LeónJMitchellAJRivera-NavarroJMorales-GonzálezJMImpaired health-related quality of life predicts progression of disability in multiple sclerosisEur J Neurol2013201798622742892
- KarampampaKGustavssonAMiltenburgerCEckertBTreatment experience, burden and unmet needs (TRIBUNE) in MS study: results from five European countriesMult Scler201218Suppl 271522623122
- GiovannoniGSouthamEWaubantESystematic review of disease-modifying therapies to assess unmet needs in multiple sclerosis: tolerability and adherenceMult Scler201218793294622249762
- CerghetMDobieEElston LafataJAdherence to disease-modifying agents and association with quality of life among patients with relapsing-remitting multiple sclerosisInt J MS Care20101225158
- UitdehaagBConstantinescuCCornelissePImpact of exposure to interferon beta-1a on outcomes in patients with relapsing–remitting multiple sclerosis: exploratory analyses from the PRISMS long-term follow-up studyTher Adv Neurol Disord20114131421339904
- TanHCaiQAgarwalSStephensonJJKamatSImpact of adherence to disease-modifying therapies on clinical and economic outcomes among patients with multiple sclerosisAdv Ther2011281516121153000
- ArnoldDKapposLHavrdovaEBrain MRI results of DECIDE: a randomized, double-blind trial of DAC HYP vs. IFN β-1a in RRMS patientsPresented at: Joint ACTRIMS/ECTRIMS MeetingSeptember 10–13, 2014Boston, MA
- DuffySSLeesJGMoalem-TaylorGThe contribution of immune and glial cell types in experimental autoimmune encephalomyelitis and multiple sclerosisMult Scler Int2014201428524525374694
