Abstract
Paraneoplastic pemphigus (PNP) is a fatal autoimmune blistering disease associated with an underlying malignancy. It is a newly recognized blistering disease, which was first recognized in 1990 by Dr Anhalt who described an atypical pemphigus with associated neoplasia. In 2001, Nguyen proposed the term paraneoplastic autoimmune multiorgan syndrome because of the recognition that the condition affects multiple organ systems. PNP presents most frequently between 45 and 70 years old, but it also occurs in children and adolescents. A wide variety of lesions (florid oral mucosal lesions, a generalized polymorphous cutaneous eruption, and pulmonary involvement) may occur in patients with PNP. The earliest and most consistent finding is severe stomatitis. There is a spectrum of at least five clinical variants with different morphology. Similarly, the histological findings are very variable. Investigations to diagnose PNP should include checking for systemic complications (to identify tumor), skin biopsies (for histopathological and immunofluorescence studies), and serum immunological studies. PNP is characterized by the presence of autoantibodies against antigens such as desmoplakin I (250 kD), bullous pemphigoid aniygen I (230 kD), desmoplakin II (210 kD), envoplakin (210 kD), periplakin (190 kD), plectin (500 kD), and a 170 kD protein. Unlike other forms of pemphigus, PNP can affect other types of epithelia, such as gastrointestinal and respiratory tract. Treatment of PNP is difficult, and the best outcomes have been reported with benign neoplasms that have been surgically excised. The first-line treatment is high-dose corticosteroids with the addition of steroid-sparing agents. Treatment failures are often managed with rituximab with or without concomitant intravenous immunoglobulin. In general, the prognosis is poor, not only because of eventual progression of malignant tumors but also because treatment with aggressive immunosuppression therapy often results in infectious complications, which is unfortunately at this time the most common cause of death in PNP.
Introduction
Paraneoplastic pemphigus (PNP) is a rare and often fatal autoimmune blistering disease accompanied by both benign and malignant neoplasms. The most frequently reported associated malignancies are lymphomatoid and hematologic, eg, B-cell lymphoma, chronic lymphocytic leukemia, Castleman’s disease, Waldenstrom’s macroglobulinemia, and thymoma (with or without myasthenia gravis). Interactions between the immune system and concomitant neoplasm seem to be the basis of pathogenesis, with autoantibodies directed against both desmosomal and hemidesmosomal antigens. In PNP, the vast majority of patients have autoantibodies to periplakins and envoplakins.
Prognosis depends on the associated tumor. Some patients experience rapid improvement after excision of a benign tumor, such as Castleman’s disease. However, malignant tumors are frequently accompanied not only by higher mortality from the associated malignancy but also because the PNP can be quite severe and unresponsive to treatment.
PNP is a rare disease whose incidence is not fully recognized. In 1990, Anhalt et alCitation1 first described five cases of patients with a rare form of atypical pemphigus that were all associated with lymphoproliferative diseases. Of 100,000 cases of non-Hodgkin’s lymphoma and chronic lymphocytic leukemia reported to the US Food and Drug Administration, a total of 12 were found to be complicated by PNP.Citation2 PNP mostly affects adults between 45 and 70 years of age, but it may also be found in children, particularly when associated with Castleman disease. There is no known correlation between incidence of the disease and specific sex, race, or place of origin.Citation3
Pathogenesis
Etiopathogenesis of PNP is not fully known.Citation4 Skin lesions are thought to be caused by an autoimmune response generated by antibodies to tumor antigens that cross-react with epithelial antigens. Tumor autoantibodies produce and release cytokines (such as interleukin-6) that favor the differentiation of B-cellsCitation5 and foster the development of the humoral branch of the immune system.
PNP is often a harbinger of benign and malignant neoplasms, most commonly malignancies of the lymphatic system. On the basis of the review of 163 cases of PNP examined between 1990 and 2003, lists the most frequent concomitant cancers.Citation6 Ohzono et alCitation7 described the associated tumors in 104 PNP cases over a period of 16 years (between January 1997 and April 2013). Their clinical and histopathological findings were generally similar to those in previous reports.Citation7
Table 1 Relative frequencies of concomitant neoplasms
Some patients have tumors that are difficult to define, such as follicular dendritic cell sarcomas located in the retroperitoneal space.Citation8 Studies of patients with non-Hodgkin lymphoma revealed that most severe lesions in the course of the PNP occur 2–3 years after diagnosis of lymphoma.Citation6 Castleman disease also known as giant lymph node hyperplasia occurs most commonly in children. When it occurs in localized form, it can be simply treated with surgical resection.
There is no consensus regarding the diagnostic criteria for PNP. The first set of criteria were made by Anhalt et alCitation1,Citation5,Citation8,Citation9 and include the following:
Characteristic clinical appearance and histopathology.
Detection of tissue bound, circulating autoantibodies via direct immunofluorescence, indirect immunofluorescence (IIF), and immunoprecipitation studies.
To expand on this later, AnhaltCitation1,Citation5,Citation8,Citation9 further developed defining characteristics of PNP such as painful inflammation of the oral mucosa, a polymorphous skin eruption with corresponding histologic findings often showing lichenoid or acantholytic changes, supportive immunofluorescence findings showing intercellular and basement membrane binding, serum antibodies that bind simple, columnar, and transitional epithelium, coexistence of lymphoproliferative disorders, and the presence of anti-dsg, desmoplakin I and II, envoplakin, periplakin, bullous pemphigoid antigen 1, and plectin antibodies.Citation9
Histology
The diagnosis is made based on clinical, histological, and immunofluorescent findings. Often diagnosis of the disease necessitates multiple biopsies.Citation10 Histology varies depending on the type of skin lesions. Acantholysis occurs over the basal layer in blisters. Additionally, there might be vacuolar degeneration of the basal layer associated with band-like infiltrate of lymphocytes in the dermis as one would typically see in lesions that are clinically and histopathologically lichenoid.
Immunology
Immunopathology plays an important role in the diagnosis of PNP. In direct immunofluorescence examination, intercellular deposits of IgG and C3 are ascertained. Unlike pemphigus vulgaris, linear C3 deposits and, to a lesser extent, IgG can be observed at epidermal–dermal junction. This is likely attributed to binding of BP180 and BP230. In fact, IIF and enzyme-linked immunosorbent assay (ELISA) testing found about 40% of patients had circulating autoantibodies to BP180, and these patients were more likely to have skin lesions involving tense bullae as seen in bullous pemphigoid.Citation11 IIF shows that IgG antibodies bind with the stratified epithelium in the esophagus and other tissues of monkeys. In sharp contrast to pemphigus vulgaris and foliaceus, they also bind with the transitional and cylindrical epithelium of the urinary bladder, bronchi, small intestine, and colon, as well as, to a lesser extent, with myocardium and skeletal muscles and thyroid epithelium.
Previously, the highest sensitivity (75%) and specificity were exhibited by rat bladder immunofluorescence testing. Immunoprecipitation and immunoblotting can demonstrate antibodies to all desmosomal proteins: desmoglein 3 (130 kD), desmoplakin 1 (250 kD), BP230, desmoplakin 2 (210 kD), envoplakin (210 kD), plectin (>400 kD), periplakin (190 kD), epiplakin,Citation12 and occasionally desmoglein 1 (160 kD). Also, studies using novel mammalia ELISAs showed that PNP sera reacted with desmocollins 1–3 in various patterns.Citation13 Fluorescence on bladder epithelium (transitional) reflects the binding with intracellular proteins. ELISA may show autoantibodies to desmoglein 3 and, less often, desmoglein 1.Citation14 New, commercially available ELISA testing to envoplakin may increase the sensitivity and specificity of serologic testing to surpass that of current immunofluorescence testing. Using immunoprecipitation and mass spectrometry, the 170 kD antigen target has been identified as a protease inhibitor, α-2 macroglobulin-like protein, a broad range protease inhibitor expressed in stratified epithelia and other tissues damaged in PNP.Citation15
Clinical
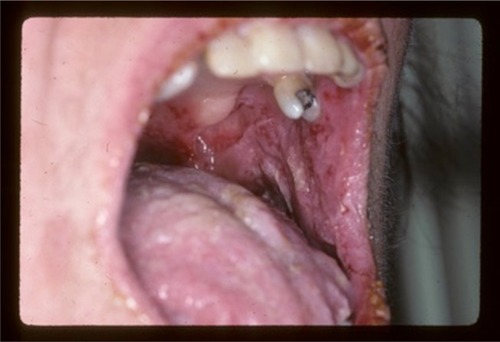
Typically, the first symptoms include severe painful oral erosions, frequently hemorrhagic, which spread to involve the entire vermilion and tongue (). The lesions are polymorphic, and symptoms such as blisters, erosions, spots, papules, and plaques can occur. These clinical findings may be accompanied by a positive Nikolsky sign. Cutaneous lesions usually appear subsequent to the onset of mucosal lesions and may involve any site, but the upper body is most usually involved.
Symptoms can be classified into several groups according to the types of alterations:
Pemphigus-like: superficial vesicles, flaccid vesicles, erosions, crust, and erythema.
Bullous pemphigoid-like: scaly erythematous papules, which may or may not be associated with stretched vesicles.
Erythema multiform-like: polymorphic alterations, mainly erythematous peeling pellets with erosions and sometimes even with hard-to-heal ulcerations.
Graft-versus-host disease: disseminated dusky red scaly papules.
Lichen planus-like: small flat scaly papules and intense mucous membrane involvement.
PNP may not only involve the buccal mucosa but also the mucous membranes of the esophagus, stomach, duodenum, and intestines and also the pulmonary epithelium.Citation2,Citation3
Patients with PNP can develop life-threatening restrictive bronchiolitis consistent with bronchiolitis obliterans. The frequency of the involvement of the respiratory system and pathological mechanisms are not known. In a study of 17 patients with PNP, restrictive bronchiolitis was found only in three patients. However, in another analysis where 28 patients with PNP and concomitant Castleman’s disease were examined, the respiratory system was affected in 26 cases.Citation16,Citation17 Pulmonary disease, when present, is irreversible despite aggressive therapy.Citation2,Citation3,Citation18 The recently discovered autoantigen, epiplakin, has demonstrated correlation with development of bronchiolitis obliterans in Japanese patients.Citation12 Epiplakin is present in the respiratory bronchiole, and mice injected with epiplakin autoantibody showed abnormal changes in the histopathology of their pulmonary epithelia. While more research is needed, these early results indicate that epiplakin may represent a specific autoantigen in PNP-related bronchiolitis obliterans.
The differential diagnosis includes pemphigus vulgaris, mucous membrane pemphigoid, erythema multiforme, Stevens–Johnson syndrome, lichen planus, graft-versus-host disease, and herpes simplex virus infection. When PNP is suspected in a patient with no know history of malignancy, an extensive baseline workup should be conducted, including: blood cell count, lactate dehydrogenase, flow cytometry, as well as computed tomography of the chest, abdomen, and pelvis. In up to a third of patients with PNP, discovery of the underlying malignancy occurred subsequent the onset of PNP symptoms.
Treatment
It is vital to define and treat the associated cancer in PNP. In patients with an operable malignancy, a surgical cure is often the best chance of inducing remission of PNP.Citation19
Concurrent to a thorough medical workup in patients without operable malignancies, several nonsurgical treatments have proven effective in reducing symptoms in patients with PNP. Initially, glucocorticosteroid therapy should be implemented – prednisone (0.5–1.0 mg/kg).Citation20 Cutaneous lesions tend to crust over and heal faster than alterations in the mucosa. Steroid-sparing agents can be added to glucocorticoid therapy to reduce the total steroid burden. Immunosuppressants such as cyclosporin, cyclophosphamide, azathioprine, and mycophenolate mofetil are often used in combination with prednisone.Citation21 However, because of the tumorgenic properties of these agents, often new alternative therapies are being used particularly in patients whose malignancy is in remission.
Rituximab – a chimeric anti-CD20 monoclonal antibody – is being used more often in patients with PNP because it appears to be more effective than alternative steroid sparing agents with perhaps reduced tumorgenicity risk. Patients can be treated with the lymphoma protocol at a dose of 375 mg/m weekly for 4 weeks, or the rheumatologic protocol of 1 g once and repeated in 2 weeks. Additional cycles may be administered every 6–12 months depending on clinical response and recovery of the B-cell (CD-20) population. Rituximab is usually very well tolerated, but notable adverse effects of treatment include infusion reactions and an allergic reaction. Severe, life-threatening anaphylactic reactions have occurred. For this reason, rituximab is infused in a monitored setting such as an infusion center where an allergy can be rapidly identified and treated. In addition, progressive multifocal leukoencephalopathy, a fatal and untreatable reactivation of JC virus in the brain, has been reported in association with rituximab. This reactivation occurs only in the setting of severe immunosuppression. This is of concern for PNP patients, who are often substantially immunosuppressed given the concomitant malignancy with or without associated chemotherapy. The first successful treatment of a patient with PNP was in one who had concomitant non-Hodgkin lymphoma that was being treated with rituximab.Citation22 Like many options for PNP, rituximab also has limited effectiveness, and often treatment success depends on controlling the patients underlying malignancy.Citation23
The concomitant use of rituximab with intravenous immunoglobulin (IVIG) has proven successful in those patients who do not respond to conventional therapy or use of rituximab alone. IVIG is dosed at 2 g/kg per cycle, and cycles are repeated on a monthly basis. IVIG is well tolerated and shown to be quite effective in reducing pathogenic autoantibodies rapidly in patients with autoimmune bullous diseases. Another benefit is that IVIG can be added into the patient’s existing treatment regimen without added concern of additional immunosuppression, making it a popular approach among clinicians who treat PNP. IVIG’s favorable safety profile makes it an obvious choice in patients who are often on complicated treatments for PNP and an underlying malignancy. However, the affordability and its inconvenience have limited its use.Citation24
Unfortunately, PNP is often resistant to treatment. The mortality rate ranges from 75% to 90%, with the main cause of death in these patients being respiratory failure.Citation25 Prompt diagnosis and early initiation of treatment are of paramount importance.
Disclosure
The authors report no conflicts of interest in this work.
References
- AnhaltGJKimSCParaneoplastic pemphigus. An autoimmune mucocutaneous disease associated with neoplasiaN Engl J Med1990323172917352247105
- NguyenVTNdoyeABasslerKDClassification, clinical manifestations, and immunopathological mechanisms of the epithelial variant of paraneoplastic autoimmune multiorgan syndrome: a reappraisal of paraneoplastic pemphigusArch Dermatol2001137219320611176692
- CzernikACamilleriMPittelkowMRGrandoSAParaneoplastic autoimmune multiorgan syndrome: 20 years afterInt J Dermatol20115090591421781058
- ZimmermannJBahmerFRoseCZillikensDSchmidtEClinical and immunopathologica spectrum of paraneoplastic pemphigusJ Dtsch Dermatol Ges20108598606 German20180886
- AnhaltGJMimouniDParaneoplastic pemphigusGoldsmithLAKatzSIGilchrestFitzpatrick’s Dermatology in General Medicine8th edNew Yory, NYMcGraw Hill20121600
- KaplanIHodakEAckermanLMimouniDAnhaltGJCalderonSNeoplasms associated with paraneoplastic pemphigus: a review with emphasis on non-hematologic malignancy and oral mucosal manifestationsOral Oncol20044055315063382
- OhzonoASogameRLiXClinical and immunological findings in 104 cases of paraneoplastic pemphigusBr J Dermatol2015173144726358412
- AnhaltGJParaneoplastic pemphigusAdv Dermatol199712778973736
- AnhaltGJParaneoplastic pemphigusJ Investig Dermatol Symp Proc200492933
- HornTDAnhaltGJHistologic features of paraneoplastic pemphigusArch Dermatol1992128109110951497365
- TsuchisakaAKawanoHYasukochiAImmunological and statistical studies of anti-BP180 antibodies in paraneoplastic pemphigusJ Invest Dermatol20141348228324658507
- TsuchisakaANumataSTeyeKEpiplakin is a paraneoplastic pemphigus autoantigen and related to bronchiolitis obliterans in Japanese patientsJ Invest Dermatol2016136239940826802236
- IshiiNTeyeKFukudaSAnti-desmocollin autoantibodies in nonclassical pemphigusBr J Dermatol20151731596825640111
- HashimotoTProduction of numerous autoantibodies in paraneoplastic pemphigusBr J Dermatol2015172484985025827731
- SchepensIJauninFBegreNThe protease inhibitor alpha-2-macroglobulin-like-1 is the p170 antigen recognized by paraneoplastic pemphigus autoantibodies in humanPLoS One20105e1225020805888
- MaldonadoFPittelkowMRRyuJHConstrictive bronchiolitis associated with paraneoplastic autoimmune multiorgan syndromeRespirology20091412913319144057
- NikolskaiaOVNousariCHAnhaltGJParaneoplastic pemphigus in association with Castleman’s diseaseBr J Dermatol2003149114314674890
- FullertonSHWoodleyDTSmollerBRAnhaltGJParaneoplastic pemphigus with autoantibody deposition in bronchial epithelium after autologous bone marrow transplantationJAMA1992267150015021538540
- FangYZhaoLYanFCuiXXiaYDurenAA critical role of surgery in the treatment for paraneoplastic pemphigus caused by localized Castleman’s diseaseMed Oncol20102790719763912
- SehgalVNSrivastavaGParaneoplastic pemphigus/paraneoplastic autoimmune multiorgan syndromeInt J Dermatol20094816219200194
- HertzbergMSSchifterMSullivanJStapletonKParaneoplastic pemphigus in two patients with B-cell non-Hodgkin’s lymphoma: significant responses to cyclophosphamide and prednisoloneAm J Hematol200063105
- HeizmannMItinPWernliMBorradoriLBargetziMJSuccessful treatment of paraneoplastic pemphigus in follicular NHL with rituximab: report of a case and review of treatment for paraneoplastic pemphigus in NHL and CLLAm J Hematol20016614214411421295
- AnanTShimizuFHatanoYOkamotoOKatagiriKFujiwaraSParaneoplastic pemphigus associated with corneal perforation and cutaneous alternariosis: a case report and review of cases treated with rituximabJ Dermatol201138108421434987
- ZhuXZhangBParaneoplastic pemphigusJ Dermatol20073450317683379
- LegerSPicardDIngen-Housz-OroSPrognostic factors of paraneoplastic pemphigusArch Dermatol20121481165117222801794