Abstract
Background
An injectable medical device containing stable hybrid cooperative complexes of high- and low-molecular-weight hyaluronic acid (HA) has been developed with characteristics suited for a global improvement of facial esthetics.
Objective
To evaluate the HA product performance in improving some key facial esthetic features. The study employed clinical scales, subjective evaluations, and facial skin objective measurements.
Methods
A single Italian site treated 64 female subjects aged 38–60 years, with injections at five predetermined points, on each side of the face, with a 4-week time lapse between the first and the second product administration. Subjects were evaluated after 4, 8, 12, and 16 weeks, using validated clinical scales, subjective evaluation, and objective quantitative outcome measures. Assessment of esthetic results included photographic documentation.
Results
Both the clinical and subjective assessments, and the majority of objective instrumental parameters indicated an improvement throughout the study and were already significant at week 4 or 8 and were still significant at week 16 (3 months after the second treatment). Minor and temporary local skin reactions were observed in 23% of subjects at the site of the injections, and the global judgment on tolerability was good or excellent, both in the investigators’ opinion and volunteers’ self-evaluation.
Conclusion
Both subjective and objective improvement of the facial parameters was consistent with the bio-remodeling purpose, and persistent and still statistically significant at the end of the study. The tolerability and safety profile of the product were judged good or excellent both by investigators and volunteers. This study supports the claim for bio-remodeling of these stable hybrid cooperative complexes of low- and high-molecular-weight HA.
Introduction
Bio-revitalization is an esthetic medicine technique for improving skin characteristics, by intra-dermal injection of hyaluronic acid (HA)-containing compounds, leading to human fibroblast modulation.Citation1
The improved understanding of the structural changes involved in face aging has shifted the focus of treatment from just concentrating on isolated problem areas to multiple facial areas, for a global revitalizing and rejuvenation effect.Citation2
It is well known that society places great deal of importance on beauty, and cultural factors inevitably influence our perception of attractiveness, but several lines of research indicate that, besides social and cultural factors, there may be biologic correlates of attractiveness.Citation3,Citation4
Evenly colored, smooth, elastic skin is viewed as attractive and healthy.Citation5 Studies have demonstrated that skin surface topography and skin coloration affect the perception of facial age, health, and attractiveness in both men and women.Citation5,Citation6
One of the primary skin aging processes, resulting in loss of skin elasticity and turgidity, consists of decreased fibroblasts activity with reduction in the biosynthesis of dermal extracellular matrix components and HA.Citation7,Citation8
HA is a polysaccharide capable of maintaining the correct moisturization of tissues and inducing optimal conditions for the proliferation of dermal cells.Citation9
The product studied (Profhilo® produced and distributed by IBSA Farmaceutici Italia Srl, Lodi, Italy) is a medical device containing a blend of high- and low-molecular-weight HA (H-HA and L-HA) packed in prefilled glass disposable syringes for local injections.
This product contains a highly purified sodium salt of HA, obtained without any chemical modification through a patented technology based on stable hybrid cooperative complexes of H-HA L-HA (NAHYCO™ technology). The concentration of HA is 3.2%, with 32 mg of H-HA and 32 mg of L-HA, in 2 mL of buffered sodium chloride physiologic solution.
The simultaneous presence in a single injection solution of different HA molecular weights (high and low) enables the integration of endogenous HA levels with balanced concentrations of stabilized HA hybrid complexes.
L-HA, which binds to specific receptors, stimulates fibroblasts and keratinocyte proliferation, providing nourishment and deep hydration to the aged skin. The technology, rheological, and biological proprieties have been described by D’Agostino et al.Citation10 On the other hand, H-HA, owing to its high capacity to bind water molecules and interact with collagen and proteoglycans, exerts a dermal scaffold action.
The aim of the study was to explore the clinical efficacy and tolerability of the product using a prospective design and, besides the standardized clinical judgment of investigatorsCitation11,Citation12 and the volunteers self-evaluation, some well-regarded quantitative outcome measures.Citation13–Citation16
Materials
The injectable medical device evaluated in this study is based on NAHYCO technology and produced following the procedure described in patent application WO2011EP65633. This product is distributed by IBSA Farmaceutici Italia Srl with the name Profhilo. The composition of each syringe, according to the International Nomenclature, was made of H-HA (1,100–1,400 kDa) and L-HA (80–100 kDa) 32 mg each in 2 mL of buffered sodium chloride physiologic solution.
The list of materials employed in the study, including those for the objective/instrumental assessments, is displayed in .
Table 1 Materials and instruments employed in the study
Subjects selection and study design
This evaluation was a monocentric, open-label, not-controlled, exploratory study, which aims to assess the global bio-remodeling effect on the facial frame of healthy female volunteers of hybrid complexes of L-HA and H-HA injected in five predetermined different areas bilaterally on the face.
The study center was the DermIng srl, a single member company, Clinical Research and Bioengineering Institute, Monza, Italy.
The study involved 64 female subjects, aged 30–60 years, who gave written informed consent for the use of their photographs in this study and for the study procedures, including the specific requests for keeping the same habits on food, exercise, make-up, cosmetics, and detergent, and avoiding ultraviolet exposure without a total block cream.
Exclusion criteria were (as per protocol): pregnancy, lactation, not being in menopause without adequate contraception or not willing to perform the pregnancy test scheduled in the protocol, performing skin treatments of esthetic correction (biomaterials implant, face lifting, Botox injection, laser, chemical peeling) in the 12 months prior to the study start, having performed permanent filler, having an anamnesis for sensitivity to the test product or its ingredients, having in the opinion of the investigator an expected lack of adhesion to study procedure, participating in a similar study within the previous 3 months, being affected with dermatological diseases of the face, as well as scars of malformations, being affected with general significant diseases (diabetes, endocrine, hepatic, renal, cardiac, pulmonary disorders, cancer, neurological or psychological diseases, inflammatory/immunosuppressive disease, drug allergy), and undergoing some pharmacological treatments (anti-inflammatory, antihistaminic, topic and systemic corticosteroids, narcotics, antidepressants, immunosuppressive drugs, and any drug able to influence results in the investigator’s opinion).
Ethical and regulatory aspects
A final version of the study protocol and appendices was submitted to the Local Ethic Committee at DermIng srl; on December 5, 2014, the study obtained the approval; an amendment to the protocol on minor study procedure modification was approved on April 30, 2015.
This study was performed in agreement with the Declaration of Helsinki. Before the screening, all subjects gave written informed consent.
Injection technique
The product, a medical device class III, is provided in prefilled syringes of 2 mL. The product was administered bilaterally in 0.2 mL bolus with the 29-G needle provided with the product injected in the deep dermis. The injections were performed in the following five predetermined points of each subject’s face, in the malar and sub-malar areas: zygomatic protuberance, nostril’s angle, inferior margin of the tragus, lip marionette lines, and mandibular angle. These points were the same described by Laurino et al.Citation17 This injection technique focuses the action of the study treatment, where the skin appears loose and with a loss of turgor and tone, concentrating a defined volume of the compound in the malar and sub-malar region. Moreover, this results in fewer injection points and less possibility of side effects. The injection treatment was performed at baseline (T0) and after 4 weeks (T4W).
Assessments
After the detailed explanation of the study procedures and the signature of the informed consent form, the subjects were checked for inclusion and exclusion criteria, and, if eligible for the study, enrolled.
After a clinical and instrumental assessment, they were treated bilaterally with the first injection treatment on the predetermined face points.
All the instrumental evaluations, at baseline and during the following visits, were carried out mono-laterally, according to a randomization list, which assigned each subject to the evaluation of the right or the left side of the face.
Four weeks after the baseline visit, clinical and instrumental assessments and the second round of bilateral injections were performed. The next assessments were scheduled at 8 weeks (T8W), 12 weeks (T12W), and 16 weeks (T16W) from the baseline. At week eight, a global tolerance evaluation of the product was carried out by the investigators and volunteers, using a five-point ordinal scale. From week 8–16, a self-assessment questionnaire on efficacy was filled by the volunteers.
Outcome measures
Efficacy
The efficacy outcome measures were both clinical and instrumental. All instrumental evaluations were carried out mono-laterally for each subject, on the right or left side, according to a randomization list.
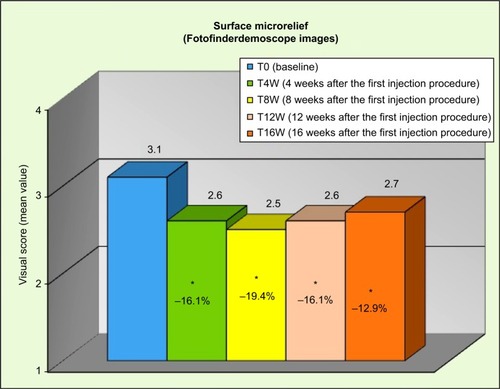
Clinical assessments employed three rating scales: The first was the Wrinkle Severity Rating Scale (WSRS),Citation10 a validated categorical five-point rating scale assessing face wrinkles, ranging from 1 (absent) to 5 (very severe). The second scale was the Facial Volume Loss Scale (FVLS),Citation11 a well-regarded categorical five-point more global scale on folds and creases, as well as volume loss in the different facial zones. The third one, Beagley and Gibson Scale, a four-point categorical one, evaluating the skin surface microrelief regularity grade, was applied by clinicians on the pictures acquired by the FotoFinderDermoscope (TeachScreen, Bad Birnbach, Germany), ranging from grade 1 (same depth of primary and secondary lines) to 4 (deep primary lines distortion and loss of secondary lines).
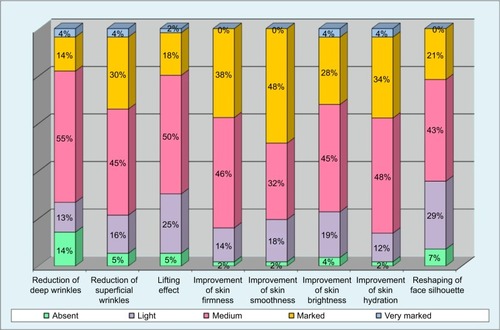
From week 8, volunteers were asked to fill a self-evaluation questionnaire, both on efficacy (superficial and deep wrinkles, skin suppleness, skin smoothness, skin brightness, skin hydration, skin lifting, face silhouette) and global tolerance (bad, poor, medium, good, excellent).
Instrumental, noninvasive evaluations employed were: optical colorimetry (Chroma meter CR-200 Minolta, Osaka, Japan), electrical capacitance of the skin (indicative of skin surface hydration) (Corneometer CM825, Courage-Khazaka, Köln, Germany), tissue dielectric constant of superficial and deep skin layers (another measure of hydration taken with the MoistureMeterD (Delfin Technologies, Kuopio, Finland), measuring skin layer up to 5 mm deep), torsiometry (measures of skin plasto-elasticity: immediate and maximum extensibility, viscoelasticity, immediate elastic recovery) (Dermal Torque Meter, Dia-Stron Ltd, Andover, UK), profilometry (replicas of nasolabial folds and marionette lines obtained with silicone rubber coupled with the Primos software elaboration), and photographic documentation (three-dimensional [3D] pictures taken with Vectra H1, Canfield, Parsippany, NJ, USA).
Moreover, face volume instrumental analysis was carried out on the 3D pictures taken at baseline, week 8, and week 16. Specifically, the Vectra analysis module overlays the images taken at two different time points and automatically calculates the volume differences through a color distance map.
Safety
The assessment of safety was based on the frequency of adverse events and the evaluation of local tolerability. Adverse events were defined in the protocol according to Good Clinical Practice and treated accordingly.
Statistical methodology
The population to be analyzed were all subjects who completed the study according to the protocol.
The statistical analysis of clinical data was to be carried out with nonparametric test while the instrumental data were planned to be analyzed with analysis of variance for repeated measure or with nonparametric tests in case of non-normality of data distribution, as proved by the Kolmogorov–Smirnov test. Alpha level was set to 0.05.
Results
A total of 64 white female volunteers, age range 38–60 years (mean 53±5.20 years), were enrolled. Slightly more than half of the volunteers (35 of 64, 55%) had had a previous esthetic treatment for the face in 2013 or the first month of 2014, mostly for a bio-revitalization purpose. The present study began on March 11, 2015, more than a year after the last esthetic treatment was performed on some of the volunteers.
After having agreed to participate in the study and signed the informed consent, the volunteers were evaluated both clinically and with the instrument devices, and received the first expected injections of HA and, the second, after 4 weeks.
Not all subjects were fully compliant with the procedures. Four volunteers withdrew due to “personal reasons” after the second microinjection session and were excluded from the analysis. Another four subjects did not come to the last visit, again due to personal reasons, but were included in the analysis, using the week 12 assessment data as the final. No major protocol violations were recorded, and there were no missing data.
Efficacy results
Results from clinical assessments were significant (P<0.05, after testing for multiplicity) from week 4 for the skin surface microrelief: −16.1% at T4W, −19.4% at T8W, 16.1% at T12W, and −12.9% at T16W (), corresponding to a reduction of the clinical score (visual evaluation on FotoFinderDermoscope acquired images) of at least 1 grade, respectively, on 52%, 58%, 55%, and 39% of included subjects compared to the baseline.
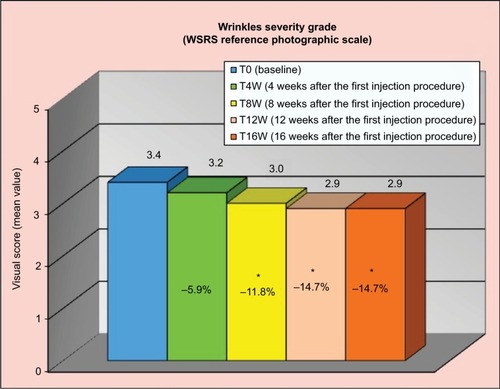
The results were also significant from week 8 for the rating in the FVLS of “cheek volume loss” and in the WSRS rating of “wrinkle severity”. FVLS showed a reduction of 21.2% at T8W, 24.2% at T12W, and 18.2% at T16W (), corresponding to a reduction of the clinical score of at least 1 grade, respectively, on 62%, 70%, and 55% of volunteers compared to the baseline. WSRS showed a decrease of 11.8% at T8W, 14.7% at T12W, and 14.7% at T16W (), corresponding to a decrease of the clinical score of at least 1 grade, respectively, on 35%, 45%, and 46% of included subjects compared to the baseline.
Figure 2 Reduction in the FVLS “cheek volume loss” throughout the study.
Abbreviation: FVLS, facial volume loss scale.
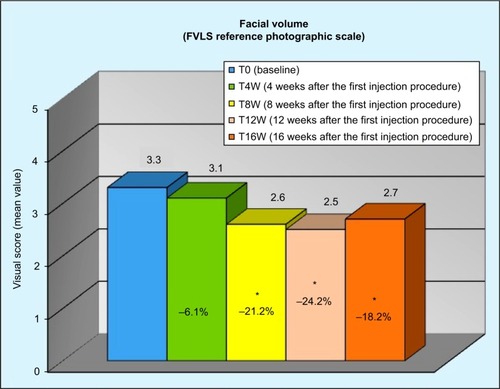
Figure 3 Reduction in the WSRS “wrinkle severity” throughout the study.
Abbreviation: WSRS, wrinkle severity rating scale.
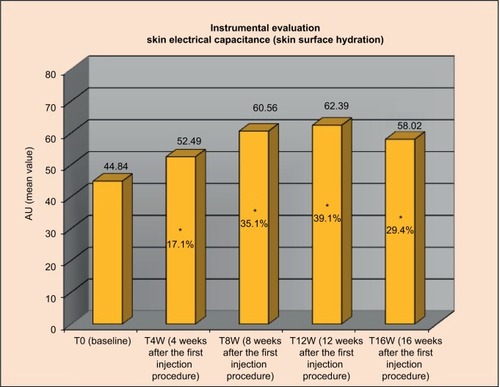
Results from instrumental assessments were statistically significant on the majority of parameters: starting from week 4 for the superficial skin hydration and from week 8 for the skin deep layers hydration; the effect size was more pronounced on the skin electrical capacitance (indicative of skin surface hydration) ().
Figure 4 Variation from baseline in the skin electrical capacitance.
Abbreviation: AU, arbitrary unit.
Regarding skin profilometry, image analysis of nasolabial folds/marionette lines skin replicas found a statistically significant reduction (P<0.05) on the following parameters: average roughness, wrinkles total high, and wrinkles’ maximum depth. The percentage of reduction from baseline at week 16 reached 9.7%, 8.2%, and 9.6%, respectively, indicating a long-lasting effect of the microinjections.
The evaluation of the principal torsiometric parameters Ue (immediate extensibility), Uf (final extensibility), Uv (viscoelasticity), and Ur (immediate elastic recovery) showed at week 16 a statistically (P<0.05) significant decrease of Uf and Uv, with a reduction of 10% and 17.1%, respectively. The improvement in the other two parameters (Ue and Ur) did not reach statistical significance.
No significant differences were observed in the optical colorimetry parameters.
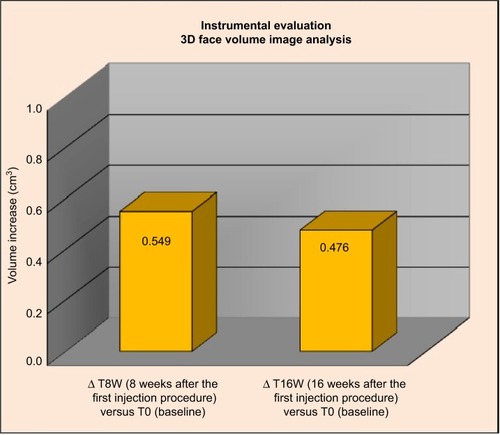
Face volume analysis highlighted an increase of volume versus T0 of 0.549 cm3 at T8W and of 0.0476 cm3 at T16W (), corresponding to an increase of at least 0.2 cm3, respectively, on the 73% and 65% of the analyzed subjects compared to the baseline.
Figure 5 Three-dimensional face volume analysis: increase of volume (cm3) T0 versus T8W and T16W versus T0.
Results of the efficacy volunteers’ self-evaluation showed an increase in positive assessments throughout the weeks, starting from week 8. Results from week 16 are displayed in , with bar plots indicating the percentages on the different items of the questionnaire.
Safety results
Regarding tolerability and safety, after the first and the second treatment, nine volunteers (14%) reported the appearance of light bruises on the injection points, while another five subjects (7.8%) reported light edema at the injection points. Both light bruises and light edema were reported to disappear between 3 and 10 days after the injections.
After the first session, one subject referred a light pinching sensation at the injection points, which were reported to disappear after 2 weeks.
Four weeks after the second treatment session, the investigators judged the product tolerance as good (30%) or excellent (70%), while the subjects’ self-assessment of tolerability was good (48%) or excellent (52%).
Discussion
The perception of facial age, health, and attractiveness has been demonstrated to be influenced by skin surface topography and appearance, and the aim of the bio-revitalization procedures is indeed to improve the global face, treating multiple facial areas.Citation1,Citation2 The technique employed in this study is performed by deep intra-dermal injection of HA; the product under investigation contains 32 mg of H-HA and 32 mg of L-HA in a 2 mL syringe. The results of this explorative prospective study, evaluating the clinical efficacy and tolerability of this class III device, clearly supports the bio-remodeling and rejuvenation claim of the hybrid cooperative complexes.
All subjective clinical outcomes and the majority of objective instrumental ones have indicated a rapid and statistically significant improvement in the face attractiveness parameters. In particular, starting 4 weeks after the first microinjection, volumetric effect and tightening effect, as measured by the Facial Volume Loss Surface score and confirmed by the 3D face volume analysis and by the Skin Surface Microrelief evaluation, were significant and maintained until the end of the study. From week 8, a filler, anti-wrinkles, plumping, and moisturizing activity become statistically significant, as measured by the reduction of WSRS score, profilometric, torsiometric, and skin electrical capacitance parameters.
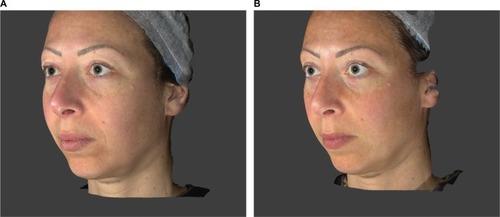
These instrumental and clinical findings are also confirmed by the photographic documentation ( and ).
Figure 7 Subject 11 (49 years old): (A) T0 Baseline and (B) T8W 8 weeks after the first injection procedure.
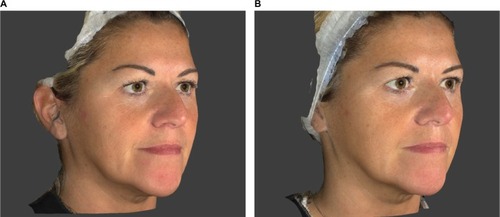
Figure 8 Subject 19 (39 years old): (A) T0 Baseline and (B) T8W 8 weeks after the first injection procedure.
A minor percentage of volunteers (23%) showed mild and temporary local reactions (light bruises and light edema) to the procedures, expected events imputable to the injections, and “nonspecific skin reactivity” in subjects unknown to have allergy and/or cosmetic intolerances. The final product tolerance was judged good or excellent in all subjects, both by the investigators and the volunteers.
The points of strength for the present study were the rapid and persistent statistical significant effect in all the subjective assessments and majority of the objective ones, indicating full face effects, consistent with the claim of bio-remodeling effect.
The main limitation of the study was the not-controlled nature of the trial, but this fact could be compensated by the presence of objective instrumental efficacy outcome measures and consistency of results across all assessments.
Disclosure
The study was supported by IBSA Farmaceutici Italia srl, the company that distributes the product used in the trial. The authors report no other conflicts of interest in this work.
References
- AvantaggiatoAGirardiAPalmieriAPascaliMCarinciFComparison of bio-revitalizing injective products: a study on skin fibroblast culturesRejuvenation Res201518327027625640228
- WeinkleSFacial assessments: identifying the suitable pathway to facial rejuvenationJ Eur Acad Dermatol Venereol200620Suppl 171116643418
- ChatterjeeAThomasASmithSEAguirreGKThe neural response to facial attractivenessNeuropsychology200923213514319254086
- LangloisJHKalakanisLRubensteinAJLarsonAHallamMSmootMMaxims or myths of beauty? A meta-analytic and theoretical reviewPsychol Bull2000126339042310825783
- FinkBGrammerKMattsPJVisual skin color distribution plays a role in the perception of age, attractiveness, and health of female facesEvol Hum Behav200627433442
- FinkBMattsPJD’EmilianoDBunseLWeegeBRöderSColour homogeneity and visual perception of age, health and attractiveness of male facial skinJ Eur Acad Dermatol Venereol201226121486149222044626
- ZimblerMSKokoskaMSThomasJRAnatomy and pathophysiology of facial agingFacial Plast Surg Clin North Am20019217918711457684
- GhersetichILottiTCampanileGGrapponeCDiniGHyaluronic acid in cutaneous intrinsic agingInt J Dermatol19943321191228157393
- RedbordKPBussoMHankeCWSoft-tissue augmentation with hyaluronic acid and calcium hydroxyl apatite fillersDermatol Ther2011241718121276160
- D’AgostinoAStevellatoABusicoTIn vitro analysis of the effects on wound healing of high- and low-molecular weight chains of hyaluronan and their hybrid H-HA/L-HA complexesBMC Cell Biol20151611926163378
- DayDJLittlerCMSwiftRWGottliebSThe wrinkle severity rating scale: a validation studyAm J Clin Dermatol200451495214979743
- AscherBColemanSAlsterTFull scope of effect of facial lipoatrophy: a framework of disease understandingDermatol Surg20063281058106916918569
- GroveGLGroveMJLeydenJJOptical Profilometry: an objective method for quantification of facial wrinklesJ Am Acad Dermatol1989213 Pt 26316372778127
- FullertonAFischerTLahtiAWilhelmKPTakiwakiHSerupJGuidelines for measurement of skin colour and erythema. A report from the Standardization Group of the European Society of Contact DermatitisContact Dermatitis19963511108896947
- SalterDCMcArthurHCCrosseJEDickensADSkin mechanics measured in vivo using torsion: a new and accurate model more sensitive to age, sex and moisturizing treatmentInt J Cosmet Sci199315520021819272125
- BloemenMCvan GervenMSvan der WalMBVerhaegenPDMiddelkoopEAn objective device for measuring surface roughness of skin and scarsJ Am Acad Dermatol201164470671521216493
- LaurinoCPalmieriBCoacciAEfficacy, safety, and tolerance of a new injection technique for high- and low-molecular-weight hyaluronic acid hybrid complexesEplasty2015E46427437