Abstract
Morgellons disease (MD) is a skin condition characterized by the presence of multicolored filaments that lie under, are embedded in, or project from skin. Although the condition may have a longer history, disease matching the above description was first reported in the US in 2002. Since that time, the condition that we know as MD has become a polemic topic. Because individuals afflicted with the disease may have crawling or stinging sensations and sometimes believe they have an insect or parasite infestation, most medical practitioners consider MD a purely delusional disorder. Clinical studies supporting the hypothesis that MD is exclusively delusional in origin have considerable methodological flaws and often neglect the fact that mental disorders can result from underlying somatic illness. In contrast, rigorous experimental investigations show that this skin affliction results from a physiological response to the presence of an infectious agent. Recent studies from that point of view show an association between MD and spirochetal infection in humans, cattle, and dogs. These investigations have determined that the cutaneous filaments are not implanted textile fibers, but are composed of the cellular proteins keratin and collagen and result from overproduction of these filaments in response to spirochetal infection. Further studies of the genetics, pathogenesis, and treatment of MD are warranted.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Morgellons disease (MD) is a disfiguring and perplexing skin condition associated with spirochetal infection and tick-borne illness.Citation1–Citation7 This poorly understood condition has a worldwide distribution, with estimated self-reported cases numbering over 14,000 in 2009.Citation5 Since that time, there has been an increasing number of individuals reported to be afflicted with this disorder (C Casey, Charles E Holman Morgellons Disease Foundation, personal communication, 2017). The distinguishing diagnostic feature of MD is spontaneously appearing ulcerative skin lesions that contain unusual filaments lying under, embedded in, or projecting from the skin. The characteristic filaments are microscopic, visually resembling textile fibers, and are white, black, or a more vibrant color, such as red or blue.Citation1–Citation7 In addition to fiber production, some patients may experience formication, described as stinging, biting, creeping and crawling sensations. The symptoms of MD are not limited to the skin. MD patients experience a variety of systemic manifestations, such as fatigue, joint pain, cardiac complications, cognitive difficulties, and neuropathy, all symptoms that are commonly reported by Lyme disease (LD) patients.Citation1–Citation7
History
The name “Morgellons” (pronounced with either a hard or soft “g”) comes from a letter written in 1674 by Sir Thomas Browne, an English physician. The letter contains a brief description of a skin disease in French children:
Hairs which have most amused me have not been in the face or head, but on the back, and not in men but children, as I long ago observed in that endemial distemper of little children in Languedock, called the Morgellons, wherein they critically break out with harsh hairs on their backs, which takes off the unquiet symptoms of the disease, and delivers them from coughs and convulsions.Citation8
The accounts by Browne and others were likely referring to a heterogeneous group of skin conditions that may have differed from the skin condition that we refer to as MD today. These early accounts describe primarily childhood illnesses, many of which were associated with convulsions. There is mention of hairs, worms (with black protruding heads), or comedones that protruded from the skin, primarily on the arms, legs, and back, and at that time there was much debate as to whether these objects were animate or inanimate.Citation8 Ettmüller, for example, provided a drawing of infesting organisms that look like various arthropods, some resembling scabies mites, while the famous Dutch microscopist Leuvenhoeck concluded that such objects were inanimate.Citation8 In 1894, Thibierge described patients who had erroneous and unshakeable beliefs of skin infestation by parasites, and proposed the name “acarophobia”.Citation9,Citation10 In 1946, Wilson and Miller suggested that “acarophobia” should be replaced by the name “delusions of parasitosis” (DOP).Citation10,Citation11
From 1902 to 1938, case studies describing “parasitophobias” or “dermatological hypochondriasis” that resulted in delusional interpretation of skin sensations were published sporadically.Citation12–Citation20 However, as early as 1935, an association between spirochetal infection and DOP was documented by the French physician Vié, who reported that six of eight of the subjects in his case studies had syphilis.Citation18 In 1938, a pivotal narrative of DOP was published by Ekbom, a series of case studies describing patients who had sensations of movement and the belief that insects were crawling on or under skin. Ekbom felt that determining the underlying cause of the formication was important, stating that “it is the underlying illness that determines the overall presentation of the beliefs” and “it is perhaps too simple that the parasitophobias should be considered as mental illness and nothing more”.Citation20 Interestingly, like Vié, Ekbom found that spirochetal infection was present in his patient cohort, and three of Ekbom’s seven patients had documented cases of syphilis. Despite the fact that syphilis was considered rare in Sweden, Ekbom did not believe that spirochetal infection was a contributing factor.Citation20
Ekbom reported that the skin sensations consisted mostly of itching, but also that there was a feeling that something was crawling on or under the skin, and that stabbing and biting sensations could also occur. He mentioned that in such cases, “little animal” specimens were sometimes brought in by patients to show to physicians and that such collections consisted of “little hairs, little threads, grains of sand, and skin scales”. He noted that apart from delusional ideas of infestation, no consistent mental problems were present.Citation20 Although Ekbom could not find any arthropods, parasites, or other microscopic animals, it is important to note that he found hairs, “little threads”, and “grains of sand” in patient specimens. His description is consistent with the findings of unusual hairs, fibers, and hardened comedo-like dermatological objects that we see in MD specimens.Citation20 Such objects will be discussed in depth later in this report.
It is possible that patients in the case studies written by other physicians and mentioned by Ekbom had syphilis or other spirochetal infections. The causative agent of syphilis was first reported in 1905 by Fritz Schaudinn and Erich Hoffmann, who used dark-field microscopy and described spiral-shaped bacteria – Spirochaeta pallida – now called Treponema pallidum.Citation21 The first test for syphilis was developed shortly afterward in 1906 by German physician and bacteriologist August von Wassermann. The Wassermann test was a complement-fixation test that detected antibodies reactive to the syphilis spirochete. The Wassermann tests performed in the 1920s and 1930s lacked accuracy,Citation22,Citation23 and cases of syphilis among patients with delusional parasitosis (DP) may have gone unacknowledged as a result.
Regardless of the test accuracy for syphilis, it is possible that some of the patients described in these historical case studies may have been infected with Borrelia spp., other treponemes or Leptospira spp. B. burgdorferi (Bb) is not a new organism: the earliest known case dates back 5,300 years in the mummy dubbed Ötzi,Citation24 and Borrelia DNA was also detected in two museum specimens of the white-footed mouse, Peromyscus leucopus, collected in 1894.Citation25 Spirochetes resembling Borrelia have also been found in amber-fossilized ticks from 15–20 million years ago.Citation26 Therefore, spirochetal infections associated with MD may have occurred periodically hundreds or even thousands of years ago in human history, yet have gone unrecognized and unreported.
There is a brief mention of “the Morgellons” by Emslie-Smith in 1946, where he proposes that the condition was a form of myiasis caused by the larva of a Hypoderma species, although his account did not provide convincing evidence to support his theory.Citation27 In a 1983 lecture, Lyell described a survey of several hundred dermatologists treating patients with DOP who reported that many of their patients exhibited specimens in matchboxes, baggies, scraps of paper, or photographs. Lyell labeled this practice the “matchbox sign”.Citation28 The survey was reported in a short editorial in the Lancet,Citation10 after which the “matchbox sign” was adopted by dermatologists as being proof of delusional mental illness.Citation29–Citation31 Likewise, the manipulation of skin to extract specimens for relief was also considered to be proof of having a delusional disorder, and this practice was labeled “the tweezer sign”.Citation29
After Emslie-Smith’s mention of MD in 1946, there were no significant references to MD in medical literature until 2002. In 2001, biologist Mary Leitao noted nonhealing lesions on her young son, who complained that he had “bugs” under his skin. She removed a scab, and upon magnification she did not see arthropods or parasites, but she did see embedded blue and red filaments. Leitao searched the Internet looking for similar conditions, and Browne’s description bore a resemblance to her son’s condition, so she appropriated the name.Citation1,Citation2 Leitao subsequently founded the not-for-profit Morgellons Research Foundation (MRF). The MRF website included a database where those with the disorder could self-report their skin and systemic symptoms.Citation5
Leitao did not get answers from the mainstream medical establishment. She had sought help from many doctors, including Fred Heldrich, a Johns Hopkins pediatrician, who arrived at the conclusion that Leitao should not use her son to “explore the problem” and that she could benefit from a psychiatric evaluation.Citation32 Leitao gathered a group of patient advocates, medical practitioners, physicians, and nurses into a volunteer board of directors, which included Georgia-based pediatrician Greg Smith, Texas-based nurse practitioner Virginia Savely, patient advocates Charles E Holman and Cindy Casey-Holman, and former National Aeronautics and Space Administration (NASA) physician and researcher William HarveyCitation32 (C Casey, Charles E Holman Morgellons Disease Foundation, personal communication 2017). Leitao also sought help from Randy Wymore, a pharmacology professor at Oklahoma State University.Citation32
In 2006, Dan Rutz, a spokesman for the US Centers for Disease Control and Prevention (CDC) contacted Leitao and said that the CDC would form a task force to investigate MD, declaring that “these people deserve more than to be blown off”.Citation32 The CDC published their study results in 2012, declaring that MD was “similar to more commonly recognized conditions, such as delusional infestation [DI]”.Citation33 As of 2012, Leitao had withdrawn from the public eye and closed the MRF. The website run by the MRF is no longer active, and the domain name was taken over by others, now promoting fringe etiologic theories of MD.
Diagnosing MD
In light of previous studies of MD,Citation1–Citation7 a case definition for MD is proposed: a somatic LD-like illness associated with spontaneously appearing, slowly healing, filamentous, ulcerative skin lesions, with the key diagnostic criterion being colored, white, or black filaments protruding from or embedded in skin. Patients diagnosed with MD, either by self-diagnosis or by a health care practitioner, are not a homogeneous group, thus highlighting the need for a universally accepted case definition.
Filaments in MD lesions usually require magnification of 50× or more to be seen, and at that magnification they can be mistaken for textile fibers. Health care providers need to be objective when viewing these fibers: a patient must have unusual filaments visible under 50× magnification or higher (as opposed to the magnification of 10× normally used in dermatology) and embedded in or extruding from skin to be diagnosed with MD. The filaments are relatively easy to see with proper visualization tools, and detectable fibers should not be automatically dismissed as “self-implanted” or composed of synthetic substances without an appropriate evaluation. Mental health status is not a diagnostic factor in MD cases, as outlined herein.
Controversy
Unlike Ekbom, who was concerned about the underlying cause of DP,Citation8 many modern-day practitioners and scientists have ignored the potential underlying causes responsible for formication and beliefs of infestation. It is easier to declare mental illness the exclusive etiologic cause, thus blaming the patient, when confronted with perplexing symptoms that the practitioner cannot explain. However, it is irresponsible to label a patient delusional without an appropriate psychiatric evaluation, and if mental illness is present a physician should bear in mind that an underlying infectious process can cause a pathological response resulting in mental illness.
A PubMed search using the keyword “Morgellons” yielded 58 articles, the earliest dating from 1946. From 2006 to present, medical literature is divided into two polarized points of view. One point of view is that MD is a form of delusional mental illness, and the other is that underlying spirochetal infection causes a filamentous dermopathy that is accompanied by an array of LD-like multisystem symptoms that may or may not include neuropsychiatric symptoms. There are approximately 40 papers in the medical literature proposing that MD is purely a delusional disorder, and only a quarter of that figure proposing that MD has an infectious etiology.
Diagnosing delusional disorder
The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM)-V makes no mention of a diagnosis of DOP. The closest diagnosis is Delusional disorder 297.1 (F22), somatic type, which is defined thus:
presence of one or more delusions with a duration of one month or longer
criteria for schizophrenia have never been met (note hallucinations if present are not prominent and are related to the delusional theme eg, the sensation of being infected with insects is associated with delusions of infestation)
apart from the impact of the delusion(s) or its ramifications, functioning is not markedly impaired, and behavior is not obviously bizarre or odd
if manic or major depressive episodes have occurred, these have been brief relative to the duration of the delusional periods
the disturbance is not better explained by another mental disorder, such as obsessive compulsive disorder, and is not due to the physiological effects of a substance or medication or another medical condition.Citation34
The second step involves determining if characteristics associated with delusions, such as confusion, agitation, perceptual disturbances, physical symptoms, and mood abnormalities are present.Citation35 The third step is performing a systematic differential diagnosis, including a thorough history, mental status examination, and laboratory/radiological evaluation to rule out other medical and psychiatric conditions that present with delusions.Citation35 The status examination, including cognitive status, is usually normal, except for the delusional beliefs: memory and cognition are intact.Citation37 Auditory or visual hallucinations are indicative of more severe psychotic disorders, such as schizophrenia, and suggest exclusion of a delusional disorder.Citation37 The differential diagnosis should exclude medical conditions that can cause delusions, such as neurodegenerative or other central nervous system disorders, vascular diseases, vitamin deficiencies, medications, metabolic and endocrine disorders, toxins, and infectious diseases.Citation37 Note that spirochetal infections such as syphilis and LD could cause symptoms that fall into this category.
Evidence supporting the hypothesis of DP
Among reports that promote a delusional etiology for MD, there are a number of review articles, opinion pieces, or editorial letters.Citation29,Citation30,Citation38–Citation53 These do not provide any new research or clinical evidence to support the claims that MD is a delusional disorder, but they do present common discussion themes that are frequently reiterated in case studies and research papers.Citation54–Citation76 Common discussion themes are:
MD is a delusional disorderCitation29,Citation30,Citation38–Citation76
MD is a variation of DOP, DP, or DICitation29,Citation30,Citation38–Citation76
MD is defined as the fixed, unshakable belief, despite lack of medical evidence, of being infested with microscopic organisms or inanimate objectsCitation29,Citation30,Citation38–Citation76
the presentation of specimens in or out of a container, whether it be a matchbox, small plastic bag, paper, pill container, or photographic image, etc, is diagnostic of DOP, DP, or DICitation29–Citation31,Citation52,Citation55,Citation59–Citation62,Citation64,Citation66,Citation70,Citation71
patients with MD tend to have psychiatric comorbiditiesCitation74,Citation68
MD is a mass delusional mental illness afflicting primarily middle-aged Caucasian femalesCitation68,Citation69,Citation74,Citation76
delusions of infestation are spread from person to person and transmitted by the InternetCitation30,Citation39,Citation41,Citation51,Citation52,Citation54,Citation58,Citation60
antipsychotic drugs are the treatment of choice for MDCitation29,Citation30,Citation38–Citation62,Citation64–Citation76
electroconvulsive therapy is an acceptable treatment for MDCitation30,Citation52
establishing rapport to gain confidence and trust helps convince patients to take antipsychotic drugsCitation30,Citation39,Citation41,Citation49,Citation54,Citation58,Citation60
using the word “Morgellons” in dialogue with patients can help establish rapport and trustCitation30,Citation39,Citation41,Citation54,Citation58,Citation60
it is acceptable for dermatologists to diagnose delusional disorder and prescribe antipsychotic medicationCitation51,Citation56
use of deceptive dialogue and strategies aimed at convincing patients to take antipsychotic drugs is a justifiable practiceCitation41,Citation50,Citation54,Citation60,Citation65
if a patient’s friend(s) or family member(s) also observe a subject’s dermatological lesions and believe the evidence, then they too are considered to share the delusional belief, and the delusion is considered to have been transmitted from one individual to another;Citation30,Citation51,Citation54,Citation68 the belief shared by two people that there are organisms present in the skin is called folie à deux (madness of two); folie à trois, folie à quatre, or folie à cinq are shared beliefs by three, four, or five people, respectively; and shared belief in a family is folie à famille.Citation30,Citation51,Citation54,Citation68
A PubMed search using the keyword “Morgellons” identified 18 publications consisting of case studies of between one and six patients. provides a summary of the case studies. Most of these patients clearly do not meet the case definition of MD. Case studies provide useful anecdotal evidence, but they have limitations. Of these case studies, the majority do not mention the observation of fibers being present in or projecting from the skin (the key defining criterion for MD), nor do they mention whether the attending health care professional looked for filaments in the skin at magnification of 50× or higher.Citation54–Citation56,Citation59–Citation68,Citation70,Citation73
Table 1 Case studies claiming delusional etiology of Morgellons disease
Some studies reported that patients presented specimens to the health care provider as evidence – fibers, lint, hair and skin scrapings, etc – and this was interpreted as being diagnostic of DI.Citation55,Citation59–Citation62,Citation64,Citation66,Citation70,Citation71 In many of these case studies, there was no mention of any analysis of patient-supplied specimens to determine composition.Citation54,Citation59,Citation60 In a few cases, the health care provider did nothing more than a gross visual identification of patient-provided specimens.Citation55,Citation62,Citation66,Citation70,Citation71 Some studies did not mention fibers associated with their cases at all.Citation55,Citation58,Citation60 Only seven of these studies indicated that the subjects had heard of MD.Citation54,Citation56,Citation58,Citation61–Citation63,Citation70
In some case studies, a patient was diagnosed with DP or DI on very little evidence. Bhandary et al diagnosed DP in patients who felt crawling sensations and thus thought they had bugs in their ears or nose.Citation55 Such conditions as seborrheic dermatitis or eczema can cause formication and crawling sensations inside the ears and nose, and should be ruled out before diagnosing mental illness. Sandhu and Steele diagnosed a patient with DI because the patient felt as though she had fibers growing into her eye. The patient had ectropion, and perhaps this was a factor contributing to the uncomfortable sensations.Citation73 In these cases, underlying causes for sensations were not thoroughly investigated before assuming the patient was having sensory hallucinations. Furthermore, given the sensations of formication, the beliefs in bugs in the first cases and of fibers in the second case are not unreasonable or inappropriate.
In cases where a health care professional did not look for filaments, it is unclear whether or not patients with MD were in these studies. Some studies did not mention if the patient had lesions.Citation54 Some reports mentioned lesions or skin abnormalities, but did not describe examination with magnification of 50× or higher for fibers in or projecting from skin.Citation56,Citation58–Citation60,Citation62–Citation67,Citation70,Citation73 Other studies mentioned that the skin was completely normal and that no skin abnormalities were present; therefore, it is very unlikely that patients in these studies actually had MD, as they did not have the diagnostic clinical finding.Citation55,Citation61,Citation65,Citation71,Citation75
There are only three case studies that specifically mention the presence of fibers either projecting from or embedded in skin.Citation57,Citation72,Citation75 Roncati et alCitation72 reported “grayish spots” under skin, then used scanning electron microscopy (SEM) and energy-dispersive spectroscopy (EDS) to study the spots. SEM showed that the spots were associated with fibers described as “synthetic wire” consistent with samples from a washing machine, as well as keratin fibers consistent with hair from the patient’s dog. The EDS analysis detected carbon, sulfur, and oxygen peaks – elements for keratin – but EDS could not reveal what type of keratin it was. Therefore, the “synthetic wire” could have been keratin filaments from the patient. The authors concluded that the keratin hairs were of canine origin, based on morphological resemblance to the patient’s dog hair, yet they provided no further proof of this conclusion. As some MD fibers are small hairs, determining whether the hairs are human or canine in origin is important. SEM shows only the outer shape of a specimen, so the scaling pattern is the only morphological feature available for comparison, and the imbricate scaling of canine and human hair is quite similar (TA Evans, TRI Princeton, personal communication, September 13, 2017). EDS analysis can only tell the chemical composition of the specimen and again, human and canine hair would be similar.Citation77
Ohn et al saw only one black fiber protruding from the skin, and then claimed that this single fiber was lost during processing for histological examination.Citation75 Therefore, one cannot draw any conclusions about the composition or origin of this fiber. Dovigi provided convincing evidence that the fibers extracted from an oral lesion on the mucosal distal tuberosity of a tooth were synthetic carbon-based fibers, but there is no evidence that the fibers were self-implanted.Citation57 Dental floss is composed of synthetic fibers, such as nylon.Citation78 Fibers could have been introduced during flossing, especially if the floss had frayed and become lodged between teeth, eventually festering and causing a lesion. Belief that the fibers had originated in the tissue would be reasonable under those circumstances.
The case studies varied in terms of looking for pathogens, but on further examination all of the studies fall short in looking for spirochetal infection. Only two of the case studies looked for Borrelia infection, and neither of these performed a thorough laboratory analysis to search for spirochetes.Citation72,Citation75 The description of testing for Borrelia in these studies is not sufficiently detailed to know what was actually done. In science, reproducibility is important, and methodologies should provide enough details that others can repeat them. Roncati et al stated: “In adjunct, the patient had noticed an increase in the viscosity of mucus, saliva, and tears, as to produce four unexplainable corneal ulcers in the last two years, without a rise in the autoimmunity or Borrelia spp. serology”.Citation72 There is no mention of whether or not the Borrelia spp. serology was interpreted as being positive or negative, what species or type of Borrelia antigens were used, what laboratory the serology was performed at, or what method was used to detect the antibodies. Furthermore, although the patient had a few gray spots containing fibers, there was no evidence they were self-implanted or related to a delusional belief. Therefore, the study cannot disprove an infectious etiology for MD.
The study by Ohn et al is no better in terms of providing methodology.Citation75 In fact, the methodology in the abstract does not match the text. While the abstract states that polymerase chain reaction (PCR) testing for Bb was negative in serum, the text states that Bb serology was negative. It is thus unclear whether serology or PCR testing was used to diagnose Bb.Citation75 There is mention that a Warthin–Starry stain was performed, and histological examination revealed only a mild lymphocytic infiltration. However, because Bb is pleomorphic, such stains can be difficult to interpret, and mild lymphocytic infiltration is precisely what one would expect in Bb skin lesions, such as erythema migrans (EM) rash sites.Citation79 Studies that do mention the search for and identification of pathogens other than Borrelia spp. are sadly lacking. The methodology in the study by Altunay et al mentioned “laboratory investigations including routine biochemical analyses of the blood and urine, cutaneous biopsy, the microscobic [sic] analysis of so-called parasites or materials emerging from the skin”.Citation66 DeBonis and Pierre described their microbiological methodology as “Evaluation by a primary care physician revealed no signs of infection”.Citation61
Many studies included cases that did not meet the DSM-V criterion for delusional disorder. Some studies featured patients who were clearly delusional and had or likely had serious underlying psychiatric illnesses, such as schizophrenia.Citation62,Citation66 Some described conditions that clearly indicated the patient had disease affecting the central nervous system: Roncati et al indicated the patient in their case study had “myoclonies” [sic], which presumably means seizures or muscle twitches; one patient in the study by Fellner et al had senile dementia; and the patient in the report of Freudenreich et al had HIV infection.Citation58,Citation67,Citation72 Some studies included cases where so-called delusional disorders could have had cultural influences.Citation55,Citation66 Some studies mentioned underlying medical conditions that may have caused psychiatric disturbance. In the study by Altunay et al, five patients had vitamin B12 deficiency and one had thyroid disease in addition to vitamin B12 deficiency.Citation66 In the study by Reid and Lio, one patient had diabetes mellitus.Citation60 Patients in other studies were using psychoactive drugs.Citation56,Citation61,Citation62,Citation67,Citation70 The patient in the case study by Roncati et al had hepatitis C virus infection.Citation72
A few case studies claimed that treatment with antipsychotic medication was curative.Citation55,Citation59,Citation60,Citation66 In contrast, many more case studies indicated that treatment with antipsychotic drugs reduced symptoms but was not curativeCitation55,Citation58,Citation61,Citation66,Citation70,Citation71 or that antipsychotics were ineffective.Citation62,Citation63,Citation65 In fact, Robles et al suggested that treatment with antibiotics was more successful than treatment with antipsychotics, although they too concluded that MD was delusional rather than infectious. They reported that treatment of two patients with doxycycline and no antipsychotics resulted in complete resolution of the condition, while one subject treated with antipsychotics and no antibiotics did not have disease resolution.Citation65 Some studies indicated that antipsychotic drugs were prescribed, but failed to report if treatment was effective,Citation54,Citation55,Citation64,Citation75 and Roncati et al failed to report what (if any) treatment was prescribed.Citation72 Some of the studies reporting cure or benefit with antipsychotic drugs used other treatment methods in addition to the antipsychotic medication, and without controls one cannot be sure which variable was responsible for the patient’s improvement.Citation55,Citation62,Citation66,Citation67,Citation73
There were only five reports of studies involving larger cohorts of patients.Citation33,Citation68,Citation69,Citation74,Citation76 Four of these were retrospective studies,Citation68,Citation69,Citation74,Citation76 and the remaining study selected its cohort by conducting a retrospective search through medical records.Citation33 Retrospective studies are limited, because data may be incomplete and cases may lack laboratory analysis or proper documentation. The retrospective study by Mohandas et alCitation74 included 35 patients and made no mention of the presence of fibers. The study reported female predominance, with a mean age of 54.6 years. Psychiatric comorbidities (anxiety and depression) were noted in 68.5%, and management of patients included treatment with psychotropic medications combined with topical and oral antibiotics. Improvement was reported in less than half of the cohort (40%), and all four patients who received low-dose oral antibiotic therapy noted improvement.Citation74 The study by Hylwa et al from the Mayo Clinic was a retrospective study of psychiatric comorbidity in a cohort of 54 patients diagnosed with DI. Comorbidities were found in 74% of these patients, and there was no mention of fiber or pathogen detection in the retrospective report.Citation69
Foster et al also conducted a retrospective study of DI at the Mayo Clinic.Citation68 Medical records of 147 patients were reviewed to determine demographic information, historical and physical findings, and treatment. In this cohort, 81% had a history of one or more psychiatric illnesses (the most common diagnosis being depression), 11% had a history of drug use (methamphetamines, cocaine, heroin, marijuana, and other street drugs) that may have contributed to their symptoms, and only 20% of subjects reported having fibers in their skin, and thus the cohort was composed predominantly of non-MD subjects. The study lacked fiber analysis, and there was no mention that any skin-associated fibers had been visualized by the investigators, so it is possible that there were no subjects in the study meeting the key diagnostic MD criterion. Methodology for detecting any pathogens was lacking, and there was no mention of detection of Borrelia or other tick-borne pathogens at all. Nevertheless, the authors stated that they did not find evidence of infestation in patient-provided specimens, biopsies, or tests for ova and parasites.Citation68
The CDC–Kaiser Permanente Northern California–Armed Forces Institute of Pathology collaborative study (CDC study) selected their cohort via a retrospective search through medical records.Citation33 This study had significant flaws. The case definition did not require the presence of fibers embedded in or projecting from skin; therefore, selection was on the basis of self-reported cases, and resulted in a heterogeneous group of subjects. Eligibility to participate in the study was limited to those enrolled in a Kaiser Permanente plan. The number of participants diminished as the study progressed: whereas 467 subjects were identified by a search of Kaiser Permanente electronic records, cultures for pathogens were conducted on only 28 subjects, and fibers were collected from only 12 subjects.Citation33 Fiber analysis was performed and cotton-textile fibers identified, but the authors admitted they did not find fibers that were embedded or projecting from skin, and they admitted that they may have introduced cotton fibers at the time of sampling. Two of the subjects identified in the electronic search died, and the cause of death was not disclosed.
Objective findings of illness that could have accounted for the symptoms were ignored: cognitive impairment, somatic complaints, neuropsychiatric symptoms, multiorgan symptoms, and the presence of inflammatory markers.Citation33,Citation80 Cases with these findings do not meet the DSM-V criteria for delusional disorder. Most importantly, although the authors acknowledged that the literature had suggested an association between MD and LD, they did not perform any specific detection methods, such as Borrelia culture or immunohistochemical staining, for any Borrelia spp., and the search for LD was limited to insensitive serologic testing. In conclusion, they used a flawed case definition, selected the wrong cohort, selected the wrong specimens, performed the wrong tests, and came to incorrect conclusions with respect to MD and the association with LD.Citation33,Citation80
Evidence of an infectious etiology
Early history
In modern times, spirochetal infection was implicated as an etiologic factor for MD as early as 2006, when William Harvey, a former medical director of a laboratory contracted to work for NASA, explained to a medical reporter, Chico Harlan with the Pittsburgh Post-Gazette, that he had been studying a group of 70 MD patients, all of whom were infected with Bb, the causative agent of LD.Citation32 Savely et al in 2006 reported that the principal author, nurse practitioner Virginia Savely, had seen 80 patients in her practice who fit the criteria for MD, and all but one of these patients had tested positive for LD.Citation2
A subsequent study by Harvey et al attempted to delineate MD characteristics in a cohort of 25 self-diagnosed MD patients.Citation81 Although these patients apparently met the case definition for DP, the authors felt the cause and effect of the symptoms were reversed from those of DP, and they suggested that an infectious process was responsible for the development of symptoms. They reported that the male:female ratio was approximately equal, that 23 of 25 subjects had prior psychiatric diagnoses, around 50% had sensations of movement, 70% had excoriations or lesions, and that fibers were present in about a third of patients.Citation81 Central nervous system symptoms, cardiac symptoms, endocrine dysfunction (hyperparathyroidism, adrenocortical hypofunction, Hashimoto’s thyroiditis, hypercalcemia, elevated fasting insulin levels, and parathyroid adenomas), a high rate of autoimmune disease, and low core body temperature were commonly encountered in their cohort. Laboratory evidence of abnormalities that were commonly encountered included anemia, leukopenia, high monocyte count, low natural-killer cells, elevated serum calcium, elevated globulin levels, and elevated inflammatory markers (CRP, TNFα, IFNγ). Skin abnormalities included excoriations, angiomas, and filament/granule production. The need for a credible MD case definition was emphasized.Citation81
Savely and Stricker analyzed clinical findings in a cohort of 122 subjects with documented presence of unusual filaments projecting from or embedded in skin.Citation4 The key objective of this study was to develop a credible case definition for MD, and because cutaneous fibers were the unique objective finding, the presence of such fibers was determined to be an obligatory part of the case definition. The link between MD and LD was explored, and the study reported that 96.8% of subjects had either positive LD tests by Western blot or clinical diagnoses of LD; many had positive tests for coinfecting tick-borne illnesses, and the demographics of the LD patients and MD patients in their practices proved to be similar. Other important findings in the cohort group were female predominance and hypothyroidism.Citation4
Middelveen and Stricker provided evidence of spirochetal involvement in the evolution of MD.Citation6 MD was compared to bovine digital dermatitis (BDD), a disease in cattle caused by various species of treponemes. Several similarities between MD and BDD were noted, including unusual filament formation, female predominance, rapid spread, exposure to unsanitary conditions or humid environments, and positive response to antibiotics. The fact that spirochetes caused unusual filament formation in cattle suggested that a similar mechanism might occur in MD patients. The fact that spirochetes were visible in BDD histological sections suggested that spirochetes might be present in MD tissue as well.Citation6
Fiber analysis
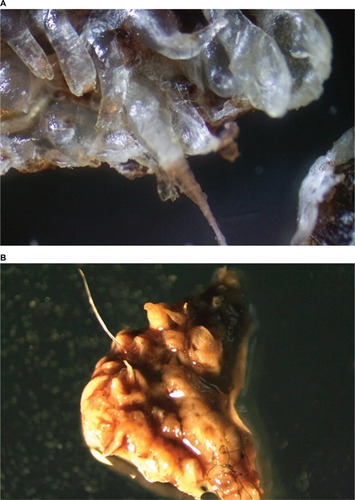
Histological studies have shown that filaments in MD tissue are not textile fibers, but are biofilaments produced by human epithelial cells and stemming from deeper epidermal layers, upper dermal layers, and the root sheath of hair follicles ().Citation7,Citation82,Citation83 MD cutaneous filaments are predominantly composed of keratin and collagen, as determined by histological studies, and appear to be produced by activated keratinocytes and fibroblasts.Citation82,Citation83 The base of filament attachment to epithelial cells demonstrates nucleation that is continuous with that of surrounding epithelial cells, indicating that the filaments are of human cellular origin ().Citation83 Histochemical staining of skin sections containing embedded filaments with Congo red resulted in apple-green birefringence suggestive of an amyloid component, although this remains to be confirmed using more specific methodologies.Citation7 Calcofluor-white staining of skin sections with embedded filaments was negative, and thus MD filaments do not have any cellulose content from plant fibers, such as cotton, or chitin from fungal cells or insect exoskeletons.Citation7
Figure 1 Embedded cutaneous blue and white filaments.
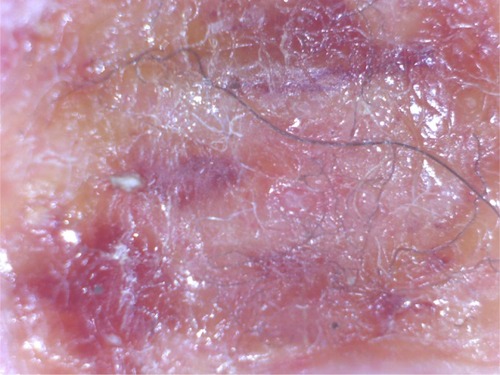
Figure 2 Longitudinal sections of filaments originating in the basal layer of the epidermis adjacent to the dermis; magnification 400×.
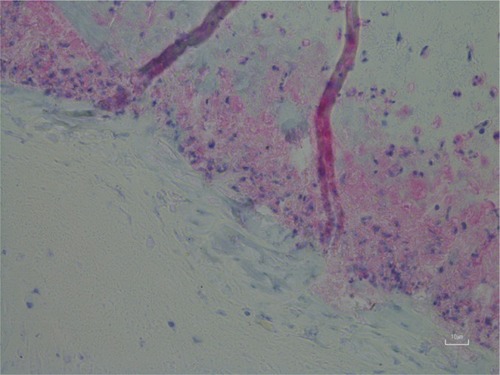
Several independent studies have shown that blue MD fibers were human hairs or hairlike extrusions and that blue coloration resulted from melanin pigmentation (). Blue textile fibers are colored by dyes, not by blue melanin pigmentation; therefore, it is not possible that blue MD fibers are textile in origin. MD filaments are hairlike extrusions, and some MD fibers are very fine human hairs.Citation7,Citation82,Citation83 The coloration of blue fibers was shown to result from melanin pigmentation, which was demonstrated by positive histochemical staining with Fontana Masson. A confirmatory study performed at a laboratory specializing in biofibers and coloration established that embedded blue fibers in MD dermatological specimens were human hairs.
Figure 3 Filaments remaining embedded in deeper layers of skin after removal of a callus; magnification 100×.
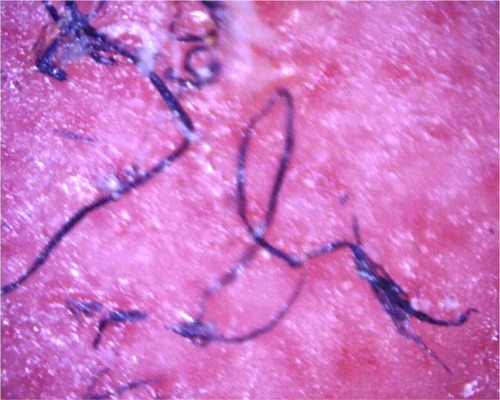
SEM of blue MD fibers shows cuticular scaling consistent with human hairs, and transmission electron microscopy shows darkly stained, disorganized melanosomes consistent with human hairs.Citation7,Citation83 Microspectrophotometry reflectance of blue fibers is consistent with that of pigmented tissues, and Raman spectroscopy results in relevant peaks corresponding to carbamate compounds and melanin aromatic rings (MD Shawkey, University of Akron, personal communication, 2013).Citation7 An investigation concluded that fibers were not self-implanted, due to the fact that they were deeply embedded in skin in a manner that a patient would not be able to achieve (MD Shawkey, University of Akron, personal communication, 2013).
Other MD findings
If MD specimens are examined, they demonstrate evidence of abnormal keratin and collagen expression. In addition to the formation of abnormal cutaneous fibers, many patients report changes to hair and fingernails.Citation82 Deformed follicular bulbs, pili multigemini (formation of multiple hair shafts within individual follicles), filamentous projections from the follicular sheath surrounding hair bulbs, and the formation of thickened keratin projections are common findings.Citation82,Citation83 The authors of this paper have had the opportunity to examine many MD lesions and MD dermatological specimens (Fesler, Middelveen, and Stricker, unpublished data, 2017). We have noted that MD lesions can begin as folliculitis that evolves into ulcerative filamentous lesions, with further evidence of keratin and collagen abnormalities, such as formation of keratin projections, formation of hardened comedo-like masses, and deformities of hairs and hair follicles, as mentioned previously.
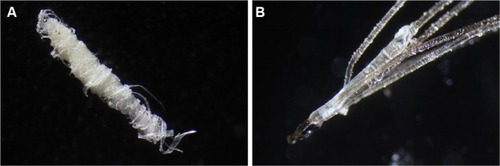
Keratin projections are thickened follicular casts. When sectioned and stained with Gömöri trichrome, these follicular casts are abnormal in that although the outer surface is composed of keratin-rich tissue, the interior can contain collagen-rich tissue. Comedo-like masses can emerge from pores spontaneously or when scratched, and are sometimes described by patients as being sand-like. Patients may misinterpret these objects as being seeds, eggs, cocoons, parasites, or even arthropods. These comedo-like masses can contain embedded keratin or collagen filaments and/or projecting filaments. When they form inside a pore or follicle, they may form a tight wad of fibers (). Hair and follicular bulb deformities include pili multigemini (mentioned previously) (), hairs or fibers growing downward deep into the dermis rather than in the opposite direction through the pore opening, and follicular sheaths with filamentous projections. These projections can completely cover the follicular sheath, and may be interpreted as caterpillars by patients.
Figure 4 (A) A filamentous follicular cast. White filaments originating on the outer follicular sheath are growing in a coiled manner. Magnification 50×. (B) Pili multigemini, a common finding in Morgellons disease patients, with multiple hairs forming from a single bulb. Magnification 50×.
Contaminating extraneous artifacts can complicate identification of legitimate dermatological findings in MD. We have found pollen, noninfesting arthropods, feathers, and mites in patient-supplied specimens. Some patients have described the production of “fuzzballs”. We performed histological sectioning and staining with Gömöri trichrome on such artifacts and determined the “fuzzballs” in patient-supplied samples that we studied were largely composed of textile fibers, but did have some keratin fibers or keratinized tissue present as well. We speculate that such artifacts may include keratin-containing material related to MD, but that textile fibers can attach to sticky exudate or can tangle into filamentous lesions. Other patients have described hexagonal crystals and “glitter” in MD skin. Spectroscopic analysis of the hexagonal crystals proved that they were contaminating man-made hexagonal objects (of the type used in cosmetics and greeting cards) with either cellulose or plastic centers and a metallic coating (V Loyd, Mount Allison University, personal communication, 2017). The “glitter” that we studied contained salts that were likely human bioproducts, and may have a role in MD (unpublished data). As extraneous artifacts can contaminate sticky lesions, it is important to collect only fibers deeply embedded in skin or clearly projecting in a hair-like manner for studies intended to determine fiber composition.
As mentioned earlier in the report, cattle with BDD form lesions that produce unusual keratin projections. Although visually different from the small slender filaments in MD lesions, we have also observed slender fibers in BDD specimens that strongly resemble the fibers in human MD () (unpublished observation).
Figure 5 (A) Thickened keratinized follicular casts in a Morgellons disease specimen that grew inward into the dermis. Note the clear inward-growing hair. Magnification 100×. (B) Specimen from a bovine digital dermatitis lesion with similarities to human Morgellons specimens. Note thickened keratin projections and the threadlike blue filament (lower part of specimen). Magnification 50×.
Detection of pathogens
Early on in MD history, a link between MD and LD was reported.Citation1–Citation7 Savely and Stricker reported that 96.8% of their cohort of 122 MD patients had either positive LD serology or an LD diagnosis.Citation4 A more recent study reported that 6% of LD patients in a cohort of Australian LD subjects had MD,Citation84 and Borrelia spirochetes have repeatedly been detected in skin and bodily fluid specimens from MD subjects. Preliminary studies reported that Bb sensu stricto (Bbss) spirochetes were detected in dermatological tissue removed from MD lesions of four North American patients,Citation83,Citation85 and an ensuing study reported the detection of Borrelia garinii in MD samples from an Australian patient.Citation86
A larger study was needed to verify the association between Borrelia infection and MD. Consequently, a study of 25 North American MD patients confirmed the presence of Borrelia spirochetes in MD-tissue and body-fluid specimens, both directly in dermatological specimens and in cultures obtained from MD patients using microscopic, histopathological, and molecular detection methods.Citation87 This study provided evidence for the presence of Borrelia DNA in MD specimens by PCR followed by DNA sequencing performed by two independent laboratories. PCR technology amplified Borrelia DNA in 13 MD whole-callus specimens (nine sequenced), four cultures inoculated with dermatological tissue (one sequenced), eight blood cultures (two sequenced), two vaginal secretion cultures (both sequenced), and one intestinal specimen. The Borrelia spirochetes detected in these studies were identified primarily as Bbss, but B. garinii and B. miyamotoi were also reported.Citation87
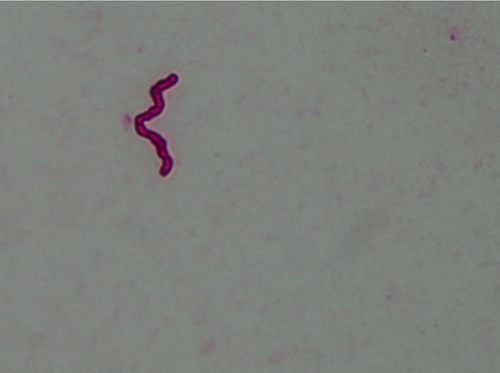
The fact that motile spirochetes identified as Borrelia spp. were detected in Barbour–Stoenner–Kelly H medium inoculated with MD dermatological tissue proves that spirochetes present in MD tissue are viable.Citation85,Citation87 Identification of Borrelia spirochetes in cultures is complicated by fastidious growth requirements and pleomorphism,Citation88,Citation89 but PCR amplification of cultured spirochetes in these studies provided confirmatory molecular identification of live Borrelia spirochetes in specimens from MD subjects.Citation87 Four laboratories independently confirmed the presence of Borrelia DNA in MD specimens using PCR technology and confirmatory DNA sequencing.Citation7 Recently, independent researchers from Canada using PCR technology and confirmatory DNA sequencing have detected Borrelia DNA in cultures inoculated with specimens from MD patients (J Lewis, V Lloyd, Mount Allison University, personal communication, 2017). Therefore, five laboratories using PCR technology have now provided confirmation of Borrelia DNA in MD specimens, including four species of Borrelia: Bbss, B. garinii, B. hermsii, and B. miyamotoiCitation7,Citation90,Citation91(J Lewis, V Lloyd, Mount Allison University, personal communication, 2017). The detection of Borrelia spirochetes is reproducible, providing that correct methods of detection are employed ().
Figure 6 Single spirochete from a Morgellons disease skin specimen immunostained for detection of Borrelia. Magnification 1,000×.
Borrelia spirochetes can invade and replicate inside fibroblasts and keratinocytes,Citation92–Citation94 and have been isolated in vitro from monolayers of keratinocytes and fibroblasts despite antibiotic treatment.Citation92,Citation93 Sequestration in these cells may be a contributing factor in the development of refractory infection in MD patients. In support of that hypothesis, Borrelia spirochetes were detected in MD skin specimens removed from patients treated with aggressive antibiotic therapy.Citation87 Hypothetically, intracellular infection of keratinocytes and fibroblasts would be able to alter keratin and collagen gene expression, respectively, resulting in unusual filament formation.Citation7 Some patients report that gel was secreted from their skin. We sectioned these dried-gel secretions, and immunostaining targeted to Borrelia was positive and demonstrated a copious quantity of well-formed spirochetes embedded in a clear matrix. This finding is consistent with biofilm formation (unpublished data). The spirochetal load in MD specimens is high and suggests biofilm formation in vivo.Citation85,Citation87 The formation of biofilms may also contribute to the severity of the dermopathy and antibiotic resistance.
The key etiologic factor contributing to the evolution of MD lesions seems to be infection with Borrelia spp., the pathogen most consistently detected in MD patients. However, we speculate that the etiology of MD is multifactorial. Factors such as genetic predisposition, endocrine influences, immune status, and the presence of other infections, particularly tick-borne coinfections, appear to play a role in the development of this phenomenon.Citation1–Citation7 Pathogens other than Borrelia spp. have been detected in MD tissue samples, including Helicobacter pylori, Treponema denticola, and Bartonella henselae.Citation87,Citation90,Citation91,Citation95 Preliminary genetic studies have demonstrated nine genes with significant sequence variation in MD patients (E Sapi, University of New Haven, unpublished observation, 2017). Examination of genetic factors that contribute to MD is currently in progress.
Recently, MD-like filamentous dermatitis was described in domestic dogs, and Bbss was detected by PCR amplification confirmed by DNA sequencing, thus providing evidence that MD-like filamentous dermatitis may be associated with LD in these dogs.Citation96 Interestingly, many of these dogs were bulldogs or other breeds with color-dilution genes, thus suggesting that genetics may predispose certain breeds of dogs infected with Borrelia to develop this skin condition. Many of the owners of these pets had no prior knowledge of MD, and thus these were not cases of delusion by proxy.Citation96 The fact that a condition analogous to MD in humans can occur in dogs and a similar animal model of spirochetal infection associated with filament formation – BDD – occurs in cattle provides supportive evidence that MD is an infectious process.
Antibiotic treatment of MD
Although there is anecdotal evidence that MD responds to antibiotics,Citation1–Citation7 controlled studies of MD treatment with antibiotics have not been conducted. Optimal treatment for MD remains undetermined. Two of the authors report success in treating LD/MD patients with antibiotics. In general, early treatment contributes to a better patient outcome. Treatment aimed at the underlying tick-borne disease is essential to resolve MD dermopathy, and treatment may require both prolonged combination-antibiotic therapy and the identification and treatment of any coinfecting tick-borne diseases or other exacerbating factors. Some patients benefit from antiparasitic therapy, although there appears to be no direct evidence of parasite infection in MD.
Clinical classification of MD
A clinical classification scheme has been proposed for MD:
early localized: lesions/fibers present for less than 3 months and localized to one area of the body (head, trunk, extremities)
early disseminated: lesions/fibers present for less than 3 months and involving more than one area of the body (head, trunk, extremities)
late localized: lesions/fibers present for more than 6 months and localized to one area of the body (head, trunk, extremities)
late disseminated: lesions/fibers present for more than 6 months and involving more than one area of the body (head, trunk, extremities).
Discussion
The diagnosis of Delusional disorder 297.1 (F22), somatic type, as described in the DSM-V, requires clinical judgment, as the delusional belief should not be better explained by another mental disorder, be caused by the effects of a substance or medication, nor caused by other medical conditions, such as infection. Many of the case studies cited in this paper mentioning MD concern patients who have medical conditions such as diabetes, vitamin B12 deficiency, substance-abuse problems, and infections that could be implicated in the development of their symptoms, and diagnosing such patients with delusional disorder is contrary to DSM-V principles. The diagnosis of a delusional disorder is best made by a professional with mental health training, such as a psychiatrist. Single, isolated delusions are quite rare, and truly delusional patients have evidence of other delusions, not just DOP.
There is significant overlap in the array of symptoms that may accompany LD, MD, and mental illness, thus complicating the diagnosis. In theory, patients who do not have MD but who are delusional could think they have MD if they have had exposure to the topic through the Internet or other means.Citation7,Citation87 To complicate the diagnosis further, MD patients may exhibit neuropsychiatric symptoms, and many have psychiatric diagnoses, such as bipolar disorder, attention-deficit disorder, obsessive compulsive disorder, and schizophrenia.Citation1,Citation7,Citation81 Therefore, many MD patients may have psychiatric comorbidities, and in some cases, patients have been misdiagnosed with a psychiatric illness that they do not have.Citation7 Some MD patients may have false beliefs that are not delusional in origin. Lack of scientific knowledge can cause patients to misinterpret symptoms, such as the presence of filaments and sensations of formication as worms, arthropods, or other infestations. In addition, MD lesions are sticky and arthropods or artifacts can adhere to exudate, and patients may incorrectly believe these external factors are associated with the dermopathy.Citation7,Citation87
A patient who (because of symptom misinterpretation or lack of scientific knowledge) believes he or she has a parasitic infestation should not be diagnosed with delusional mental illness. It is logical for a patient to speculate that a complex of symptoms, including abnormal skin fibers coupled with formication, could be caused by a parasite. Furthermore, patients with MD are not always aware that they have filaments, because magnification is needed to visualize the filaments and many are diagnosed with other conditions, such as lichen sclerosus or prurigo nodularis, which lack filament formation.Citation7,Citation87 In addition, systemic LD is commonly associated with dermatological conditions and neurological symptoms, such as paresthesias.Citation7,Citation87,Citation97,Citation98 As such, multiorgan MD symptoms overlap with LD because MD is associated with LD, and this dermatological and neurological symptom overlap may partly explain the odd movement or stinging sensations that MD patients experience.
The DSM-V does not mention DOP, DI, or DP. In the case of Delusional disorder 297.1 (F22), delusions are not the beliefs themselves, but the way they are interpreted by patients. Delusions are profound, intensely held beliefs that seem barely swayed by evidence to the contrary, even to the point of believing in the bizarre.Citation34–Citation36 In contrast, the presence of human biofibers embedded in skin of MD patients is factual: the patients are not hallucinating imaginary fibers, and they are not implanting textile fibers or hallucinating an imaginary infestation. MD is not a case of fixed belief despite lack of medical evidence, because if fibers are present and they are visible under magnification, then there is medical evidence. MD fibers projecting from and embedded in skin may have elaborate configurations with branching, and the filaments may have tapered ends. Even skilled microsurgeons could not implant the fibers in that configuration.
The evidence is present in patients who have MD, but in order to be recognized, a physician must be willing to look. If patients meet the case definition for MD with visible skin fibers and do not believe they are infested, these patients do not meet the criteria for a diagnosis of Delusional disorder 297.1, somatic type, as described in the DSM-V. Creeping and crawling sensations unaccompanied by delusions of infestation are not enough to give a patient a diagnosis of delusional disorder. These sensations are consistent with the well-recognized symptom of formication that occurs with peripheral neuropathy and is associated with many medical conditions, such as diabetes, chronic infections, menopause, skin cancer, and multiple sclerosis, and exposure to various chemical substances, such as toxins, certain medications, alcohol, or recreational drugs. It is contrary to proper psychiatric diagnosis to label a patient as having a delusional mental illness based solely on a complaint of formication.
LD and associated tick-borne diseases may be accompanied by mental illness.Citation99–Citation101 Chronic progressive neurodegenerative diseases can be caused by infection and resulting prolonged inflammation.Citation101,Citation102 The spectrum of mental illnesses associated with LD varies in severity, and includes anxiety, depression, paranoia, schizophrenia, bipolar disorder, sensory hallucinations, and homicidal tendencies.Citation101,Citation103 Some of these neuropsychiatric conditions involve a delusional component. Delusions resulting from infectious processes do not meet the DSM-V criteria for delusional disorder. Furthermore, the presence of psychiatric comorbidities is not proof that a patient is delusional. Some patients have a component of posttraumatic stress disorder and are hypervigilant and overreactive to physical symptoms, rather than being delusional. If a health care provider cannot tell the difference between a hypervigilant patient and a delusional patient, the provider is not qualified to diagnose delusional disorder.
The presentation of specimens or pictorial evidence to a doctor is not an indication of delusional disorder or mental illness. This action was never included as an indication of delusional disorder in the DSM-V. Likewise, the fact that a patient with beliefs of infestation accompanied by movement sensation has psychiatric comorbiditiesCitation69,Citation74 is not proof of delusional disorder and is contrary to the recommended practices of the DSM-V.Citation80 In most of the case studies that equate MD with DOP, DP, or DI, patient-supplied evidence was dismissed, evidence of disease (physical and laboratory) was dismissed, fibers were identified as being textile in origin based solely on visual examination, physicians were unwilling to examine skin at sufficient magnification to see microscopic fibers, and all too quickly patients were diagnosed as being delusional in a manner that is contrary to the DSM-V approach to psychiatric diagnosis.
The bar set for burden of proof is higher for those proposing an infectious etiology than for those proposing that MD is delusional. Many of the papers reviewed in this analysis relied solely on visual identification for “textile fibers”. Others did not select the correct patients to study (ie, patients with documented embedded or projecting cutaneous filaments) or did not collect the correct specimens, collecting instead superficial artifacts, including some that might have been introduced at the time of sampling. The analysis of MD fibers requires that the patient meets the case definition with the key diagnostic criterion of having colored, white, or black filaments protruding from or embedded in skin, and the correct specimens must be collected.
Fibers that are embedded in deeper layers of skin or that are firmly attached, originating underneath the stratum corneum and projecting either outward to the surface or inward into the dermis, are the only specimens that are suitable for fiber analysis. Most often, MD fibers are present as inclusions in callus material that resembles scabs. These can be removed for analysis by a health care practitioner, or in rare cases patient-supplied specimens of calluses can contain fibers suitable for analysis, provided that calluses composed of skin and fibers are embedded throughout the specimen. Histological sectioning and staining to detect keratin and collagen can visually and chemically determine the keratin and collagen nature of these fibers.Citation7,Citation83
When diagnosing mental illness, it is imperative first to determine if there is an underlying cause of the psychiatric symptom, such as an infection. None of the case studies reviewed in this paper or the research studies involving larger cohorts of MD patients looked adequately for infections, in particular LD. Science has to be reproducible, and there has to be enough detail provided in the methodology description for the study to be replicated. This was not the case for detecting LD in many of the case studies. Borrelia spirochetes are readily detectable in MD tissue, but sensitive and specific methods are required.Citation7,Citation87 Although sensitive and specific direct-detection methods, such as antigen detection, culture of Borrelia spirochetes, and PCR detection of Borrelia DNA, exist, these methods are not standardized, and vary in sensitivity and specificity.Citation104,Citation105 They are not recommended by the CDC, which only endorses two-tier serological LD testing.Citation7,Citation87,Citation106 Unfortunately, two-tier serological testing for LD, although specific for Bbss, lacks sensitivity and is little better than a coin toss in detecting LD.Citation107,Citation108
False negatives can occur when using two-tier testing for a number of reasons, including the fact that some patients with known LD are seronegative.Citation107,Citation109 In addition, there is significant genetic diversity in Borrelia spp. capable of causing LD and LD-like illnesses, but commercial two-tier testing is based on the antigens of one laboratory strain, and testing may not detect other Borrelia species.Citation110–Citation112 The fact that the CDC does not consider any direct detection method, not even culture, as being diagnostic for LD as proof of infection is unjustifiable. It should be noted that culture is considered to be the gold standard for detection of organisms by the American Society for Microbiology.Citation113 The reluctance of the CDC to accept more sensitive testing methods for LD makes the evidence showing the association between LD and MD controversial.Citation7,Citation87 Those who maintain that MD is a form of DOP or DI and rely on the two-tier test for Borrelia detection claim that studies supporting an infectious etiology and an association with LD are flawed,Citation72,Citation75 yet these critics have not used adequate methodologies, and by failing to do so have not proved that methods for detecting Borrelia spp. used in more sophisticated studies are unreproducible or false.
Patients diagnosed with DOP in case studies are frequently prescribed antipsychotic medication with potentially serious side effects.Citation30 These patients are often talked into taking antipsychotic medication by health care providers using deceptive unethical dialogue, which can compromise patient autonomy.Citation41,Citation43,Citation50,Citation54,Citation60,Citation114 Many published articles mentioning MD make claims that antipsychotic drugs are effective treatment for MD, but careful review of the literature suggests otherwise. Two systematic reviews concerning the use of these medications to treat DI, DOP, or DP cases conclude that treatment efficacy was unproven.Citation115,Citation116 In many of the case studies reviewed in this paper, authors claimed that antipsychotics were used to treat MD cases, yet the patients received treatment in addition to antipsychotic drugs, including antibiotics, wound dressings, antiseptics, and antipruritic drugs.Citation59,Citation65,Citation66 Therefore, it is not certain which treatments were providing benefit, as the studies were uncontrolled. In fact, one study reported complete remission using antibiotics and not with antipsychotics.Citation65
Antipsychotic medications can have off-label effects, such as reduced growth of parasites and anti-pruritic properties.Citation117 Evidence showing an association between antipsychotic treatment in DI, DOP, or DP patients and resolution of symptoms or benefit is very limited and is more limited in MD cases. There has been only one randomized, double-blind, placebo-controlled study that evaluated the effectiveness of the antipsychotic drug pimozide.Citation118 This study of a small cohort of eleven DI patients reported that pimozide was better than placebo at controlling formication, but was not better at controlling delusions of vermin infestation or excoriation.Citation118 One study of 14 DI/DOP/DP patients reported that although seven patients remained in remission 19–48 months after pimozide treatment, four patients had no response to this antipsychotic medication.Citation119 The variation seen in reported effectiveness in this study and the various studies reviewed here may have arisen from the fact that patients diagnosed with DI are a heterogeneous group of individuals, some of whom are truly delusional and some of whom are not.
Recent studies using advanced brain magnetic resonance imaging (MRI) technology have found that patients diagnosed with DI have significant gray-matter changes that differ from findings in both patients with nonsomatic delusions and healthy controls.Citation120–Citation122 These MRI abnormalities involve altered cortical thickness and surface area in various parts of the brain, indicating that selective delusional symptoms in patients may be based on specific somatic brain alterations. It is tempting to speculate that these brain alterations are related to spirochetal infection in MD, either via direct brain invasion or an inflammatory response in genetically susceptible individuals.Citation123,Citation124 The intriguing link between spirochetal infection and brain pathology detected by advanced imaging methods merits further study.
Conclusion
The history of MD has taught us that scientific evidence must be carefully considered before a disease is written off as a purely psychiatric disorder. Delusional disorder is a diagnosis of exclusion that requires clinical judgment, and all underlying causes for delusional symptoms need to be ruled out before jumping to erroneous conclusions. Medical practitioners continue to consider MD a delusional disorder, although studies have shown that MD is strongly associated with spirochetal infection. According to the best-available scientific evidence, MD should be considered a dermopathy associated with tick-borne disease. Further study of the genetics, pathogenesis, and treatment of MD is warranted.
Acknowledgments
The authors thank Jesus Walker-Salas, Diana Canchola and Jeannie Ramos for technical assistance. This work was supported in part by a grant from the Lindorf Family Foundation, Newark, OH.
Disclosure
The authors report no conflicts of interest in this work.
References
- SavelyGLeitaoMMSkin lesions and crawling sensations: disease or delusion?Adv Nurse Pract20051351617
- SavelyVRLeitaoMMStrickerRBThe mystery of Morgellons disease: infection or delusion?Am J Clin Dermatol2006711516489838
- SavelyVRStrickerRBMorgellons disease: the mystery unfoldsExpert Rev Dermatol200725585591
- SavelyVRStrickerRBMorgellons disease: analysis of a population with clinically confirmed microscopic subcutaneous fibers of unknown etiologyClin Cosmet Investig Dermatol201036778
- LelandDKTouched by Lyme: threading your way through Morgellons disease2017 Available from: https://www.lymedisease.org/touched-lyme-threading-path-morgellons-diseaseAccessed September 9, 2017
- MiddelveenMJStrickerRBFilament formation associated with spirochetal infection: a comparative approach to Morgellons diseaseClin Cosmet Investig Dermatol20114167177
- MiddelveenMJStrickerRBMorgellons disease: a filamentous borrelial dermatitisInt J Gen Med2016934935427789971
- KellettCESir Thomas Browne and the disease called MorgellonsAnn Med Hist19357467479
- ThibiergeGLes acaraphobesJ Prat Rev Gen Clin Ther18948373376
- LeeWRThe matchbox signLancet198328347457458
- WilsonJWMillerHEDelusion of parasitosis (acarophobia)Arch Derm Syphilol194654395620992505
- RaeckeJUber hypochondrieAllg Z Psychiatr190259319410
- GrønKLes dermatophobiesPoster presented at: 6th meeting of the Nordic Dermatological AssociationAugust 26–28, 1924Helsinki, Finland
- MacNamaraEDCutaneous and visual hallucinationsLancet1928214807808
- EllerJJNeurogenic and psychogenic disorders of the skinMed J Rec1929129481485554559616620675679
- SchwarzHCircumscripte hypochondrienMonatsschr Psychiatr Neurol192972150164
- MalletRMalePDélire cénesthesiqueAnn Med Psychol (Paris)193088198201
- ViéJL’idée délirante d’anthropathie internePoster presented at: 39th Congress of Alienist Physicians and Neurologists of FranceJuly 22–28, 1935Brussels, Belgium
- KlauderJVPsychogenic aspects of skin diseasesJ Nerv Ment Dis1936843249273
- EkbomKAPraeseniler dermat-zooenwahnActa Psychiatr Scand193813227259
- KohlPKWinzerIThe 100 years since discovery of Spirochaeta pallidaHautarzt200556211211515657727
- BiggerJWThe reliability of the Wassermann test as performed by different pathologistsJ Hyg (Lond)192120438338920474749
- GilbertRStandardization of the Wasserman testAm J Public Health Nations Health1930201474818012919
- KeanWFToccioSKeanMRainsfordKDThe musculoskeletal abnormalities of the Similaun iceman (Ötzi): clues to chronic pain and possible treatmentsInflammopharmacology2013211112023096483
- MarshallWFTelfordSRRysPNDetection of Borrelia burgdorferi DNA in museum specimens of Peromyscus leucopusJ Infect Dis199417041027327930700
- PoinarGSpirochete-like cells in a Dominican amber Amblyomma tick (Arachnida: Ixodidae)Hist Biol2015275565570
- Emslie-SmithAHMyiasis, fillan, and the morgellonsBr Med J1946196220988616
- LyellADelusions of parasitosisJ Am Acad Dermatol1983868958976863652
- RoblesDTMorgellons disease and the ‘tweezer sign’Clin Exp Dermatol2008336793794
- FreudenmannRWLeppingPDelusional infestationClin Microbiol Rev200922469073219822895
- FreudenmannRWKölleMSchönfeldt-LecuonaCDieckmannSHarthWLeppingPDelusional parasitosis and the matchbox sign revisited: the international perspectiveActa Derm Venereol201090551751920814630
- HarlanCMom fights for answers on what’s wrong with her son2006 Available from: http://www.post-gazette.com/local/2006/07/23/Mom-fights-for-answers-on-what-s-wrong-with-her-son/stories/200607230221Accessed August 4, 2017
- PearsonMLSelbyJVKatzKAClinical, epidemiologic, histopathologic and molecular features of an unexplained dermopathyPLoS One201271e2990822295070
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders5th edSt LouisAPA2016
- ManschreckTCDelusional disorder: the recognition and management of paranoiaJ Clin Psychiatry199657Suppl 33238 discussion 49
- FennigSFochtmannLJBrometEJDelusional and shared psychotic disorderSadockBJSadockVAKaplan and Sadock’s Comprehensive Textbook of Psychiatry8th edPhiladelphiaLWW200515251533
- BourgeoisJAKhanRAHiltyDMDelusional disorder2015 Available from: http://emedicine.medscape.com/article/292991-overviewAccessed September 4, 2017
- KoblenzerCSPimozide at least as safe and perhaps more effective than olanzapine for treatment of Morgellons diseaseArch Dermatol2006142101364
- KoblenzerCSThe challenge of Morgellons diseaseJ Am Acad Dermatol200655592092217052516
- WaddellAGBurkeWAMorgellons disease?J Am Acad Dermatol200655591491517052510
- AccordinoREEnglerDGinsburgIHKooJMorgellons disease?Dermatol Ther200821181218318880
- MolyneuxJAKA ‘Morgellons’Am J Nurs200810852526
- RoblesDTRommSCombsHOlsonJKirbyPDelusional disorders in dermatology: a brief reviewDermatol Online J20081462
- LustigAMackaySStraussJMorgellons disease as Internet memePsychosomatics20095019019213978
- WildnerMDo they understand Morgellons disease?Gesundheitswesen20097112795796 German20039225
- FairBMorgellons: contested illness, diagnostic compromise and medicalisationSociol Health Illn201032459761220149149
- HalvorsonCRAn approach to the evaluation of delusional infestationCutis2012904E1E4
- SchmidtELevittJDermatologic infestationsInt J Dermatol201251213114122250620
- MiseryLMorgellons syndrome: a disease transmitted via the mediaAnn Dermatol Venereol20131401596223328363
- TeyHLWallengrenJYosipovitchGPsychosomatic factors in pruritusClin Dermatol2013311314023245971
- WongJWKooJYDelusions of parasitosisIndian J Dermatol2013581495223372213
- VulinkNCDelusional infestation: state of the artActa Derm Venereol201696217586327282746
- FerreiraBRRocciaMGCardosoJCHistory of Morgellons disease: the same name for different psychodermatologic diseases?Wien Med Wochenschr2017167Suppl 14951
- MuraseJEWuJJKooJMorgellons disease: a rapport-enhancing term for delusions of parasitosisJ Am Acad Dermatol2006555913417052509
- BhandarySKPeterRBhatSDelusional parasitosis in ENTIndian J Otolaryngol Head Neck Surg2008604387923120590
- Vila-RodriguezFMacewanBGDelusional parasitosis facilitated by web-based disseminationAm J Psychiatry200816512161219047336
- DovigiAJIntraoral Morgellons disease or delusional parasitosis: a first case reportAm J Dermatopathol201032660360520489569
- FreudenreichOKontosNTranulisCCatherCMorgellons disease, or antipsychotic-responsive delusional parasitosis, in an HIV patient: beliefs in the age of the InternetPsychosomatics201051645345721051675
- HarthWHermesBFreudenmannRWMorgellons in dermatologyJ Dtsch Dermatol Ges20108423424219878403
- ReidEELioPASuccessful treatment of Morgellons disease with pimozide therapyArch Dermatol2010146101191119320956673
- DeBonisKPierreJMPsychosis, ivermectin toxicity, and “Morgellons disease”Psychosomatics2011523295296
- DewanPMillerJMustersCTaylorREBewleyAPDelusional infestation with unusual pathogens: a report of three casesClin Exp Dermatol201136774574821933231
- GartnerAMDolanSLStanfordMSElkinsGRHypnosis in the treatment of Morgellons disease: a case studyInt J Clin Exp Hypn201159224224921390982
- GrosskopfCDesaiBStooplerETAn oral ulceration associated with Morgellons disease: a case reportOral Surg Oral Med Oral Pathol Oral Radiol Endod20111122e19e2321749875
- RoblesDTOlsonJMCombsHRommSKirbyPMorgellons disease and delusions of parasitosisAm J Clin Dermatol201112116
- AltunayIKAtesBMercanSDemirciGTKayaogluSVariable clinical presentations of secondary delusional infestation: an experience of six cases from a psychodermatology clinicInt J Psychiatry Med201244433535023885516
- FellnerMJNew findings in delusions of parasitosisSkinmed2012102727422545320
- FosterAAHylwaSABuryJEDavisMDPittelkowMRBostwickJMDelusional infestation: clinical presentation in 147 patients seen at Mayo ClinicJ Am Acad Dermatol2012674673.e11022264448
- HylwaSAFosterAABuryJEDavisMDPittelkowMRBostwickJMDelusional infestation is typically comorbid with other psychiatric diagnoses: review of 54 patients receiving psychiatric evaluation at Mayo ClinicPsychosomatics201253325826522458994
- MortillaroGRodgmanCKinzieERyalsSA case report highlighting the growing trend of Internet-based self-diagnosis of “Morgellon’s disease”J La State Med Soc2013165633433625073260
- RankaNGodseKNadkarniNPatilSAgarwalSMorgellons disease: a myth or reality?Indian Dermatol Online J20167543043227730047
- RoncatiLGattiAMPusiolTPiscioliFBarboliniGMaioranaAThe first investigative science-based evidence of Morgellons psychogenesisUltrastruct Pathol201640524925327269255
- SandhuRKSteeleEAMorgellons disease presenting as an eyelid lesionOphthal Plast Reconstr Surg2016324e85e87
- MohandasPBewleyATaylorRMorgellons disease: experiences of an integrated multidisciplinary dermatology team to achieve positive outcomesJ Dermatolog Treat Epub2017714
- OhnJParkSYMoonJChoeYSKimKHMorgellons diseaseAnn Dermatol201729222322528392653
- ShahRTaylorREBewleyAExploring the psychological profile of patients with delusional infestationActa Derm Venereol20179719810127026055
- CargnelloJAPowellJJThompsonRPCrockerPRWattFElemental hair analysis using nuclear microscopy and X-ray energy dispersive spectroscopyAnalyst1995120783787
- KruszelnickiKSDental floss 12001 Available from: http://www.abc.net.au/science/articles/2001/03/30/268342.htmAccessed September 9, 2017
- BöerABreschMDayritJFalkTMErythema migrans: a reassessment of diagnostic criteria for early cutaneous manifestations of borreliosis with particular emphasis on clonality investigationsBr J Dermatol200715661263127117535225
- StrickerRBMiddelveenMJMorgellons disease: more questions than answersPsychosomatics201253550450522959062
- HarveyWTBransfieldRCMercerDEWrightAJRicchiRMLeitaoMMMorgellons disease, illuminating an undefined illness: a case seriesJ Med Case Rep20093824319830222
- MiddelveenMJRasmussenEHKahnDGStrickerRBMorgellons disease: a chemical and light microscopic studyJ Clin Exp Dermatol Res20123140
- MiddelveenMJMaynePJKahnDGStrickerRBCharacterization and evolution of dermal filaments from patients with Morgellons diseaseClin Cosmet Investig Dermatol20136121
- MaynePJClinical determinants of Lyme borreliosis, babesiosis, bartonellosis, anaplasmosis, and ehrlichiosis in an Australian cohortInt J Gen Med20158152625565883
- MiddelveenMJBuruguDPoruriAAssociation of spirochetal infection with Morgellons diseaseF1000Res201322524715950
- MaynePEnglishJSKilbaneEJBurkeJMMiddelveenMJStrickerRBMorgellons: a novel dermatological perspective as the multisystem infective disease borreliosisF1000Res20132118
- MiddelveenMJBandoskiCBurkeJExploring the association between Morgellons disease and Lyme disease: identification of Borrelia burgdorferi in Morgellons disease patientsBMC Dermatol201515125879673
- MursicVPWannerGReinhardtSWilskeBBuschUMargetWFormation and cultivation of Borrelia burgdorferi spheroplast L-form variantsInfection19962432182268811359
- BrorsonOBrorsonSHAn in vitro study of the susceptibility of mobile and cystic forms of Borrelia burgdorferi to tinidazoleInt Microbiol20047213914215248163
- ShahJSMorgellons disease: chronic form of Borrelia infection?Poster presented at: 9th Annual Medical Scientific Conference on Morgellons DiseaseApril 30–May 1, 2016Austin, TX
- AllenLSaylor-HefleyCMorgellons under investigation: identification of associated microorganisms by molecular analysis of epithelial samplesPoster presented at: 7th Annual Medical Scientific Conference on Morgellons DiseaseMarch 29–30, 2014Austin, TX
- GeorgilisKPeacockMKlempnerMSFibroblasts protect the Lyme disease spirochete, Borrelia burgdorferi, from ceftriaxone in vitroJ Infect Dis199216624404441634816
- KlempnerMSNoringRRogersRAInvasion of human skin fibroblasts by the Lyme disease spirochete Borrelia burgdorferiJ Infect Dis19931675107410818486939
- ChmielewskiTTylewska-WierzbanowskaSInteractions between Borrelia burgdorferi and mouse fibroblastsPol J Microbiol201059315716021033577
- BandoskiCEvidence for the presence of human pathogens Borrelia and Helicobacter in Morgellons patients’ skin samplesPoster presented at: 7th Annual Medical Scientific Conference on Morgellons DiseaseMarch 29–30, 2014Austin, TX
- MiddelveenMJRotaruGMMcMurrayJLCanine filamentous dermatitis associated with Borrelia infectionJ Vet Sci Med Diagn2016561000212
- MalaneMSGrant-KelsJMFederHMJrLugerSWDiagnosis of Lyme disease based on dermatologic manifestationsAnn Intern Med19911144904981994797
- VrethemMHellblomLWidlundMChronic symptoms are common in patients with neuroborreliosis: a questionnaire follow-up studyActa Neurol Scand2002106420520812225315
- FallonBASchwartzbergMBransfieldRLate-stage neuropsychiatric Lyme borreliosis: differential diagnosis and treatmentPsychosomatics19953632953007638317
- GaoHMHongJSWhy neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progressionTrends Immunol200829835736518599350
- BransfieldRCThe psychoimmunology of Lyme/tick-borne diseases and its association with neuropsychiatric symptomsOpen Neurol J20126889323091569
- KristenssonKMicrobes’ roadmap to neuronsNat Rev Neurosci201112634535721587289
- MattingleyDWKoolaMMAssociation between Lyme disease and schizoaffective disorder, bipolar type: is it inflammation mediated?Indian J Psychol Med201537224324625969618
- LangeRSeyyediSEvidence of a Lyme borreliosis infection from the viewpoint of laboratory medicineInt J Med Microbiol20022913312012412141736
- SchmidtBLPCR in laboratory diagnosis of human Borrelia burgdorferi infectionsClin Microbiol Rev19971011852018993863
- US Centers for Disease Control and PreventionLyme disease: diagnosis and testing2015 Available from: https://www.cdc.gov/lyme/diagnosistesting/index.htmlAccessed September 30, 2017
- StrickerRBJohnsonLSerologic tests for Lyme disease: more smoke and mirrorsClin Infect Dis20084781111111218800935
- CookMJPuriBKCommercial test kits for detection of Lyme borreliosis: a meta-analysis of test accuracyInt J Gen Med2016942744027920571
- DattwylerRJVolkmanDJLuftBJHalperinJJThomasJGolightlyMGSeronegative Lyme diseaseN Engl J Med198831922144114463054554
- GirardYAFederovaNLaneRSGenetic diversity of Borrelia burgdorferi and detection of B. bissettii-like DNA in serum of north coastal California residentsJ Clin Microbiol201149394595421177909
- OgdenNHMargosGAanensenDMInvestigation of genotypes of Borrelia burgdorferi in Ixodes scapularis ticks collected during surveillance in CanadaAppl Environ Microbiol201177103244325421421790
- PéterOBretzAGBeeDOccurrence of different genospecies of Borrelia burgdorferi sensu lato in Ixodid ticks of Valais, SwitzerlandEur J Epidemiol19951144634678549716
- WilsonMLWeinsteinMPRellerBarthLaboratory detection of bacteremia and fungemiaJorgensonJHPfallerMACarrollKCManual of Clinical Microbiology11th edWashingtonASM Press20151529
- SöderfeldtYGrossDInformation, consent and treatment of patients with Morgellons disease: an ethical perspectiveAm J Clin Dermatol2014152717624671866
- LeppingPRussellIFreudenmannRWAntipsychotic treatment of primary delusional parasitosis: systematic reviewBr J Psychiatry200719119820517766758
- SultanaAMcMonagleTPimozide for schizophrenia or related psychosesCochrane Database Syst Rev20002CD001949
- WebsterJPLambertonPHDonnellyCATorreyEFParasites as causative agents of human affective disorders? The impact of anti-psychotic, mood-stabilizer and anti-parasite medication on Toxoplasma gondii’s ability to alter host behaviourProc Biol Sci200627315891023103016627289
- HamannKAvnstorpCDelusions of infestation treated by pimozide: a double-blind crossover clinical studyActa Derm Venereol198262155586175138
- LindskovRBaadsgaardODelusions of infestation treated with pimozide: a follow-up studyActa Derm Venereol19856532672702411090
- EcclesJAGarfinkelSNHarrisonNASensations of skin infestation linked to abnormal frontolimbic brain reactivity and differences in self-representationNeuropsychologia20157790626260311
- HirjakDHuberMKirchlerECortical features of distinct developmental trajectories in patients with delusional infestationProg Neuropsychopharmacol Biol Psychiatry201776727928257853
- HuberMWolfRCLeppingPRegional gray matter volume and structural network strength in somatic vs. non-somatic delusional disordersProg Neuropsychopharmacol Biol Psychiatry Epub20171124
- LivengoodJAGilmoreRDJrInvasion of human neuronal and glial cells by an infectious strain of Borrelia burgdorferiMicrobes Infect2006814–152832284017045505
- FallonBALevinESSchweitzerPJHardestyDInflammation and central nervous system Lyme diseaseNeurobiol Dis201037353454119944760
