Abstract
Lupus erythematosus tumidus (LET) is an uncommon and photosensitive inflammatory skin disorder which is characterised by erythematous urticarial plaques. In the last 20 years, extensive research on clinical and histological aspects of the disease have led to a better characterization of this nosological entity and to differentiate it from other similar or related diseases. Today, LET is considered as a separate subtype of cutaneous lupus erythematosus (CLE) with a benign, intermittent clinical course (intermittent CLE, ICLE) and only rarely associated with systemic lupus erythematosus (SLE).
Introduction
Lupus erythematosus tumidus (LET) is a chronic relapsing inflammatory disease of the skin and was first described by Erich Hoffmann in 1909,Citation1 presenting two patients with rounded, elevated, erythematous and non-scaling urticarial lesions. This article was followed by a report in 1930 with subsequent reports describing LET in the 1950s.Citation2,Citation3 LET occurs predominantly on sun exposed areas. Sometimes the skin lesions have an annular or semi-annular (“arciform”) appearance but there are no epidermal changes and therefore no scale, ulceration or crust formation. Every lesion heals without scarring or postinflammatory hyper- or hypopigmentation and therefore LET does not result in chronic skin damage.Citation4 ()
Figure 1 Clinical picture of Lupus erythematosus tumidus: erythematous, urticarial plaque and papules on the neck of a 43-year old male smoker. Consent was received for the publication of this image.
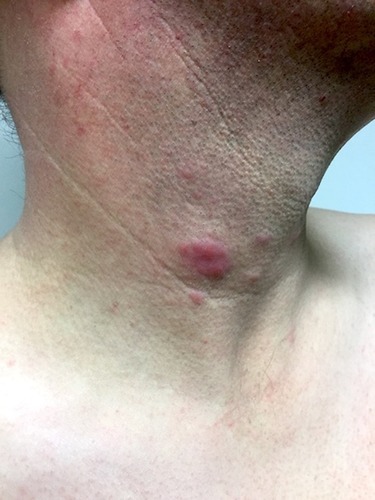
The high photosensitivity of LET patients and the evolution of standardized photoprovocation protocols has helped to improve the scientific evaluation and identification of the disease in recent years.Citation5–Citation7 The development of histologic and immunohistologic evaluation procedures supported important similarities with other forms of cutaneous lupus erythematosus (CLE) and has resulted in a better differentiation of LET from other CLE subtypes at the microscopic level.Citation8–Citation10 This has substantially increased awareness of LET, resulting in a higher reporting rate in the literature.
LET is included in the classification of CLE even if it can be well differentiated, both clinically and histologically, from the most common forms of CLE, such as discoid lupus erythematosus (DLE), subacute cutaneous lupus erythematosus (SCLE), as well as acute cutaneous lupus erythematosus (ACLE). LET was previously subclassified as a form of chronic CLE together with DLE, chilblain lupus erythematosus (ChLE) and lupus erythematosus panniculitis/profundus (LEP).Citation11 In the last two decades, the extensive works of Kuhn and co-workers on LET demonstrated that LET is a distinct entity and suggested the term intermittent CLE (ICLE) to include LET in the Duesseldorf Classification in 2004 ().Citation12 The question whether LET is regarded either as a specific cutaneous manifestation of CLE (an opinion widely accepted in Europe) or an unspecific cutaneous manifestation of LE, such as Raynaud’s phenomenon, diffuse alopecia, vasculitis/vasculopathy etc is a topic of ongoing debate.Citation13–Citation18 Some aspects of this debate will be addressed in the present review. In 2012 Systemic Lupus International Collaboration Clinics (SLICC) derived and validated new classification criteria (SLICC-12) for SLE, including LET to other forms of chronic CLE (DLE, LEP, ChLE, mucosal lupus and Lichen planus-DLE overlap) as a criterion for the diagnosis of SLE ().Citation19
Table 1 Duesseldorf classification of cutaneous lupus erythematosus (CLE)a
Table 2 Systemic Lupus International Collaborating Clinics (SLICC) classification criteria for systemic lupus erythematosus (SLE)b
However, there are many clinical and histological similarities to other clinical entities, such as reticular erythematous mucinosis (REM), polymorphic light eruption (PLE) and lymphocytic infiltration of the skin Jessner-Kanof (LIS). The latter is barely distinguishable from LET and many physicians consider it as the same disease as LET.Citation20–Citation23
Epidemiology
LET is considered a rare disease and, because of its benign nature and its clinical course with relapses and spontaneous remissions, it may subsequently be more rarely reported. Information regarding the prevalence and incidence of LET is still lacking. One reason for this is that there is no specific international classification of diseases (ICD) code for LET, and it is currently merely included in the ICD L93.2 (other local lupus erythematosus) together with LEP or ChLE, for example. In a large population cohort of 1088 patients in Sweden, which has shown an incidence of CLE to be 4/100 000, only 49 (4.5%) patients were given the code L93.2.Citation24
In 2013, the European Society of Cutaneous Lupus Erythematosus (EUSCLE) provided clinical data from 1002 CLE patients from 13 European countries and Brazil.Citation25–Citation27 65 of these were diagnosed with LET and a further 41 were diagnosed with LET together with one or more different CLE subtypes, most commonly ACLE or DLE. In the group of 65 LET patients, 60% were female, showing less gynecotropy in comparison with other CLE or SLE patients.Citation27 In a cohort of 100 LET patients referred to a specialized tertiary dermatology centre in Germany, we reported similar gender findings (females 68%), as did a recent multicentre study of CLE in Italy, in which 72/108 LET patients (66.7%) were females.Citation28,Citation29
Relation to other (C)LE subtypes
According to the EUSCLE report from Biazar et al, 39% of patients with LET suffer concomitantly from other subtypes of CLE.Citation27 This appears to be a high proportion, taking into account the literature regarding LET as a separate entity in the CLE group.Citation16 A possible explanation is the higher confidence of physicians to diagnose LET in the presence of other LE subtypes, or even that isolated LET is still frequently diagnosed as LIS or pseudolymphoma in some European countries. However, these results support the notion that LET is a form of (C)LE, since LET is commonly associated with other CLE subtypes. Moreover, there are numerous case reports presenting the coexistence of LET with other CLE subtypes or even with SLE.Citation30–Citation36 Nevertheless, LET most commonly occurs without other autoimmune signs. Furthermore, LET rarely correlates with systemic disease (SLE) or specific antibodies against extractable nuclear antigens (ENA), such as anti-Ro/SSA, anti-La/SSB, anti-Sm, anti-U1-RNP, or antibodies against ds-DNA. The presence of antinuclear antibodies in serum (especially in higher titrations), which is a hallmark of some other LE subtypes (SLE, ACLE, SCLE), is uncommon in LET.Citation28 Therefore, the sole presence of LET is considered as a separate entity in the LE disease group showing a favourable prognosis.Citation16
Histology
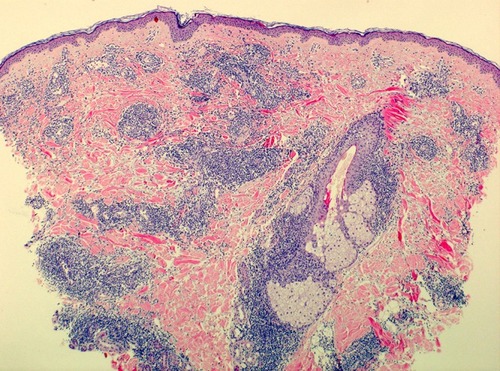
Histologically, LET shows a superficial and deep perivascular and periadnexal lymphocytic infiltration of the skin with prominent mucinous dispositions. The epidermis is intact. () Important features of other cutaneous LE subtypes, such as vacuolization of the basal membrane and epidermal changes are missing.Citation8,Citation9
Figure 2 Histology of Lupus erythematosus tumidus: superficial and deep perivascular and periadnexal lymphocytic infiltrates with prominent mucinous dispositions and without epidermal changes.
Direct immunofluorescence (DIF) is supposed to be negative in LET patients (in contrast to non-inflamed, non-UV-exposed skin of ACLE/SLE patients showing IgG, IgM and/or complement C3 deposits at the dermoepidermal junction- “lupus band test”).Citation8,Citation9,Citation37,Citation38 However, a retrospective study of 21 LET patients showed a positive DIF in 16 of 19 patients.Citation39 Common problems, such as false positive DIF in inflamed or sun exposed skin have to be addressed before evaluating this result. In addition, a positive DIF result could have been due to a selection bias leading to the diagnosis of LET since it was previously regarded as a diagnostic criterion for CLE.
Although LET was previously clearly described and classified, histopathological findings of this disease may show certain variations. Undoubtedly, most LET patients have monomorphic lesions and a distinct disease with an indolent and benign course. Nevertheless, there are some forms with overlapping clinical and/or histological features providing even better evidence for the classification of LET in the CLE group.Citation10 Some of them are minimal alterations of the epidermis or the dermoepidermal junction, such as slight vacuolar degeneration, or even the absence of mucin dispositions.Citation40 Such alterations are acceptable and do not exclude the diagnosis of LET, since other clinical criteria are present. These are, for example, the presence of urticarial and succulent lesions without scaling, dyspigmentation or scarring, positive phototesting with delayed evolution of characteristic lesions, as well as a rapid and excellent response to antimalarials.Citation40
The body area biopsied, the specific location on the plaque selected (central or peripheral) and the time of taking a biopsy (recent or older lesion) are factors that may play a role in the histologic interpretation of skin specimens in LET, as in many other inflammatory conditions. This could also explain a certain variability of the histological results. It has been reported that dermal or subcutaneous fat inflammation of LE (LET and LEP respectively) could be misinterpreted as pseudolymphoma or even as cutaneous lymphoproliferative disorders.Citation41,Citation42 This pitfall applies also to other CLE-infiltrates with histological findings resembling mycosis fungoides or aggressive angiocentric lymphomas.Citation43
Antinuclear antibodies (ANA)
In contrast to some other LE subtypes, such as SLE and SCLE, LET is characteristically not associated with the presence of ANA. In a large cohort of 100 LET patients evaluated for the presence of ANA, and even with a threshold in the low titration of 1:80, only 25% of LET patients were ANA positive, much less than with other CLE patients. Antibodies to extractable nuclear antigens (ENA) were even rarer with 8 (8%) patients tested positive for autoantibodies against Ro/SSA, 6 (6%) against La/SSB and 1 (1%) against Smith (Sm) antigen. Anti-ds-DNA antibodies were detected in 3 out of 100 patients (3%).Citation28 However, a positive ANA test does not exclude LET. The 65 patients included in the EUSCLE register were tested positive for ANA in 36.9% of cases (titration of ≥1:160), whereas anti-Ro/SSA-, anti-La/SSB- and anti-ds-DNA positivity was seen in 17.9%, 10.7% and 3.3% of the patients respectively (please note the high coincidence rate of LET with other CLE subtypes in this study, as previously commented). Verdelli et al included 108 LET patients in their recent, multicentre Italian study of 619 patients with CLE. 47 of them (43.5%) were ANA positive (titration not mentioned), while 24 (22.2%) were tested positive for anti-Ro/SSA-, 5 (4.6%) for anti-La/SSB-, 4 (3.7%) for anti-Sm- and 4 (3.7%) for anti-ds-DNA antibodies.Citation29 These are the highest scores of positive ANA tests ever reported in patients with LET. A possible explanation could be that physicians are more confident making a diagnosis of LET or in referring patients to a tertiary centre treating LE patients more frequently in the case of positive ANA testing (negative selection of ANA negative CLE patients). Conversely, these results could implicate that elevated ANA in serum (most commonly at a low titration) are not such an infrequent finding in LET.
Pathogenesis
There is no specific theory concerning the pathogenesis of LET, most hypotheses are derived from the study of other CLE subtypes.Citation44 UV-induced damage of the skin, impaired clearance of apoptotic cells and externalized autoantigens as well as innate and acquired immune system dysfunction are all implicated in the development of LET lesions.
Ultraviolet light
UV-light is one of the environmental factors with a high impact on human health and disease. LET is a highly photosensitive disease, characterized by a retarded induction of skin lesions following UV-exposition, compared to other photodermatoses.Citation6 LET lesions can be experimentally reproduced after photoprovocation with UVB, UVA and/or a combination of UVA and UVB light. Standardized photoprovocation protocols include the initial determination of the minimal erythema dose (MED) for UVB and possible hypersensitivity against UVA, followed by irradiation of three non-lesional and non-UV-exposed areas (eg on the upper back of the patient) on three consecutive days. The irradiation doses are 1.5xMED/day for UVB and a dose between 40–100 J/cm2/day, depending on the skin type and possible hypersensitivity of the subject under evaluation, for UVA. The aforementioned doses may be subsequently reduced over the next 2 days in cases of very severe reactions (such as severe sunburn). After photoprovocation, LET lesions occur more than 48 hrs after irradiation and present as erythematous papules or plaques with a self-limiting course within several days and up to some weeks.Citation45 Histopathologic evaluation of biopsies from these specimens show similar findings to genuine CLE lesions.Citation46,Citation47
In various studies LET has shown the highest positive photoprovocation rates (70–81%), compared to the positive photoprovocation rates of other CLE subtypes, such as of SCLE with 50–100% and of DLE with 10–64%.Citation27
Smoking
Cigarette smoking may have phototoxic effects.Citation48,Citation49 In a large cohort of 405 patients with CLE or/and SLE, 186 (45.9%) were smokers, significantly more frequent compared to 33.2% of smokers in the matched general population. Among them, 63 LET patients were included of which 52 (82.5%) smoked, which was the highest rate of all subgroups.Citation50 Similar results were provided from the international European registry for CLE (EUSCLE). 84.2% of the 65 included LET patients smoked at any given time, more frequently than any other CLE groups (55.9–60.4%) and 90.6% of them smoked at the time of first diagnosis.Citation25 Italian data from 108 LET patients have shown much lower rates of smokers among them (42.6%), although there were still significantly more than in the other CLE subpopulations (21.6%).Citation29
Not only does smoking negatively influence the course of the disease, it also correlates with a worse response to antimalarials. As antimalarials are the most common prescribed systemic treatment for CLE (about 75% of CLE patients receive or have received antimalarials) and LET, showing a highly positive profile regarding good effectiveness and few side effects, smoking is an important factor with significant impact for some patients.Citation25 The overall efficacy of hydroxychloroquine in CLE among non-smokers is 93.8%, with the smokers’ group having significantly lower efficacy rates of 82.1%. The overall efficacy in LET patients is lower with 77.4% of all treated patients, possibly reflecting the high smoking rates of LET patients.Citation51,Citation52 Furthermore, a monocentric German study of 36 patients with LET demonstrated low activity scores with clear or almost clear disease (score of 0 or 1 measured with the Cutaneous Lupus erythematosus disease Area and Severity Index-CLASI) under treatment with antimalarials in 88% of non-smokers in contrast to only 57% in the group of smokers.Citation53
Medications
Although drug inductions are considered a minor risk factor for the development of LET, there are several reports associating various medications with the disease. TNF-α inhibitors are known to induce the production of ANA and anti-ds-DNA antibodies and, less commonly, a clinical picture similar to SLE (“lupus-like syndrome” or “rhupus syndrome”).Citation54 Three reports associated the induction of LET after treatment with infliximab, adalimumab and etanercept.Citation55–Citation57 Thiazide diuretics are common medications which can induce photosensitivity and two cases of LET in patients taking thiazide diuretics have been reported.Citation58 Further medications have also been associated with the induction of LET: the proteasome inhibitor bortezomib (in two cases), the angiotensin converting enzyme (ACE) inhibitor enalapril, a highly active antiretroviral therapy (HAART) and the IL-12/23 inhibitor ustekinumab.Citation59–Citation63 Recently, two patients receiving the IL-17 inhibitor ixekizumab were described with injection-site inflammatory reactions that were histologically similar to LET.Citation64 LET has also been reported after sex reassignment surgery.Citation65
Genetics
As with other complex autoimmune diseases, the pathogenesis of LET is multifactorial and one of its basic components is genetic predisposition, although no studies have to date focussed solely on the genetics of LET. In a genome-wide association study of 183 CLE patients including 30 LET patients, Kunz et al identified polymorphisms in genes responsible for antigen presentation, regulation of apoptosis, RNA processing and interferon response (HLA-DQA1, MICA, MICB, MSH5, TRIM39 and RPP21).Citation66 Some of them are already known to play a role in SLE patients or even more in patients with SLE and CLE.
Further immunological aspects
Dendritic cells (DC), plasmacytoid DC (pDC) and macrophages recruited to the skin of LET (and overall CLE) patients, are thought to drive inflammation through IFN production, which can be completely prevented through the use of sunscreens.Citation67–Citation69 The lymphocyte infiltrate in LET consists mainly of CD4+ T lymphocytes and much less CD8+ lymphocytes.Citation67 T-regulatory lymphocytes are significantly fewer in LET than in DLE.Citation70 Furthermore, the cytokine and chemokine profile of LET differs from those of other CLE subtypes.Citation71
Relation to other (non-LE) diseases
PLE, REM and LIS are skin conditions with similar clinical and histologic findings with LET. The first two entities can easily be distinguished from LET through their clinical picture or their clinical course ().
Table 3 Clinical and histological comparison of Lupus erythematosus tumidus (LET), reticulated erythematous mucinosis (REM), polymorphic light eruption (PLE) and lymphocytic infiltration of the skin (LIS)
PLE is a relatively common photosensitive condition. It occurs within hours after sun or UV-radiation exposure presenting with small papules, vesicles or plaques on sun exposed areas.Citation72,Citation73 Conversely, LET, though showing a high sensitivity too, occurs more than 48 hrs after sun exposure and is characterised through wider, urticarial lesions/plaques. PLE lesions are highly itchy (pruritus is not a common feature of LET) and respond more rapidly to topical therapies than LET lesions. However, there is a coincidence of LET with PLE in 24.6% of the LET patients.Citation27 Although sharing some characteristics, both entities can be easily clinically differentiated.
REM has a very distinctive clinical presentation, showing erythematous, firm papules or plaques and/or macules in reticulated distribution on the breast and back of young patients. REM also heals without scarring or dyspigmentations.Citation74 Cinotti et al identified some histologic differences between REM and LET, such as the higher representation of plasmacytoid dendritic cells (pDCs) in LET.Citation75 Furthermore, a more scattered and superficial lymphocytic infiltrate as well as more superficial mucin dispositions were observed, in addition to a lower frequency of immunoglobulin and complement depositions along the dermo-epidermal junction.Citation75 REM responds similarly well to antimalarials as for LET.Citation76
In contrast, there are no features that can clearly differentiate LET with LIS. LIS was first described by Jessner and Kanof in 1953 as a cutaneous disease characterized by asymptomatic or -rarely- itching erythematous papules and/or papulonodules, sometimes grouped with an arciform disposition located on the face or upper back. LIS heals without scarring and shows a benign course and is still regarded as a trivial clinical entity.Citation22,Citation23,Citation61,Citation77–Citation79 Histologic examination of skin lesions shows dense perivascular or periadnexal lymphocyte infiltration (“sleeve like”) under a usually normal or only slight modified epidermis.Citation22,Citation80 Most clinical and histological findings of LIS correlate highly with LET. One differentiating histologic feature is the presence of higher amounts of mucin dispositions in LET compared to LIS. However, this differentiation feature was doubted through an evaluation of 210 LIS cases by a group of French dermatopathologists.Citation20 Similar doubts have arisen regarding the ambiguous photosensitivity of LIS in contrast to the well-known photosensitivity of LET. Weber et al could show high reaction rates of LIS patients after photoprovocation with UV-light, similar to these described for LET patients, concluding that LIS may be a photosensitive CLE variant.Citation21 Moreover, both entities show analogical responses to suggested treatments, such as topical steroids and systemic antimalarials.Citation81
With increasing awareness of LET due to its better characterisation, physicians more frequently diagnose LET instead of lymphocytic infiltration, a fact reflected in the more recent published literature. It is therefore conceivable that the term LET will gradually replace the term LIS.
Diagnostic and further workout suggestions
The recognition of consistent clinical manifestations of the skin (erythematous, infiltrated plaques without epidermal alterations) of sun exposed areas should give rise to a suspicion of LET. The diagnosis can be established through routine examination of a 4-mm punch biopsy (hematoxylin and eosin stain), where the well-described criteria of perivascular and periadnexal lymphocytic infiltration with interstitial mucin disposition supports the diagnosis. In case of atypical clinical or histological manifestations, standardised photoprovocation can also support the diagnosis. Furthermore, phototesting could be a useful diagnostic tool in asymptomatic patients (at the time of presentation) with a medical history indicative for LET. In extensive and atypical cases, the rapid and effective responsiveness to antimalarials is a further criterion which is indicative for LET. However, as previously discussed, not all patients respond to antimalarials and in these cases the negative influence of smoking should be taken into consideration.Citation25,Citation26,Citation51,Citation53
Given the rare association with SLE, patients should be questioned for potential symptoms from other systems and be appropriately physically examined. Evaluation of full blood count, urine analysis for proteinuria and blood cell casts, and testing for ANA and -if positive- a search for antibodies against ENA and ds-DNA are also suggested.
Treatment ()
General measures
Affected patients should be reassured about the benign nature of the condition and its rare correlation with SLE. Because of the association of their disease with UV light and smoking, LET patients are advised to regularly use sunscreens with high protection against UVA and UVB light (LSF ≥30), as well as to join smoking cessation programs.Citation25,Citation45,Citation53 The elimination of photosensitizing drugs should be also considered, especially in refractory disease, as well as vitamin D supplementation, since vitamin D deficiency can follow lack of sun exposure, as suggested by the S2 guidelines for the treatment of CLE.Citation82
Table 4 Lupus erythematosus tumidus treatment options
Although singular LET lesions are frequently self-limiting, there is a high relapse rate.Citation16 Singular lesions responding quickly to topical therapies may not need any further treatment. However, the experience of the authors from several tertiary referral centres in Germany indicates that a systemic treatment is frequently used in patients with LET. Similar experience has been reported in the EUSCLE registry (73.8% of the LET patients included were treated systemically at least once).Citation26 The decision for initiating systemic treatment has to be taken respecting the needs of the patient.
Topical corticosteroids
Topical corticosteroids are the first line topical anti-inflammatory therapy for LET. They are applied twice a day for one to two weeks and, depending on their efficacy, they are tapered in the following two weeks. In the retrospective evaluation of the 65 included patients of the EUSCLE study, treatment with topical corticosteroids was successful in 33 of the 38 patients.Citation26 High or very high potency corticosteroids may be more effective, however scientific data supporting this is limited.Citation37,Citation40,Citation83 In facial involvement, lower potency agents are preferred because of their smaller risk for side effects, such as atrophy of the skin and induction of telangiectasias. The efficacy of topical corticosteroids is observed within about 1–2 weeks, but relapses after tapering the dose are common. LET patients who do not respond to topical corticosteroids after two to four weeks should be considered for alternative treatments, most commonly systemic antimalarials. Furthermore, in cases of good efficacy but relapsing disease or multiple lesions, intermittent courses of topical corticosteroid treatment could be considered, although alternative treatments should be preferred in cases where frequent use is required.
Topical calcineurin inhibitors
Topical calcineurin inhibitors (including 0.03% and 0.1% tacrolimus ointment and 1% pimecrolimus cream) are licenced for the treatment of patients with atopic eczema but are frequently used as steroid sparing agents for other indications because of their anti-inflammatory effects and their positive side effect profile (no proven chronic cutaneous side effects). Only a few patients with LET have been included and treated with such agents in prospective studies, showing promising results.Citation84,Citation85 They can be used twice a day (use of an occlusive dressing may enhance their efficacy) until a response is noticeable (generally up to four weeks). However, they are less commonly used (18.5% vs 69.2%) and much less effective (36.4% vs 86.8%) than topical corticosteroids.Citation26
Pulsed dye lasers
The treatment of LET with pulsed dye lasers (PDL) has been evaluated in a monocentric prospective study. All of the ten patients showed clinical improvement. However, relapses were not prevented, and new lesions developed in 50% of the patients so that PDL is not considered as a treatment option for patients with LET.
Systemic treatments
Systemic antimalarials
Systemic treatment with antimalarials is the established cornerstone in the treatment of CLE and SLE and the first-line systemic therapy for LET. Their favorable efficacy-side effect profile makes the decision for a systemic therapy easier, especially in frequently relapsing or refractory to topical treatments disease.Citation86 A recent meta-analysis of all studies including treatments of CLE patients with antimalarials reported 145 courses of antimalarials in LET patients and an overall response rate of 68%.Citation51
The most widely used antimalarials are hydroxychloroquine (HCQ) and chloroquine (CQ), with a typical dose of 200–400 mg/day (up to 5 mg/kg real bodyweight) and 125–250 mg/day (up to 2.3 mg/kg real bodyweight) respectively. Higher doses are not recommended since HCQ and CQ can cause irreversible retinal toxicity. Patients should be screened at the beginning of the treatment and regularly thereafter.Citation87,Citation88 Further but rare side effects include maculopapular rash, gastrointestinal upset, hemolytic anemia -especially in case of glucose-6-phosphate-dehydrogenase deficiency (G6PD)- and blue-gray discoloration of the skin or the mucous membranes, which may be permanent. HCQ has a somewhat superior side effect profile and since it could be more easily dosed depending on the patient’s bodyweight, HCQ is more commonly prescribed than CQ.Citation89
Combination therapy with mepacrine (quinacrine) in a dose of 50–100 mg/day is suggested if disease control cannot be reached with the sole use of HCQ or CQ. Mepacrine is a further antimalarial but it does not induce retinal toxicity and therefore it can be used as a combination therapy with HCQ or CQ, or as a monotherapy in case HCQ or CQ are contraindicated. Mepacrine-specific side effects include a reversible yellow discoloration of the skin and sclera and the very rare -but serious- induction of aplastic anemia. Since mepacrine is not available in many countries, there can be problems in importing and reimbursing treatment costs.Citation82
Systemic corticosteroids
The use of systemic corticosteroids in the treatment of LET is uncommon because of their well-known side effects. Steroid pulse therapy tapered and discontinued within 4–8 weeks could be used for extensive, exacerbated disease. In the opinion of the authors, low doses of prednisolone tapered to a maximum dose of 5–7.5 mg/day could have a significantly positive impact on patients with highly photosensitive LET who are non-responsive to topical therapies and antimalarials. One reason for choosing this treatment regimen is that other second-line systemic treatments are neither more efficient nor have a more favorable side effect profile.
Second-line systemic treatments
Data regarding the efficacy of all systemic agents used as second-line treatment of CLE are lacking in terms of therapy for LET. Such treatments include methotrexate, retinoids (acitretin), dapsone, mycophenolate mofetil, thalidomide, all of which are preferably used in combination with antimalarials.Citation82 Recently, Kreuter et al reported a patient with generalized and refractory LET who was successfully treated with the anti-CD20 monoclonal antibody rituximab, a rather counterintuitive approach since B-lymphocytes targeted through rituximab are not recognized as important players in the immunologic response in LET.Citation90
Conclusions
LET is a rare and highly photosensitive form of CLE with a high association with smoking. It is regarded as the most benign form of CLE, because it only rarely correlates with systemic autoimmune disease (especially SLE) and does not induce skin damage (scarring or dyspigmentations). Sunscreens, topical corticosteroids and systemic antimalarials are the most common and most frequently highly effective therapeutic measures. Increasing awareness of the clinical course and histologic picture of LET may lead to even higher diagnostic rates and better management of this previously neglected disease.
Disclosure
The authors report no conflicts of interest in this work.
References
- Hoffmann E. Demonstrationen: lupus erythematodes tumidus. Derm Zeitschr. 1909;16:159–160.
- Gougerot H, Burnier R. Lupus érythémateux tumidus. Bull Soc Fr Dermatol Syphil. 1930;37:1219–1292.
- Rockl H. [Erythematodes tumidus; a case history with reference to the problem of erythematodic lymphocytoma.]. Hautarzt. 1954;5:422–423.13221143
- Kuhn A, Bein D, Bonsmann G. The 100th anniversary of lupus erythematosus tumidus. Autoimmun Rev. 2009;8(6):441–448. doi:10.1016/j.autrev.2008.12.01019162244
- Lehmann P, Hölzle E, Kind P, Goerz G, Plewig G. Experimental reproduction of skin lesions in lupus erythematosus by UVA and UVB radiation. J Am Acad Dermatol. 1990;22(2):181–187.2179293
- Kuhn A, Sonntag M, Richter-Hintz D, et al. Phototesting in lupus erythematosus tumidus—review of 60 patients. Photochem Photobiol. 2001;73(5):532. doi:10.1562/0031-8655(2001)073<0532:PILETR>2.0.CO;211367576
- Kuhn A, Sonntag M, Richter-Hintz D, et al. Phototesting in lupus erythematosus: a 15-year experience. J Am Acad Dermatol. 2001;45(1):86–95. doi:10.1067/mjd.2001.11458911423840
- Kuhn A, Sonntag M, Ruzicka T, Lehmann P, Megahed M. Histopathologic findings in lupus erythematosus tumidus: review of 80 patients. J Am Acad Dermatol. 2003;48(6):901–908. doi:10.1067/mjd.2003.43512789183
- Alexiades-Armenakas MR, Baldassano M, Bince B, et al. Tumid lupus erythematosus: criteria for classification with immunohistochemical analysis. Arthritis Rheum. 2003;49(4):494–500. doi:10.1002/art.1120612910555
- Rodriguez-Caruncho C, Bielsa I, Fernández-Figueras MT, Roca J, Carrascosa JM, Ferrándiz C. Lupus erythematosus tumidus: a clinical and histological study of 25 cases. Lupus. 2015;24(7):751–755. doi:10.1177/096120331456020425413356
- Obermoser G, Sontheimer RD, Zelger B. Overview of common, rare and atypical manifestations of cutaneous lupus erythematosus and histopathological correlates. Lupus. 2010;19(9):1050–1070. doi:10.1177/096120331037004820693199
- Kuhn A, Ruzicka T. Classification of cutaneous lupus erythematosus In: Kuhn A, Lehmann P, Ruzicka T, editors. Cutaneous Lupus Erythematosus. New York: Springer; 2005:53–57.
- Callen JP. Clinically relevant information about cutaneous lupus erythematosus. Arch Dermatol. 2009;145(3):316–319. doi:10.1001/archdermatol.2008.58219289766
- Maize JCJ, Costner M. Tumid lupus erythematosus: a form of lupus erythematosus. Arch Dermatol. 2010;146(4):451 author reply 450-1. doi:10.1001/archdermatol.2010.15620404245
- Callen JP. Tumid lupus erythematosus: a form of lupus erythematosus - reply. Arch Dermatol. 2010;146(4):451–452. doi:10.1001/archdermatol.2010.5420404245
- Schmitt V, Meuth AM, Amler S, et al. Lupus erythematosus tumidus is a separate subtype of cutaneous lupus erythematosus. Br J Dermatol. 2010;162(1):64–73. doi:10.1111/j.1365-2133.2009.09401.x19712116
- Mutasim D. Lupus erythematosus tumidus as a separate subtype of cutaneous lupus erythematosus. Br J Dermatol. 2010;163(1):230 author reply 231-2.20408831
- Callen JP. Cutaneous lupus erythematosus: reflecting on practice-changing observations over the past 50 years. Clin Dermatol. 2018;36(4):442–449. doi:10.1016/j.clindermatol.2018.04.00230047428
- Petri M, Orbai AM, Alarcõn GS, et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677–2686. doi:10.1002/art.3447322553077
- Lipsker D, Mitschler A, Grosshans E, Cribier B. Could Jessner’s lymphocytic infiltrate of the skin be a dermal variant of lupus erythematosus? An analysis of 210 cases. Dermatology. 2006;213(1):15–22. doi:10.1159/00009283216778421
- Weber F, Schmuth M, Fritsch P, Sepp N. Lymphocytic infiltration of the skin is a photosensitive variant of lupus erythematosus: evidence by phototesting. Br J Dermatol. 2001;144(2):292–296. doi:10.1046/j.1365-2133.2001.04017.x11251561
- Rémy-Leroux V, Léonard F, Lambert D, et al. Comparison of histopathologic-clinical characteristics of Jessner’s lymphocytic infiltration of the skin and lupus erythematosus tumidus: multicenter study of 46 cases. J Am Acad Dermatol. 2008;58(2):217–223. doi:10.1016/j.jaad.2007.09.03918083273
- Kim IS, Kim BR, Youn SW. Differentiation of Jessner’s lymphocytic infiltration of the skin from various chronic cutaneous lupus erythematosus subtypes by quantitative computer-aided image analysis. Dermatology. 2016;232(1):57–63. doi:10.1159/00044064826458204
- Grönhagen CM, Fored CM, Granath F, Nyberg F. Cutaneous lupus erythematosus and the association with systemic lupus erythematosus: a population-based cohort of 1088 patients in Sweden. Br J Dermatol. 2011;164(6):1335–1341. doi:10.1111/j.1365-2133.2011.10272.x21574972
- Kuhn A, Sigges J, Biazar C, et al. Influence of smoking on disease severity and antimalarial therapy in cutaneous lupus erythematosus: analysis of 1002 patients from the EUSCLE database. Br J Dermatol. 2014;171(3):571–579. doi:10.1111/bjd.1300624673427
- Sigges J, Biazar C, Landmann A, et al. Therapeutic strategies evaluated by the European Society of Cutaneous Lupus Erythematosus (EUSCLE) core set questionnaire in more than 1000 patients with cutaneous lupus erythematosus. Autoimmun Rev. 2013;12(7):694–702. doi:10.1016/j.autrev.2012.10.00523220353
- Biazar C, Sigges J, Patsinakidis N, et al. Cutaneous lupus erythematosus: first multicenter database analysis of 1002 patients from the European Society of Cutaneous Lupus Erythematosus (EUSCLE). Autoimmun Rev. 2013;12(3):444–454. doi:10.1016/j.autrev.2012.08.01923000206
- Patsinakidis N, Gambichler T, Lahner N, Moellenhoff K, Kreuter A. Cutaneous characteristics and association with antinuclear antibodies in 402 patients with different subtypes of lupus erythematosus. J Eur Acad Dermatol Venereol. 2016;30(12):2097–2104. doi:10.1111/jdv.1376927431977
- Verdelli A, Coi A, Marzano AV, et al. Autoantibody profile and clinical patterns in 619 Italian patients with cutaneous lupus erythematosus. J Eur Acad Dermatol Venereol. 2019;33(4):742–752. doi:10.1111/jdv.15147.29924416
- Stead J, Headley C, Ioffreda M, Kovarik C, Werth V. Coexistence of tumid lupus erythematosus with systemic lupus erythematosus and discoid lupus erythematosus: a report of two cases of tumid lupus. J Clin Rheumatol. 2008;14(6):338–341. doi:10.1097/RHU.0b013e31817d118318664992
- Jolly M, Laumann AE, Shea CR, Utset TO. Lupus erythematosus tumidus in systemic lupus erythematosus: novel association and possible role of early treatment in prevention of discoid lupus erythematosus. Lupus. 2004;13(1):64–69. doi:10.1191/0961203304lu473cr14870920
- Rupec RA, Petropoulou T, Belohradsky BH, et al. Lupus erythematosus tumidus and chronic discoid lupus erythematosus in carriers of X-linked chronic granulomatous disease. Eur J Dermatol. 2000;10(3):184–189.10725815
- Chen X, Wang S, Li L. A case report of lupus erythematosus tumidus converted from discoid lupus erythematosus. Medicine (Baltimore). 2018;97(16):e0375. doi:10.1097/MD.000000000001037529668590
- Ruiz H, Sánchez JL. Tumid lupus erythematosus. Am J Dermatopathol. 1999;21(4):356–360.10446777
- Lehrhoff S, Tzu J, Patel R, Sanchez M, Franks A. Lupus erythematosus tumidus with discoid lupus erythematosus-induced alopecia of the scalp. Dermatol Online J. 2011;17:10.
- Wozniacka A, Robak E, McCauliffe DP, Sysa-Jedrzejowska A. The evolution of multiple forms of cutaneous lupus erythematosus in the same patient over time. J Eur Acad Dermatol Venereol. 2008;22(10):1237–1239. doi:10.1111/j.1468-3083.2007.02594.x18826552
- Choonhakarn C, Poonsriaram A, Chaivoramukul J. Lupus erythematosus tumidus. Int J Dermatol. 2004;43(11):815–818. doi:10.1111/j.1365-4632.2004.02073.x15533063
- Reich A, Marcinow K, Bialynicki-Birula R. The lupus band test in systemic lupus erythematosus patients. Ther Clin Risk Manag. 2011;7(1):27–32. doi:10.2147/TCRM.S1014521339940
- Cozzani E, Christana K, Rongioletti F, Rebora A, Parodi A. Lupus erythematosus tumidus: clinical, histopathological and serological aspects and therapy response of 21 patients. Eur J Dermatol. 2010;20(6):797–801.21030340
- Vieira V, Del Pozo J, Yebra-Pimentel MT, Martínez W, Fonseca E. Lupus erythematosus tumidus: a series of 26 cases. Int J Dermatol. 2006;45(5):512–517. doi:10.1111/j.1365-4632.2004.02574.x16700782
- Willemze R, Dijkstra A, Meijer CJLM. Lymphocytic infiltration of the skin (Jessner): a T‐cell lymphoproliferative disease. Br J Dermatol. 1984;110(5):523–529. doi:10.1111/j.1365-2133.1984.tb04674.x6372848
- Cerroni L. Pseudolymphomas of the skin In: Cerroni L, editor. Skin Lymphoma. 4th ed. Oxford, UK: Wiley Blackwell; 2014:374–375.
- Pereira A, Ferrara G, Calamaro P, et al. The histopathological spectrum of pseudolymphomatous infiltrates in cutaneous lupus erythematosus. Am J Dermatopathol. 2018;40(4):247–253. doi:10.1097/DAD.000000000000094228654469
- Privette ED, Werth VP. Update on pathogenesis and treatment of CLE. Curr Opin Rheumatol. 2013;25(5):584–590. doi:10.1097/BOR.0b013e32836437ba23872903
- Patsinakidis N, Wenzel J, Landmann A, et al. Suppression of UV-induced damage by a liposomal sunscreen: a prospective, open-label study in patients with cutaneous lupus erythematosus and healthy controls. Exp Dermatol. 2012;21(12):958–961. doi:10.1111/exd.1203523171460
- Kuhn A, Wozniacka A, Szepietowski JC, et al. Photoprovocation in cutaneous lupus erythematosus: a multicenter study evaluating a standardized protocol. J Invest Dermatol. 2011;131(8):1622–1630. doi:10.1038/jid.2011.10121593767
- Ruland V, Haust M, Stilling RM, et al. Updated analysis of standardized photoprovocation in patients with cutaneous lupus erythematosus. Arthritis Care Res. 2013;65(5):767–776. doi:10.1002/acr.21867
- Placzek M, Kerkmann U, Bell S, Koepke P, Przybilla B. Tobacco smoke is phototoxic. Br J Dermatol. 2004;150(5):991–993. doi:10.1111/j.1365-2133.2004.05818.x15149514
- Mentens G, Lambert J, Nijsten T. Polymorphic light eruption may be associated with cigarette smoking and alcohol consumption. Photodermatol Photoimmunol Photomed. 2006;22(2):87–92. doi:10.1111/j.1600-0781.2006.00204.x16606413
- Böckle BC, Sepp NT. Smoking is highly associated with discoid lupus erythematosus and lupus erythematosus tumidus: analysis of 405 patients. Lupus. 2015;24(7):669–674. doi:10.1177/096120331455963025411260
- Chasset F, Bouaziz JD, Costedoat-Chalumeau N, Francès C, Arnaud L. Efficacy and comparison of antimalarials in cutaneous lupus erythematosus subtypes: a systematic review and meta-analysis. Br J Dermatol. 2017;177(1):188–196. doi:10.1111/bjd.1531228112801
- Chasset F, Francès C, Barete S, Amoura Z, Arnaud L. Influence of smoking on the efficacy of antimalarials in cutaneous lupus: a meta-analysis of the literature. J Am Acad Dermatol. 2015;72(4):634–639. doi:10.1016/j.jaad.2014.12.02525648824
- Kreuter A, Gaifullina R, Tigges C, Kirschke J, Altmeyer P, Gambichler T. Lupus erythematosus tumidus response to antimalarial treatment in 36 patients with emphasis on smoking. Arch Dermatol. 2009;145(3):244–248. doi:10.1001/archdermatol.2008.59219289751
- Shovman O, Tamar S, Amital H, Watad A, Shoenfeld Y. Diverse patterns of anti-TNF-α-induced lupus: case series and review of the literature. Clin Rheumatol. 2018;37(2):563–568. doi:10.1007/s10067-017-3884-229063464
- Schneider SW, Staender S, Schlüter B, Luger TA, Bonsmann G. Infliximab-induced lupus erythematosus tumidus in a patient with rheumatoid arthritis. Arch Dermatol. 2006;142(1):115–116. doi:10.1001/archderm.142.1.11516415403
- Sohl S, Renner R, Winter U, et al. [Drug-induced lupus erythematosus tumidus during treatment with adalimumab]. Hautarzt. 2009;60(10):826–829. doi:10.1007/s00105-008-1699-419229504
- Chogle AR, Shah CV, Murthy AK. Role of anti-tumor necrosis factor-α blockers in inducing lupus erythematosus tumidus in “rhupus syndrome.”. J Rheumatol. 2011;38(6):1218–1219. doi:10.3899/jrheum.10102021632691
- Brown CW, Deng JS. Thiazide diuretics induce cutaneous lupus-like adverse reaction. Clin Toxicol. 1995;33(6):729–733.
- Bockle BC, Baltaci M, Weyrer W, Sepp NT. Bortezomib-induced lupus erythematosus tumidus. Oncologist. 2009;14(6):637–639. doi:10.1634/theoncologist.2008-019719474161
- Aguayo-Leiva I, Vano-Galvan S, Carrillo-Gijon R, Jaén-Olasolo P. Lupus tumidus induced by bortezomib not requiring discontinuation of the drug. J Eur Acad Dermatol Venereol. 2010;24(11):1363–1364. doi:10.1111/j.1468-3083.2010.03643.x20337821
- Schepis C, Lentini M, Siragusa M, Batolo D. ACE-inhibitor-induced drug eruption resembling lymphocytic infiltration (of Jessner-Kanof) and lupus erythematosus tumidus. Dermatology. 2004;208(4):354–355. doi:10.1159/00007784915178923
- Chamberlain AJ, Hollowood K, Turner RJ, Byren I. Tumid lupus erythematosus occurring following highly active antiretroviral therapy for HIV infection: a manifestation of immune restoration. J Am Acad Dermatol. 2004;51(5SUPPL). doi:10.1016/j.jaad.2004.04.020
- Guarneri C, Lentini M, Polimeni G, Giuffrida R, Cannavò SP. Ustekinumab-induced drug eruption resembling lymphocytic infiltration (of Jessner-Kanof) and lupus erythematosus tumidus. Br J Clin Pharmacol. 2016;81(4):792–794. doi:10.1111/bcp.1283726616890
- Prieto-Barrios M, Rodriguez-Peralto J, Vico-Alonso C, et al. Lupus-like injection-site reaction to ixekizumab: report of two cases. Indian J Dermatol Venereol Leprol. 2018;84(5):610–613. doi:10.4103/ijdvl.IJDVL_786_1730073991
- Zandman-Goddard G, Solomon M, Barzilai A, Shoenfeld Y. Lupus erythematosus tumidus induced by sex reassignment surgery. J Rheumatol. 2007;34(9):1938–1940.17696264
- Kunz M, Konig IR, Schillert A, et al. Genome-wide association study identifies new susceptibility loci for cutaneous lupus erythematosus. Exp Dermatol. 2015;24(7):510–515. doi:10.1111/exd.1270825827949
- Wenzel J, Zahn S, Tüting T. Pathogenesis of cutaneous lupus erythematosus: common and different features in distinct subsets. Lupus. 2010;19(9):1020–1028. doi:10.1177/096120331037004620693195
- Obermoser G, Schwingshackl P, Weber F, et al. Recruitment of plasmacytoid dendritic cells in ultraviolet irradiation-induced lupus erythematosus tumidus. Br J Dermatol. 2009;160(1):197–200. doi:10.1111/j.1365-2133.2008.08873.x
- Zahn S, Graef M, Patsinakidis N, et al. Ultraviolet light protection by a sunscreen prevents interferon-driven skin inflammation in cutaneous lupus erythematosus. Exp Dermatol. 2014;23(7):516–518. doi:10.1111/exd.1242824758584
- Gambichler T, Pätzholz J, Schmitz L, Lahner N, Kreuter A. FOXP3+ and CD39+ regulatory T cells in subtypes of cutaneous lupus erythematosus. J Eur Acad Dermatol Venereol. 2015;29(10):1972–1977. doi:10.1111/jdv.1312325808110
- Gambichler T, Genc Z, Skrygan M, et al. Cytokine and chemokine ligand expression in cutaneous lupus erythematosus. Eur J Dermatol. 2012;22(3):319–323.22562806
- Lembo S, Raimondo A. Polymorphic light eruption: what’s new in pathogenesis and management. Front Med. 2018;5(5):252. doi:10.3389/fmed.2018.00252
- Dawe RS. Polymorphic Light Eruption. In: Katsambas AD, Lotti TM, Dessinioti C, D’Erme AM, editors. European Handbook of Dermatological Treatments. Berlin, Heidelberg: Springer; 2015;757–761.
- Thareja S, Paghdal K, Lien MH, Fenske NA. Reticular erythematous mucinosis - a review. Int J Dermatol. 2012;51(8):903–909. doi:10.1111/j.1365-4632.2011.05292.x22788804
- Cinotti E, Merlo V, Kempf W, et al. Reticular erythematous mucinosis: histopathological and immunohistochemical features of 25 patients compared with 25 cases of lupus erythematosus tumidus. J Eur Acad Dermatol Venereol. 2015;29(4):689–697. doi:10.1111/jdv.1265425087914
- Rongioletti F, Merlo V, Riva S, et al. Reticular erythematous mucinosis: a review of patients’ characteristics, associated conditions, therapy and outcome in 25 cases. Br J Dermatol. 2013;169(6):1207–1211. doi:10.1111/bjd.1257723937648
- Kaatz M, Zelger B, Norgauer J, Ziemer M. Lymphocytic infiltration (Jessner-Kanof): lupus erythematosus tumidus or a manifestation of borreliosis? [10]. Br J Dermatol. 2007;157(2):403–405. doi:10.1111/j.1365-2133.2007.07997.x17573889
- Weyers W, Bonczkowitz M, Weyers I, Weyers W, Bonczkowitz M, Weyers I. LE or not LE-that is the question: an unsuccessful attempt to separate lymphocytic infiltration from the spectrum of discoid lupus erythematosus. Am J Dermatopathol. 1998;20(3):225–232.9650693
- Sparsa L, Afif N, Goetz J, et al. Jessner-Kanof disease induced by leflunomide: a dermal variant of cutaneous lupus? Rheumatol Int. 2011;31(2):255–258. doi:10.1007/s00296-009-1169-z19823837
- Akasu R, Kahn HJ, From L. Lymphocyte markers on formalin‐fixed tissue in Jessner’s lymphocytic infiltrate and lupus erythematosus. J Cutan Pathol. 1992;19(1):59–65.1556268
- Wuyts L, Dandelooy J, Siozopolou V, Lambert J, Aerts O. Mepacrine as successful monotherapy for refractory Jessner–kanof disease: still an important drug in the dermatologic armamentarium. J Dermatolog Treat. 2017;28(3):276–278. doi:10.1080/09546634.2016.121466827686749
- Kuhn A, Aberer E, Bata-Csörgő Z, et al. S2k guideline for treatment of cutaneous lupus erythematosus – guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV). J Eur Acad Dermatol Venereol. 2017;31(3):389–404. doi:10.1111/jdv.1405327859683
- Kuhn A, Richter-Hintz D, Oslislo C, Ruzicka T, Megahed M, Lehmann P. Lupus erythematosus tumidus A neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136(8):1033–1041.10926740
- Kreuter A, Gambichler T, Breuckmann F, et al. Pimecrolimus 1% cream for cutaneous lupus erythematosus. J Am Acad Dermatol. 2004;51(3):407–410. doi:10.1016/j.jaad.2004.01.04415337984
- Kuhn A, Gensch K, Haust M, et al. Efficacy of tacrolimus 0.1% ointment in cutaneous lupus erythematosus: a multicenter, randomized, double-blind, vehicle-controlled trial. J Am Acad Dermatol. 2011;65(1):54–64. doi:10.1016/j.jaad.2010.03.03721501887
- Ochsendorf F. Use of antimalarials in dermatology. J Der Dtsch Dermatologischen Gesellschaft. 2010;8:829–844.
- Marmor MF, Kellner U, Lai TYY, Melles RB, Mieler WF, Lum F. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016;123(6):1386–1394. doi:10.1016/j.ophtha.2016.01.05826992838
- Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014;132(12):1453–1460. doi:10.1001/jamaophthalmol.2014.345925275721
- Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis. 2010;69(1):20–28. doi:10.1136/ard.2008.10176619103632
- Kreuter A, Tigges C, Hunzelmann N, Oellig F, Lehmann P, Hofmann SC. Rituximab in the treatment of recalcitrant generalized lupus erythematosus tumidus. JDDG - J Ger Soc Dermatol. 2017;15(7):729–731.
