Abstract
Background
Rosacea is a chronic facial skin disorder characterized by inflammation and vascular abnormalities. The pathophysiology of rosacea involves increased activation of the capsaicin receptor, TRPV1, the vascular endothelial growth factor (VEGF) pathway, and cathelicidin LL-37, MMP-9, and KLKs. We evaluated the activity of four compounds (dextran sulfate, 4-t-butylcyclohexanol [BCH; TRP-regulin®], pongamia oil, and hesperidin methyl chalcone [HMC]) on inflammatory and vascular responses implicated in rosacea.
Materials and methods
The anti-inflammatory activity of dextran sulfate was evaluated on PGE2 production after PMA stimulation of NCTC-2544 keratinocytes, and on normal human epidermal keratinocytes (NHEKs) after proinflammatory stimulation to mimic a rosacea environment. The anti-angiogenic activity of dextran sulfate was measured by analyzing pseudotube formation in co-cultured human microvascular endothelial cells/normal human dermal fibroblasts. HMC modulation of vascular responses and IL-8 cytokine production after SP stimulation was evaluated in human skin explants. We also assessed the effect of BCH on TRPV1 activation, and the effect of combined BCH and pongamia oil on the inflammatory response of NHEKs.
Results
Dextran sulfate strongly and significantly inhibited PMA-induced PGE2 production, inhibited KLK5 and MMP-9 mRNA expression, and IL-8, IL-1α and VEGF production, and displayed a highly significant inhibitory effect on VEGF-induced pseudotube formation. In SP-stimulated human skin explants, HMC significantly decreased the proportion of dilated vessels, total vessel area, and IL-8 production. BCH significantly and dose-dependently inhibited TRPV1 activation, and BCH and pongamia oil inhibited CXCL1 and CXCL6 mRNA expression and IL-8 production in NHEKs. Combined BCH/pongamia oil inhibited IL-8 production synergistically.
Conclusion
These in vitro results showed that dextran sulfate, BCH, pongamia oil and HMC, possess complementary soothing and anti-redness properties, supporting their combination in Avène redness-relief cosmetic products for sensitive skin prone to redness, and for topical adjunctive rosacea treatment.
Introduction
Rosacea, a chronic skin disorder with poorly understood etiology, is characterized by inflammation and vascular abnormalities of the facial skin. Rosacea skin biopsies show perivascular infiltrates associated with vasodilation and increased activation of the capsaicin receptor, transient receptor potential vanilloid 1 (TRPV1).Citation1 Vascular endothelial growth factor (VEGF), a vasoactive and inflammatory factor, and the VEGF receptor, have also been shown to be expressed in rosacea skin biopsies. The endothelium expresses both VEGF receptors (VEGF R1 and VEGF R2). Although VEGF is present in the epidermis and epithelium, it is not expressed by the endothelium but rather by infiltrating immune cells such as lymphocytes and macrophages. Therefore, the VEGF pathway may contribute to the vascular changes and immune infiltration that are observed in rosacea.Citation2
Molecular studies also show a common link between the triggers of rosacea and the cellular response, suggesting that an altered innate immune response is involved in disease pathogenesis.Citation3 Rosacea skin is susceptible to environmental changes, altered hormone balance, and microbe challenges because of increased toll-like receptor 2 (TLR2).Citation3 The activation of TLR2 induces an increase in effector molecules: cathelicidin antimicrobial peptide (CAMP) and kallikrein 5 (KLK5).Citation3 Elevated KLK5 results in the generation of active peptides such as LL-37, which stimulates vascular changes and inflammatory cell recruitment.Citation3,Citation4 The matrix metalloproteinases (MMPs) MMP-2 and MMP-9 are also increased in rosacea skin.Citation5 In this pathology, proinflammatory cytokines trigger the release of MMPs, especially MMP-1, -3, and -9, leading to the degradation of extracellular matrix components,Citation6 and inflammatory damage in the form of papulopustular lesions.Citation7 Moreover, MMP has a role in LL-37 activation by activating KLKs.Citation8
The aim of this study was to assess the effectiveness of different active ingredients incorporated into the Avène range of redness-relief products dedicated to skin which is prone to redness and rosacea. Thus, dextran sulfate, 4-t-butylcyclohexanol (BCH; TRP-regulin®), pongamia oil and hesperidin methyl chalcone (HMC) were evaluated on the inflammatory and vascular responses implicated in rosacea.
Materials and methods
Cells and cell culture
The NCTC-2544 human keratinocyte cell line was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and grown on DMEM supplemented with 10% fetal calf serum (FCS).
Normal human epidermal keratinocytes (NHEKs) were produced from skin explants (abdominoplasty or breast reduction, obtained with written and informed patient consent). NHEKs were grown in Keratinocyte Serum-Free Growth Medium (Gibco®, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with bovine pituitary extract and epidermal growth factor (Gibco).
Human microvascular endothelial cells (HMVECs) or normal human dermal fibroblasts (NHDFs) were grown in co-culture medium: Endothelial Cell Basal Medium 2 and DMEM supplemented with 1% FCS.
Prostaglandin E2 (PGE2) production
The keratinocyte cell line NCTC-2544 was stimulated with phorbol-12-myristate-13-acetate (PMA; 0.1 µg/mL) for 24 hours. Dextran sulfate (0.2 and 2 mg/mL) was pre-incubated with the cells for 24 hours before PMA stimulation. Indomethacin (1 µM) was used as a positive control. Prostaglandin E2 (PGE2) production (a marker for inflammation) was analyzed in culture supernatants by enzyme-linked immunosorbent assay (ELISA) quantification. Results were expressed as absolute quantity of PGE2, and as the percentage of inhibition to the stimulated condition.
NHEK rosacea model: ELISA and mRNA expression
NHEKs were exposed for 1 hour with dextran sulfate 10 µg/mL (for IL-8, IL-1α, KLK5, and MMP-9 experiments) or 4, 13 and 40 µg/mL (for VEGF experiments), or the positive control I kappa B kinase (IKK) inhibitor (10 µM; a specific NF-κB inhibitor), then stimulated for 24 hours with a proinflammatory stimulus to mimic a rosacea-like environment (LL37 [3 µM], FSL1 [0.3 µg/mL], TNF-α [3 ng/mL]). The culture supernatants were removed, centrifuged, and then frozen at −20°C and VEGF, IL-8 and IL-1α were quantified by ELISA (DuoSet Kit; R&D Systems, Lille, France) according to the manufacturer’s instructions. To assess the effects of dextran sulfate on KLK5 and MMP-9 expression, cells were also harvested for mRNA extraction. RNA was extracted with the Qiacube (Qiagen NV, Venlo, the Netherlands), according to the supplier’s instructions. Total RNA was converted into complementary DNA (cDNA) with the SuperScript VILO cDNA synthesis kit (Thermo Fisher Scientific), according to the manufacturer’s instructions. The cDNA was then used for real-time quantitative PCR, according to the instructions provided by the manufacturer. Relative quantities (RQs) were calculated using Expression Suite software and with respect to the control. Regulation of the expression of the gene of interest was taken into account on the basis of an RQ ≥2 (induction) or an RQ ≤0.5 (inhibition). RQ was 1 for non-stressed cells.
Using the same methodology, the anti-inflammatory response of BCH (300 µM, corresponding to 47 µg/mL) and/or pongamia oil (10 and 20 μg/mL) was also evaluated in NHEKs exposed to a rosacea environment for 24 hours. Cells were harvested for IL-8, CXCL1, and CXCL6 mRNA analysis expression. Culture supernatants were also collected and IL-8 was quantified by ELISA.
Pseudotube formation
The HMVEC/NHDF co-culture was seeded in 96-well plates in co-culture medium and incubated for 24 hours. The medium was then removed and replaced by co-culture medium containing, or not (control), dextran sulfate (10, 30, and 100 µg/mL) or the positive reference (suramin 100 µM) and then the cells were stimulated with VEGF (100 ng/mL). In parallel, a non-stimulated control was performed. Cells were incubated for 7 days with treatment renewal after 72 hours of incubation. After incubation, the co-culture medium was discarded and the cells were rinsed, fixed, permeabilized, and labeled using an anti-collagen IV primary antibody. The primary antibody was then revealed using an appropriate fluorescent secondary antibody (GAR-Alexa 568), and the cell nuclei were stained in parallel using Hoechst 33,258 solution (bis-benzimide). The formation of pseudotubes was observed using a NIKON Diaphot 300 microscope (objective lens ×4). Images were captured using a NIKON DS-Fi1 camera and NIS-Elements 4.13.04 software. The analysis of pseudotube formation was performed through collagen IV labeling using Image J software. The percentage inhibition of VEGF-induced pseudotube formation was calculated using the mean of the pseudotube area (mm2) in the different conditions.
Transient receptor potential cation channel subfamily V member 1 (TRPV1) antagonist activity
TRPV1 antagonist activity (BCH 10, 30, and 100 µg/mL) was analyzed on CHO human recombinant cells after 30 minutes of stimulation with capsaicin (30 nM). Intracellular calcium was measured by fluorimetry and the percentage inhibition of control agonist response was calculated.
Vascular response induced by substance P (SP) in a normal human skin model
Fragments of normal human skin were obtained from plastic surgery (eight different donors) and placed in inserts positioned over culture wells, as developed by Boisnic et al.Citation9 The medium used was DMEM containing antibiotics (100 U/mL penicillin and 100 µg/mL streptomycin), 200 µg/mL l-glutamine and growth factors (bovine pituitary extract and FCS). HMC (0.2 mg/mL) and the NK1 inhibitor L-703,606 oxalate (10 µM; positive control inhibitor for SP activation) were diluted in skin model culture medium at Day 0. Compounds were then preincubated for 24 hours. At Day 1, SP (10 µM) and test compounds were added for 24 hours. At Day 2, supernatants were frozen for IL-8 analysis; skin explants were fixed then paraffin-imbedded for histological analysis. After staining with H&E, vascular modulation was evaluated by counting the number of dilated vessels on the entire histological section. Vascular modulation was determined by the proportion of dilated vessels among the total number of vessels counted on the entire histological section (16 fields at 40× magnification). Morphometric analysis of the surface (µm2) occupied by the light of the vessels was performed to determine the average area (µm2) occupied by the vessels in the dermis. The cytokine IL-8 immunoassay was performed with the Gen-Probe kit (Eurobio, Courtaboeuf, France), according to the manufacturer’s instructions. CD34 immunohistochemistry was performed according to standard procedures using CD34 antibody (QBEnd 10; Dako, Agilent Technologies, Santa Clara, CA, USA) and universal labelled streptavidin biotin Kit (Dako).
Statistical analysis
Statistical significance was determined using a one-way analysis of variance (ANOVA) and using Dunnett’s test as the post-test for ELISA assays. Intergroup comparisons were performed by an unpaired Student’s t-test for pseudotube formation and TRPV1 antagonist activity and by a paired Student’s t-test for experiments performed on human skin explants.
Ethics statement
Experiments on human cells and tissues (obtained from surgical waste, generally abdominal or breast surgical reduction) were conducted according to French Ethical Practice and approved by the Ministère de l” Enseignement Supérieur et de la Recherche (CODECOH statement delivery: approval no. DC-2011-1457). In accordance with this ethical statement, patients provided written informed consent.
Results
Anti-inflammatory activity of dextran sulfate
PMA strongly induced PGE2 production and the positive control, indomethacin, completely inhibited PGE2 production (P<0.01). Dextran sulfate (0.2 and 2 mg/mL) strongly and significantly inhibited PMA-induced PGE2 production (68% and 70% inhibition, respectively; both P<0.01 vs PMA-stimulated control cells).
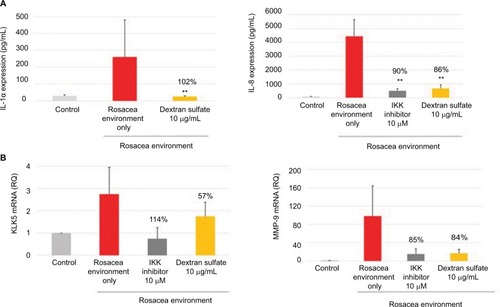
IL-1α and IL-8 production, and KLK5 and MMP-9 mRNA expression, was induced in NHEKs exposed to a rosacea environment for 24 hours. The positive control, IKK inhibitor (10 µM; a specific NF-κB inhibitor), inhibited IL-1α and IL-8 production and KLK5 and MMP-9 mRNA expression induced by the rosacea environment. Dextran sulfate (10 µg/mL) strongly inhibited IL-1α and IL-8 production (), as well as KLK5 and MMP-9 mRNA expression ().
Figure 1 Mean (pg/mL) and percentage inhibition of IL-1α and IL-8 expression (A), and RQ and percentage inhibition of KLK5 and MMP-9 mRNA expression (B) after incubation of NHEK with dextran sulfate for 24 hours in a rosacea environment. IKK inhibitor was used as a positive control.
Abbreviations: RQ, relative quantity; MMP-9, matrix metalloproteinase 9; NHEK, normal human epidermal keratinocyte; IKK, I kappa B kinase.
Anti-redness activities of dextran sulfate
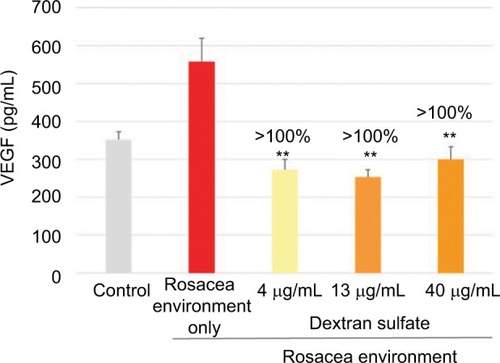
In keratinocytes exposed to a rosacea environment for 24 hours, VEGF expression was induced. At the three concentrations tested (4, 13, and 40 µg/mL), dextran sulfate completely inhibited VEGF production ().
Figure 2 Mean (pg/mL) and percentage inhibition of VEGF expression after incubation of keratinocytes with dextran sulfate for 24 hours in a rosacea environment.
Abbreviation: VEGF, vascular endothelial growth factor.
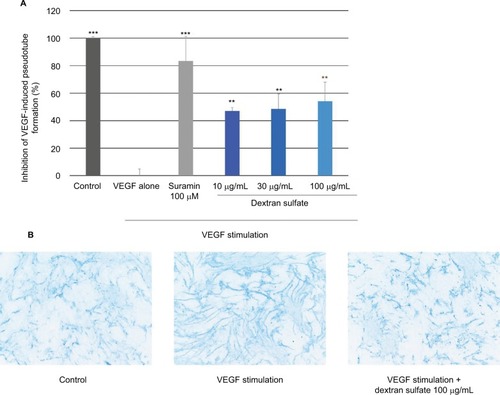
The anti-angiogenic activity of dextran sulfate was assessed by analyzing the formation of pseudotubes on HMVEC/NHDF co-culture. shows that VEGF strongly induced pseudotube formation and, importantly, suramin (100 µM; positive control) inhibited this effect. Dextran sulfate 10, 30, and 100 µg/mL, displayed a highly significant inhibitory effect on VEGF-induced pseudotube formation in the HMVEC/NHDF co-culture (47%, 49%, and 54% inhibition, respectively).
Figure 3 Effect of dextran sulfate on pseudotube formation.
Abbreviation: VEGF, vascular endothelial growth factor.
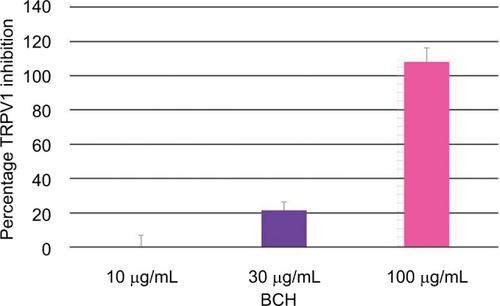
Anti-TRPV1 activity of 4-t-butylcyclohexanol (BCH)
BCH significantly inhibited TRPV1 activation by capsaicin in CHO-TRPV1 recombinant cells, in a dose-dependent manner, with complete inhibition at 100 µg/mL ().
Anti-inflammatory activity of BCH and pongamia oil
As shown previously (), the rosacea environment induced IL-8 production (); in addition, IL-8, CXCL1, and CXCL6 mRNA expressions were increased (). The positive control, IKK inhibitor (10 µM), strongly inhibited this chemokine activity. Moreover, BCH and pongamia oil inhibited the mRNA expression of the chemokines CXCL1, CXCL6, and IL-8 (). In addition, the combination of BCH and pongamia oil demonstrated a synergistic effect on the inhibition of IL-8 production ().
Figure 5 Mean (pg/mL) and percentage inhibition of IL-8 expression after incubation of NHEK with BCH, pongamia oil, or BCH + pongamia oil, for 24 hours in a rosacea environment. IKK inhibitor was used as a positive control.
Abbreviations: IKK, I kappa B kinase; BCH, 4-t-butylcyclohexanol; NHEK, normal human epidermal keratinocyte.
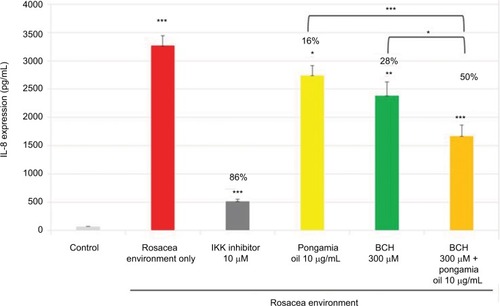
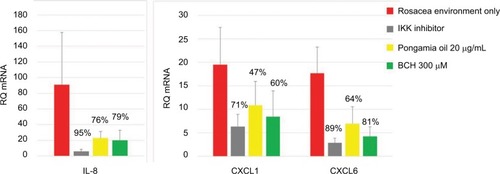
Figure 6 The RQ and percentage inhibition of IL-8, CXCL1, and CXCL6 mRNA expression after incubation of NHEK with BCH or pongamia oil for 24 hours in a rosacea environment. IKK inhibitor was used as a positive control.
Abbreviations: RQ, relative quantity; IKK, I kappa B kinase; BCH, 4-t-butylcyclohexanol; NHEK, normal human epidermal keratinocyte.
Anti-redness activity of hesperidin methyl chalcone
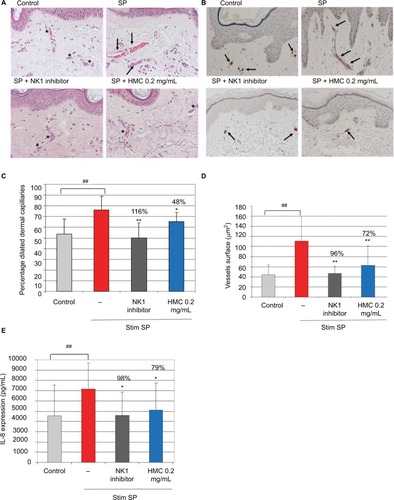
In human skin explants, SP stimulation induced significant vasodilation, an increase in the vessel surface, and increased IL-8 production, relative to the control skin (). The NK1 inhibitor completely inhibited the vasodilation, the vessel surface, and IL-8 production. In this model of SP-stimulated skin, HMC (0.2 mg/mL [0.02%]) significantly decreased the proportion of dilated vessels (48% inhibition), total vessel area (72% inhibition), and IL-8 production (79% inhibition) ().
Figure 7 The activity of HMC in modulating vascular responses and IL-8 cytokine production after SP stimulation in human skin explants. Human skin explants were pre-incubated (or not) with HMC and then stimulated with SP for 24 hours. L-703,606 oxalate (10 µM), an NK1 inhibitor, was used as a positive control.
Abbreviations: HMC, hesperidin methyl chalcone; SP, substance P.
Discussion
Rosacea is a chronic relapsing inflammatory and vascular disease of the facial skin, characterized by flushing, chronic inflammation, and fibrosis.Citation10 The prevalence of rosacea is estimated to range from 2-22%, with a particularly high prevalence in fair-skinned Caucasian adults.Citation11–Citation14 Although the occurrence of rosacea is common, the complex pathophysiology, representing dysregulation of the immune, vascular and nervous systems, is relatively poorly understood.Citation10,Citation15 At the molecular level, aberration of the innate immune response and antimicrobial peptides (eg, CAMP, KLK5, and MMPs) coupled with neurogenic inflammation and vascular hyperreactivity (eg, TRPV, IL-1, IL-8, CXCLs) play key roles in the pathophysiology of rosacea.Citation6,Citation8,Citation15 In addition, VEGF has been identified as an important epidermal marker for reddened skin in rosacea.Citation2
The in vitro experiments reported herein show that dextran sulfate strongly and significantly inhibited PMA-induced PGE2 production. Furthermore, dextran sulfate inhibited cytokine (IL-1α and IL-8) production in a rosacea environment. By inhibiting these different inflammatory markers, dextran sulfate demonstrates potential soothing properties. Moreover, dextran sulfate inhibited the mRNA expression of proteases KLK5 and MMP-9 in keratinocytes exposed to a rosacea environment. By inhibiting KLK5 and MMP-9 mRNA expression, dextran sulfate may also inhibit LL-37 activity, an important cathelicidin that is associated with rosacea.Citation3 Dextran sulfate also inhibited the pro-angiogenic factor VEGF in a rosacea environment and showed a highly significant inhibitory effect on VEGF-induced pseudotube formation in co-cultured HMVEC/NHDF cells. Therefore, dextran sulfate appears to possess interesting soothing and anti-redness properties.
Rosacea is also associated with increased TRPV1 activity.Citation1 Inhibition of this activity by BCH appears to represent an interesting approach to limit rosacea. Our results showed that, individually, BCH and pongamia oil inhibited IL-8 production and CXCL1 and CXCL6 mRNA expression in keratinocytes exposed to a rosacea environment. Moreover, the combination of BCH and pongamia oil demonstrated a synergistic effect on the inhibition of IL-8 production. Therefore, these two compounds possess soothing properties in a rosacea environment.
Finally, we demonstrated that HMC decreased the proportion of dilated vessels, the total vessel area and IL-8 production in a model of SP-stimulated skin. Thus, in this model, HMC inhibited inflammation and vasodilation. HMC is used to treat chronic venous disease (CVD).Citation16,Citation17 The inflammatory response is a key player in the pathophysiology of CVD,Citation17 and previous preclinical studies have reported that HMC inhibits oxidative stress and inflammation induced by ultraviolet B irradiation.Citation18–Citation20
Currently, the main focus for the treatment of patients with rosacea is symptom suppression to improve quality of life, prevent disease progression, and maintain remission.Citation15,Citation21 Treatment generally starts with patient education and general measures including gentle skin cleansing, photoprotection, and avoidance of exacerbating factors such as changes in temperature, ultraviolet light, stress, alcohol, and certain foods.Citation15,Citation21 Depending on the rosacea subtype, pharmacological therapy includes topical metronidazole, ivermectin, azelaic acid, or brimonidine as monotherapy or in combination, or systemic doxycycline, tetracycline or isotretinoin.Citation15,Citation22 In general, many of the available therapeutic options for rosacea are used as monotherapy and, as such, there is currently a lack of data on the simultaneous and complementary treatment of different pathophysiological features of rosacea. Although the current study, designed to assess the effectiveness of four active compounds for rosacea treatment, only reports in vitro data, it highlights the potential clinical importance of combining agents which complement each other to target different aspects of the multifactorial pathophysiology of rosacea.
Conclusion
Rosacea is a chronic vascular and inflammatory skin disease. Understanding the role of factors that trigger the onset of rosacea symptoms and exacerbate the condition (eg, TRPV1, VEGF, KLK5, MMP-9, IL-1α,IL-8, CXCL1, and CXCL6) is important in treating this skin disease. Overall, our in vitro results showed that dextran sulfate, BCH, pongamia oil, and HMC possess complementary soothing and anti-redness properties and, as such, they could potentially be suitable candidates for topical adjunctive treatment in patients with rosacea.
Acknowledgments
The authors thank David P. Figgitt PhD, ISMPP CMPP™, Content Ed Net, for providing editorial assistance in the preparation of the manuscript, with funding from Pierre Fabre Dermo-Cosmétique, Lavaur, France.
The abstract of this paper was presented at the following conferences as a poster presentation with interim findings: IFSCC (International Federation of Societies of Cosmetic Chemists) 2017, SID (Society for Investigative Dermatology) 2017, ESDR (European Society for Dermatological Research) 2017, and WCI (World Congress on Inflammation) 2017 (http://www.iloveweb.kr/IFSCC2017/file/abstract/Abstract%20P-175.pdf). The poster’s abstract was published in “Poster Abstracts” (Oct 2017), J Invest Dermatol. 2017;137(10):S276, October 2017; https://www.researchgate.net/publication/320151861_492_Properties_of_TRP-regulin_R_pongamia_oil_and_hesperidin_methyl_chalcone_on_anti-redness_treatment.
Disclosure
All authors are employees of Pierre Fabre Dermo-Cosmétique R&D Center, Toulouse, France. A patent has been deposited (FR 1750059) by Hernandez-Pigeon H and Castex-Rizzi N. The authors report no other conflicts of interest in this work.
References
- SulkMSeeligerSAubertJDistribution and expression of non-neuronal transient receptor potential (TRPV) ion channels in rosaceaJ Invest Dermatol201213241253126222189789
- KajiyaKKajiya-SawaneMOnoTSatoKIdentification of an epidermal marker for reddened skin: vascular endothelial growth factor AJ Dermatol201744783683728677138
- YamasakiKGalloRLRosacea as a disease of cathelicidins and skin innate immunityJ Investig Dermatol Symp Proc20111511215
- ReinholzMRuzickaTSchauberJCathelicidin LL-37: an antimicrobial peptide with a role in inflammatory skin diseaseAnn Dermatol201224212613522577261
- JangYHSimJHKangHYKimYCLeeESImmunohistochemical expression of matrix metalloproteinases in the granulomatous rosacea compared with the non-granulomatous rosaceaJ Eur Acad Dermatol Venereol201125554454820698913
- KähäriVMSaarialho-KereUMatrix metalloproteinases in skinExp Dermatol1997651992139450622
- KangSChoSChungJHHammerbergCFisherGJVoorheesJJInflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivoAm J Pathol200516661691169915920154
- KanadaKNNakatsujiTGalloRLDoxycycline indirectly inhibits proteolytic activation of tryptic kallikrein-related peptidases and activation of cathelicidinJ Invest Dermatol201213251435144222336948
- BoisnicSBranchet-GumilaMCBenslamaLLe CharpentierYArnaud-BattandierJLong term culture of normal skin to test the efficacy of a hydroxy acid-containing creamEur J Dermatol199774271273
- SteinhoffMSchauberJLeydenJJNew insights into rosacea pathophysiology: a review of recent findingsJ Am Acad Dermatol2013696 Suppl 1S15S2624229632
- BergMLidénSAn epidemiological study of rosaceaActa Derm Venereol19896954194232572109
- AbramKSilmHOonaMPrevalence of rosacea in an Estonian working population using a standard classificationActa Derm Venereol201090326927320526544
- ChosidowOCribierBEpidemiology of rosacea: updated dataAnn Dermatol Venereol2011138Suppl 3S179S18322183096
- SpoendlinJVoegelJJJickSSMeierCRA study on the epidemiology of rosacea in the U.KBr J Dermatol2012167359860522564022
- RainerBMKangSChienALRosacea: epidemiology, pathogenesis, and treatmentDermatoendocrinology201791e1361574
- GuexJJAvrilLEnriciEEnriquezELisCTaïebCQuality of life improvement in Latin American patients suffering from chronic venous disorder using a combination of Ruscus aculeatus and hesperidin methyl-chalcone and ascorbic acid (quality study)Int Angiol201029652553221173734
- JawienABouskelaEAllaertFANicolaïdesANThe place of Ruscus extract, hesperidin methyl chalcone, and vitamin C in the management of chronic venous diseaseInt Angiol2017361314128124877
- Pinho-RibeiroFAHohmannMSBorghiSMProtective effects of the flavonoid hesperidin methyl chalcone in inflammation and pain in mice: role of TRPV1, oxidative stress, cytokines and NF-κBChem Biol Interact2015228889925617481
- MartinezRMPinho-RibeiroFASteffenVSHesperidin methyl chalcone inhibits oxidative stress and inflammation in a mouse model of ultraviolet B irradiation-induced skin damageJ Photochem Photobiol B201514814515325916506
- MartinezRMPinho-RibeiroFASteffenVSTopical formulation containing hesperidin methyl chalcone inhibits skin oxidative stress and inflammation induced by ultraviolet B irradiationPhotochem Photobiol Sci201615455456327021784
- RiveroALWhitfeldMAn update on the treatment of rosaceaAust Prescr2018411202429507456
- van ZuurenEJFedorowiczZCarterBvan der LindenMMCharlandLInterventions for rosaceaCochrane Database Syst Rev20154CD003262