Abstract
Background
We evaluated the effects of 3-month prebiotic oral supplementation with fructo-oligosaccharides (FOS) and galacto-oligosaccharides (GOS) on glucose and lipid metabolic parameters in women with adult acne (female adult acne).
Methods
Twelve women, mean age 35 years, with mild to moderate acne were enrolled. Exclusion criteria were severe acne, body mass index (BMI) >25, history of diabetes mellitus, polycystic ovary syndrome, regular intake of prebiotics or probiotics, and history of inflammatory intestinal diseases. At baseline visit (T0), at month 1 (T1), and at month 3 (T2) fasting glucose, blood insulin, glycated hemoglobin (HbA1c), C-peptide, triglycerides, total cholesterol levels, and BMI were measured. Subjects were treated with a food supplement containing FOS (100 mg) and GOS (500 mg), one sachet daily, for 3 months. Subjects were instructed to follow their regular diet, and no dietary restrictions were suggested.
Results
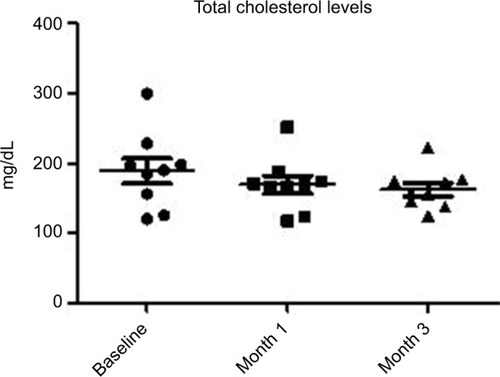
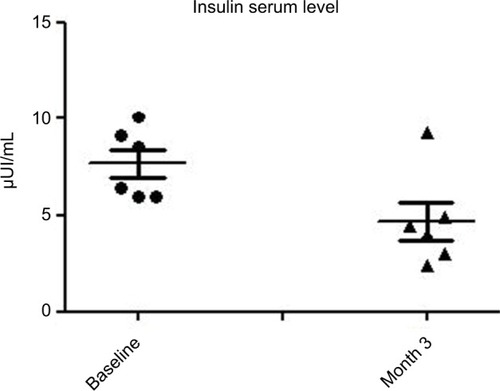
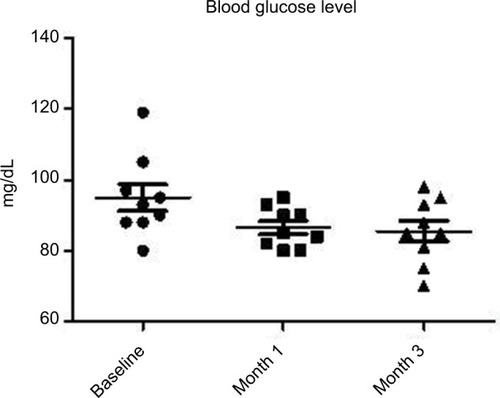
At baseline, the BMI, mean ± SD, was 23±0.7. No modification of BMI was observed during the study. At baseline, fasting blood glucose levels were 92±7 mg/dL. A significant (P=0.02) reduction was observed at month 1 (86±5 mg/dL) and at month 3 (85±7 mg/dL) (–10%). Total cholesterol levels were reduced significantly (P=0.018) from 184±19 to 161±10 mg/dL (–13%) at the end of the study. Triglycerides at baseline were 51 mg/dL and were reduced to 46 mg/dL (P=0.05). Insulin and C-peptide plasma levels showed a nonsignificant reduction trend from baseline to the end of the study. In subjects with baseline insulin level >6 µUI/mL (n=6) the FOS/GOS supplementation induced a significant (P=0.03) reduction from 7.8 to 4.3 µUI/mL at day 90 (–45%). C-peptide was reduced from 2.1 to 1.6 ng/mL (month 3). HbA1c at baseline was 35 mg/dL and 32 mg/dL at the end of the study (NS).
Conclusion
In adult female acne, supplementation with prebiotic FOS and GOS was associated with positive effects on glycemic and lipid metabolic parameters.
Introduction
Adult female acne (AFA) is a skin disease of increasing incidenceCitation1 that may affect up to 50% of women above 25 years of age.Citation2 Its pathogenesis is unknown. Recent epidemiologic data demonstrated that AFA may be associated with metabolic syndrome and high glycemic indexCitation3 caused by elevated blood level of IGF-1, which may induce comedogenesis and inflammation of sebaceous glands.Citation4 Some epidemiological data have shown that a high-carbohydrate (>55% of energy from carbohydrate) and high-fat diet worsens acne.Citation5 Aizawa and NiimuraCitation6 demonstrated that adult women with acne presented mild insulin resistance during oral glucose tolerance test. Chronic and acute hyperinsulinemia upregulates the production of IGF-1, which is a potent mitogen stimulating cellular growth at the follicle level as well.Citation7 In animal models, overexpression of IGF-1 induces hyperkeratosis and epidermal hyperplasia.Citation8 Furthermore, IGF-1 increases the production of sebum.Citation9 IGF-1 serum levels are elevated in women with postadolescent acne.Citation10 Both insulin and IGF-1 stimulate the production of androgens in ovarian and testicular tissues.Citation11,Citation12 These data support the hypothesis that the endocrine cascade induced by hyperinsulinemia enhances sebum production and acne development. Modification of intestinal microbiota has been implicated in insulin resistance.Citation13 Larsen et alCitation14 have shown that gut microbiota in type 2 diabetic subjects differs from nondiabetic adults. In obese subjects, therapeutic modification of intestinal microbiota is associated with an improvement of insulin sensitivity.Citation15 Fructo-oligosaccharides (FOS) and galacto-oligosaccharides (GOS) are prebiotic substances able to improve intestinal microbiota and oral supplementation with both increases the amount of intestinal Bifidumbacterium spp. and Lactobacillus spp.Citation16 Experimental data show that oral supplementation with FOS and GOS could have positive effect on glucose metabolism in healthy nondiabetic individuals, thereby reducing the glycemic diet load effects.Citation17 So far, no data regarding the effects of FOS/GOS dietary supplementation on glycemic parameters in subjects with AFA are available.
Study aim
To evaluate, in a pilot study, the effects of prebiotic oral supplementation with FOS and GOS on glucose and lipid metabolic parameters in women with adult acne.
Subjects and methods
Study design
The study (“S.O. Sweet trial”) was designed as open prospective proof-of-concept trial. The clinical setting was the acne outpatient service at the Dermatology Clinic of Catania University. The study took place from July 2017 to December 2017. The institutional review board of the Catania University approved the study protocol. The trial was conducted according to Good Clinical Practice Guidelines and Helsinki Declaration (update 2014).Citation18
Subjects
Eligible subjects included women aged ≥25 years with mild to moderate acne. A total of 12 women, mean age 35 years, were included in the study after their written informed consent. Exclusion criteria were severe acne, body mass index (BMI) >25, smoking habits, use of oral contraceptives, history of diabetes mellitus, regular intake of prebiotics or probiotics, and history of inflammatory intestinal diseases.
Study outcomes
At baseline visit (T0), at day 30 (month 1), and at day 90 (month 2), fasting glucose, blood insulin, glycated hemoglobin (HbA1c), C-peptide, triglycerides, total cholesterol levels, and BMI were measured. HbA1c levels were measured by low-pressure liquid chromatography. Serum C-peptide levels were measured by radioimmunoassay using commercial kits (Diagnostic Systems Laboratories Inc, Webster, TX, USA).
Intervention
Subjects were treated with a food supplement containing FOS (100 mg) and GOS (500 mg), one sachet daily (Sebogard oral, Cantabria Labs Difa Cooper, Caronno Pertusella, Italy), for 3 consecutive months. Subjects were instructed to follow their regular diet, and no dietary restrictions were suggested.
Statistical analysis
Statistical analysis was performed using GraphPad Statistical Software (GraphPad Software, Inc., La Jolla, CA, USA). Continuous variables were expressed as mean ± SD. The primary outcomes of the study were to evaluate the evolution of glycemic and lipidic parameters. For inferential statistical analysis, we used the ANOVA multiple comparison test and the paired Student’s t-test. In view of the proof-of-concept nature of the present trial, a formal sample size calculation was not performed. We decided to enroll at least ten evaluable subjects.
Results
All enrolled women completed the 3-month study period. At baseline the BMI, mean ± SD, was 23±0.7. No modification of BMI was observed during the study duration (BMI 23±6 at month 3). At baseline, fasting blood glucose levels were 92±7 mg/dL. A progressive and significant (P=0.02; ANOVA) reduction was observed at month 1 (86±5 mg/dL) and at month 3 (85±7 mg/dL) (–10%) (). Total cholesterol levels were reduced significantly (P=0.05) from 184±19 to 161±10 mg/dL (–13%) at the end of the study (). Triglycerides at baseline were 51 mg/dL and were reduced to 46 mg/dL (P=0.05). Insulin and C-peptide plasma levels showed a nonsignificant reduction trend from baseline to the end of the study. In subjects with baseline insulin level >6 µUI/mL (n=6) the FOS/GOS supplementation induced a significant (P=0.03) reduction from 7.8 to 4.3 µUI/mL at day 90 (–45%) (). C-peptide was reduced from 2.1 to 1.6 ng/mL at day 90. HbA1c at baseline was 35 mg/dL, and it was 32 mg/dL (difference not significant) at the end of the study. The product was very well tolerated. No serious adverse events were reported during the entire study duration. Even if it was not an endpoint of this trial, a general improvement of acne lesion count was also observed in comparison with baseline condition.
Figure 1 Evolution of glucose serum levels at baseline and during 1 and 3 months of FOS/GOS dietary supplementation (P=0.022; ANOVA).
Discussion
Acne is a common and complex skin disease that affects individuals of all ages.Citation19 Acne is most common in teenagers, but acne can be observed in 54% of adult females and 40% of adult males, and its prevalence does not decrease substantially with age.Citation20 AFA is a skin disease of increasing incidence that may affect up to 50% of women above 25 years of age.Citation1,Citation2 AFA is classically distinguished into three subtypes:Citation21 “persistent acne” (adolescent acne that is persistent after adolescence), “late onset acne” (characteristically starts after 25 years of age with no previous history of acne), and “recurrent acne” (distinguished by the disappearance of acne for years and by its recurrence). Factors involved in the pathogenesis of adult acne include genetic factors, hypercolonization of resistant strains of Propionibacterium acnes, hyperandrogenism with or without polycystic ovarian syndrome,Citation1 increased serum levels of insulin and IGF-1,Citation22 and the use of comedogenic substances.Citation23 Also, stress and smokingCitation24 seem to be associated with AFA, even if some epidemiological data do not support this link.Citation25 Recent evidences show that glycemic load and glycemic index of whole diet may participate in the pathogenesis of AFA by increasing serum levels of insulin that in turn may increase the blood levels of IGF-1 and adiponectin responsible for comedogenesis and inflammation.Citation26 In a trial conducted by Smith et al,Citation27 a low glycemic load diet significantly reduced acne lesion count improving insulin sensitivity. However, in this trial there was a concomitant weight loss in the group assigned to low glycemic load diet, which could have interfered with the net clinical outcome. An alteration of intestinal microbiota has also been claimed in one study, with 66% of 57 patients affected by AFA showing positive reactivity to stool-isolated coliforms compared to none of the control patients without active skin disease.Citation28 Finally, high fat and sugar diet with low fibers may lead to gut motility alterations, loss of normal microbial biofilm (Bifidobacterium in particular), and increased intestinal permeability (loss of intestinal barrier).Citation29 The latter may cause efflux of lipopolysaccharide endotoxins into systemic circulation, promoting low-grade inflammation, oxidative stress, release of substance P, and increased permeability of IGF-1, which in turn cause insulin resistance, hyperinsulinemia, type 2 diabetes, and likely onset of acne.Citation30 Finally, oral nutritional supplementation with prebiotics like FOS and GOS has been demonstrated to increase stool colony counts of Bifidobacteria and Lactobacilli, contributing to maintain efficient intestinal mucosal barrier.Citation31 Prebiotics could improve insulin sensitivity, thus controlling the role of high glycemic diets in acne exacerbation.Citation32 The results of our study support the hypothesis that dietary supplementation with some prebiotics, in particular FOS and GOS, may improve some blood parameters of sugar and lipid metabolism in women with AFA. Some limitations should be taken in account while evaluating our results. This was an open, uncontrolled trial performed in 12 subjects. The sample size was small. However, we wanted to enroll subjects with specific inclusion and exclusion criteria to reduce confounding factors (no diabetes, no polycystic ovarian syndrome, etc), and these aspects have substantially reduced the eligible population. Therefore, our study should be considered as a pilot trial.
Conclusion
In AFA, oral supplementation with prebiotic FOS and GOS was associated with positive effects on glycemic and lipid metabolism parameters. Further controlled comparative studies are warranted to better evaluate these effects in a larger population of AFA subjects.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
FDo and GM have disclosed that they have no significant relationships with or financial interests in any commercial companies related to this study or article. MM is an employee of Cantabria Labs Difa Cooper. MM reports grants from Difa Cooper, during the conduct of the study, and grants from Difa Cooper, outside the submitted work.
References
- DrénoBLaytonAZouboulisCCAdult female acne: a new paradigmJ Eur Acad Dermatol Venereol20132791063107023302006
- ZeichnerJABaldwinHECook-BoldenFEEichenfieldLFFallon-FriedlanderSRodriguezDAEmerging Issues in Adult Female AcneJ Clin Aesthet Dermatol20171013746
- KaymakYAdisenEIlterNBideciAGurlerDCelikBDietary glycemic index and glucose, insulin, insulin-like growth factor-I, insulin-like growth factor binding protein 3, and leptin levels in patients with acneJ Am Acad Dermatol200757581982317655968
- KurokawaIDanbyFWJuQNew developments in our understanding of acne pathogenesis and treatmentExp Dermatol2009181082183219555434
- BoweWPJoshiSSShalitaARDiet and acneJ Am Acad Dermatol201063112414120338665
- AizawaHNiimuraMMild insulin resistance during oral glucose tolerance test (OGTT) in women with acneJ Dermatol19962385265298854583
- DeplewskiDRosenfieldRLGrowth hormone and insulin-like growth factors have different effects on sebaceous cell growth and differentiationEndocrinology199914094089409410465280
- BolDKKiguchiKGimenez-ContiIRuppTDigiovanniJOverexpression of insulin-like growth factor-1 induces hyperplasia, dermal abnormalities, and spontaneous tumor formation in transgenic miceOncogene19971414172517349135074
- SmithTMGillilandKClawsonGAThiboutotDIGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathwayJ Invest Dermatol200812851286129317989724
- BaltaIEkizOOzuguzPInsulin resistance in patients with postadolescent acneInt J Dermatol201554666266624961925
- BarbieriRLSmithSRyanKJThe role of hyperinsulinemia in the pathogenesis of ovarian hyperandrogenismFertil Steril19885021972123294042
- BebakarWMHonourJWFosterDLiuYLJacobsHSRegulation of testicular function by insulin and transforming growth factor-betaSteroids19905562662702201104
- DumasMEBartonRHToyeAMetabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant miceProc Natl Acad Sci U S A200610333125111251616895997
- LarsenNVogensenFKvan den BergFWGut microbiota in human adults with type 2 diabetes differs from non-diabetic adultsPLoS One201052e908520140211
- VriezeAvan NoodEHollemanFTransfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndromeGastroenterology2012143491391622728514
- CollinsMDGibsonGRProbiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gutAm J Clin Nutr19996951052s105710232648
- CostaGTGuimarãesSBSampaioHAFructo-oligosaccharide effects on blood glucose: an overviewActa Cir Bras201227327928222460261
- General Assembly of the World Medical AssociationWorld Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjectsJ Am Coll Dent20148131425951678
- WilliamsHCDellavalleRPGarnerSAcne vulgarisThe Lancet20123799813361372
- BergfeldWFA lifetime of healthy skin: implications for womenInt J Fertil Womens Med1999442839510338266
- Ramos-E-SilvaMRamos-E-SilvaSCarneiroSAcne in womenBr J Dermatol2015172Suppl 1202625597414
- MelnikBCSchmitzGRole of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgarisExp Dermatol2009181083384119709092
- WhiteGMRecent findings in the epidemiologic evidence, classification, and subtypes of acne vulgarisJ Am Acad Dermatol1998392 Pt 3S34S379703121
- YangYSLimHKHongKKCigarette smoke-induced interleukin-1 alpha may be involved in the pathogenesis of adult acneAnn Dermatol2014261111624648681
- YounSWThe role of facial sebum secretion in acne pathogenesis: facts and controversiesClin Dermatol201028181120082943
- CappelMMaugerDThiboutotDCorrelation between serum levels of insulin-like growth factor 1, dehydroepiandrosterone sulfate, and dihydrotestosterone and acne lesion counts in adult womenArch Dermatol2005141333333815781674
- SmithRNMannNJBraueAMäkeläinenHVarigosGAA low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trialAm J Clin Nutr200786110711517616769
- BoweWPLoganACAcne vulgaris, probiotics and the gut-brain-skin axis – back to the future?Gut Pathog201131121281494
- TilgHKaserAGut microbiome, obesity, and metabolic dysfunctionJ Clin Invest201112162126213221633181
- MelnikBCLinking diet to acne metabolomics, inflammation, and comedogenesis: an updateClin Cosmet Investig Dermatol20158371
- HaarmanMKnolJQuantitative real-time PCR assays to identify and quantify fecal Bifidobacterium species in infants receiving a prebiotic infant formulaAppl Environ Microbiol20057152318232415870317
- KumarSMahajanBBKamraNFuture perspective of probiotics in dermatology: an old wine in new bottleDermatol Online J201420913030/qt8br333fc