 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background:
Quantitative numerical analysis of skin displacement triggered by muscles inserting the overlaying skin would be useful for monitoring the inhibition of mimetic muscles.
Methods:
By using removable grid markings and digital photographs, skin displacement analysis (SDA) was performed on 13 patients pre-treatment and on Days 1, 2, 3, and 7 after injection of 18 units of botulinum toxin type A (BoNT/A) in the fronto-glabellar area.
Results:
At baseline, amplitudes of horizontal skin displacement with fronto-glabellar contraction showed a linear increase along the eyebrow laterally from the midline; mean values (±standard deviation [SD]) 15 and 30 mm lateral to the midline were 3.2 ± 1.0 mm (range, 1.9–4.9 mm) and 6.5 ± 1.4 mm (range 4.0–8.5 mm), respectively. After injection of BoNT/A, maximum horizontal skin displacement 30 mm lateral to the midline showed a mean reduction (±SD) to 62% ± 23% at Day 2 and to 17% ± 16% at Day 7; corresponding values 15 mm lateral to the midline were 62% ± 29% and 15% ± 20%, respectively. In all cases, the reduction in horizontal skin displacement compared with pre-injection levels was statistically significant (P < 0.001).
Conclusion:
SDA is a feasible method for the quantitative evaluation of skin movements elicited by muscles inserting the overlaying skin in the face and neck area.
Introduction
Injection of botulinum toxin type A (BoNT/A) is a frequently administered aesthetic treatment for the reduction or prevention of hyperkinetic facial lines and rhytids. However, since the introduction of BoNT/A to aesthetic medicine,Citation1 studies evaluating the effects of this treatment have predominantly relied on semi-quantitative evaluation tools, such as the rating scale for hyperkinetic facial lines, as proposed by Kim et alCitation2 or other self-designed analog scores adapted to the specific design of their associated studies.Citation3,Citation4 Even in recent trials, semi-quantitative analog scale-derived analyses of subjects’ and investigators’ subjective perceptions were used for comparing differences between injection volumes and dilutionsCitation5 and for comparing BoNT/A versus BoNT/A with epinephrine.Citation6
The reference method for quantitative evaluation of the BoNT/A blocking action on skeletal muscle in neurology is electromyography. While quantitative analysis of BoNT/A efficacy with evaluation of the compound muscle action potential (CMAP) M-waveCitation7 seems reasonable in neurological diseases, the spatial resolution, in general, is too limited for distinctive monitoring of BoNT/A efficacy in aesthetics. Despite this, it has recently been proposed for follow-up of global BoNT/A action on the frontalis muscle.Citation8 To date, quantitative image analyses of the aesthetic effects of BoNT/A have been limited to the evaluation of the eyebrow height or eyebrow mobility after injections into the glabellar area, with respect to the mid-pupillary line or to directly related nearby locations.Citation9,Citation10
To overcome some of the above-mentioned limitations of current quantitative image analyses, and to avoid semi-quantitative analog scale-based methods and electromyography, a new quantitative, two-dimensional method – skin displacement analysis (SDA) – with a high resolution of a few millimeters is presented here. It is applicable to almost any given place in the face and neck area. Specifically, the proof-of-principle of the method is demonstrated by analysis of skin displacement before and after BoNT/A injection in the fronto-glabellar area.
Methods
Study design
Consenting individuals requesting routine fronto-glabellar BoNT/A injections were treated with BoNT/A at a standardized dosage. Patients were marked on the right and central frontal area with a removable 0.25 cm2 grid (Thermage Inc, Hayward, CA, USA), thus providing a length of 5 mm between 2 crossings (). Facial photo-documentation was carried out pre-treatment (Day 0) and during the week after administration of the toxin (Days 1, 2, 3, and 7), as described below. The grid was placed immediately before each measurement and removed afterwards.
Figure 1 A) Pre-treatment patient with relaxed fronto-glabellar area and a removable 5 mm grid on the central and right frontal region. Numbers indicate the injection sites with corresponding number of BoNT/A units injected. B) Relaxed muscle tension. The point of interest 30 mm lateral to the midline, immediately above the eyebrow, was marked with two perpendicular black lines, coordinates (X2; Y2). The reference point of the geometric system at the inner canthus of the right eye was marked (X1; Y1) and subtracted from any point of interest for the purpose of calibration. C) Maximum fronto-glabellar contraction with otherwise identical markings as in . The horizontal movement of the point of interest from (relaxed) to (contraction) was calculated in pixels as ΔX = [(X2 contraction − X1) − (X2 relaxed − X1)].
![Figure 1 A) Pre-treatment patient with relaxed fronto-glabellar area and a removable 5 mm grid on the central and right frontal region. Numbers indicate the injection sites with corresponding number of BoNT/A units injected. B) Relaxed muscle tension. The point of interest 30 mm lateral to the midline, immediately above the eyebrow, was marked with two perpendicular black lines, coordinates (X2; Y2). The reference point of the geometric system at the inner canthus of the right eye was marked (X1; Y1) and subtracted from any point of interest for the purpose of calibration. C) Maximum fronto-glabellar contraction with otherwise identical markings as in Figure 1B. The horizontal movement of the point of interest from Figure 1B (relaxed) to Figure 1C (contraction) was calculated in pixels as ΔX = [(X2 contraction − X1) − (X2 relaxed − X1)].](/cms/asset/9d9e7a87-537a-43fc-a8a1-0406272f8aa6/dcci_a_18185_f0001_c.jpg)
Injection pattern of BoNT/A
Each 100 unit vial of lyophilized BoNT/A (Xeomin®, Merz Pharmaceuticals, Frankfurt, Germany) was dissolved in 2.5 mL physiological saline and then injected intramuscularly using a 0.3 mL insulin syringe with an 8 mm 30G needle. This purified BoNT/A preparation is free from complexing proteins, has demonstrated equivalence to the first market-available BoNT/A preparation for efficacy and duration of action,Citation11 and has shown a lack of development of neutralizing antibodies in preclinical studies.Citation12 In total, 18 units of BoNT/A were injected into the fronto-glabellar area and 6 units were injected laterally to both eyebrows (). First, 4 units were injected in the midline into the central part of the procerus, followed by 2 units 15 mm above this (). Three units were then injected 10 mm above and 15 mm laterally to the first procerus injection, into the medial parts of right and left corrugator supercilii close to the medial ending of the eyebrow (); another 3 units were injected 15 mm laterally and 5 mm above this injection (). Finally, 3 units were injected into the orbital rim, immediately at the lateral end of the eyebrows into the lateral and upper parts of the orbicularis oculi ().
Skin displacement analysis
Horizontal skin displacement due to maximum contraction of the fronto-glabellar area was analyzed at Day 0 immediately before the injection of BoNT/A, and at Days 1, 2, 3, and 7 post-injection. shows a patient with relaxed fronto-glabellar muscle tonus and the removable marking grid in place with an additionally drawn midline. Vertical and horizontal black lines cross at the medial canthus of the right eye (X1, Y1), which serves as the fixed-point of the geometric system, thereby allowing shifts or movements of the face at other points to be calculated from a standardized reference point. Vertical and horizontal black lines also cross at one sample point of interest above the right eyebrow (X2, Y2). The same 2 points are marked during maximum fronto-glabellar contraction, as shown in . The horizontal shift of the point of interest above the eyebrow during maximum fronto-glabellar contraction (X2 contraction; ) compared with its position under relaxed conditions (X2 relaxed; ) forms the basis of numeric calculations for SDA. The amplitude of the horizontal shift from the relaxed to the contracted situation relative to the fixed reference point X1 at the inner canthus was calculated as follows:
where ΔX is given in the dimension of pixels in the digital photograph. To obtain movement amplitudes in millimeters, the value of ΔX is normalized for the number of pixels along the length of each 5 mm box in each digital photograph.
Digital photographs were taken at Day 0, before injection of BoNT/A, and at Days 1, 2, 3, and 7 post-injection. At each time-point, 3 cycles of relaxed muscle tension and maximum contraction were captured. The pixels of grid data of each photograph were analyzed using Windows Paint™ Software.
Statistical analyses, including calculation of means, standard deviations (SD) and P-values of t-tests, were performed using Microsoft Excel™ Software. The study was carried out in accordance with the Declaration of Helsinki and its later extensions.
Results
Patients
Thirteen patients were enrolled and included in the study (female, n = 12; male, n = 1). The median age of the patients was 39 years (range, 19–62 years). Ten of the participants had never previously received BoNT/A, 2 had not received BoNT/A during the past 12 months, and 1 had received BoNT/A 6 months previously but did not show any remaining action.
Skin displacement analysis
Skin displacement before BoNT/A injection
Horizontal skin displacement from a relaxed to a contracted state above the eyebrow showed a linear correlation up to 35 mm lateral to the midline; horizontal skin displacement started to plateau at 40 and 45 mm (). The mean (±SD) amplitude of horizontal skin displacement 15 mm lateral to the midline was 3.2 ±1.0 mm, with a range of 1.9−4.9 mm (). The mean (±SD) amplitude of horizontal skin displacement doubled at 30 mm lateral to the midline to 6.5 ±1.4 mm, with a range of 4.0−8.5 mm ().
Figure 2 A) Pre-treatment horizontal skin displacement by maximum glabellar contraction in 13 individual patients. Analyzed grid points of interest ran laterally from the midline, immediately above the eyebrow. B) Mean values ± standard deviation of the 13 patients in .
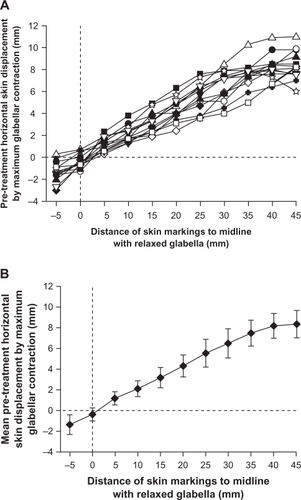
Table 1 Skin displacement amplitudes elicited by maximum fronto-glabellar contraction evaluated immediately above the eyebrow from 13 patients prior to injection of BoNT/A
A linear regression analysis of pre-injection horizontal skin displacement amplitudes from the midline up to 35 mm laterally ( and ) was performed with the equation [y = m · x + t], where y is the horizontal skin displacement amplitude and x is the distance of the point of interest from the midline under relaxed conditions. The constant parameters m and x were calculated as 0.22 and −0.12 mm, respectively, with t close enough to zero to support calculating distances from the midline. A slope of m = 0.22 resulted in horizontal skin displacement at maximum contraction of 22% of the distance to the midline under relaxed conditions. For example, a specific point 30 mm lateral to the midline under relaxed conditions would be 22% closer to the midline under contracted conditions, resulting in a new position 23.5 mm lateral from the midline.
BoNT/A-induced reduction of fronto-glabellar contraction
shows the horizontal skin displacement amplitudes from a relaxed to a contracted state for the patient shown in at Days 0 (pre-injection), 1, 2, 3, and 7. Skin displacement analysis was performed on the grid points located immediately above the right eyebrow. Similar to the variability of horizontal skin displacement before BoNT/A injection in different patients (), the BoNT/A-induced reduction in horizontal skin displacement amplitude in the fronto-glabellar area also showed intersubject variability. To allow better comparison of BoNT/A action kinetics, data obtained at Days 1, 2, 3, and 7 (post-injection) were normalized to each subject’s skin displacement data obtained at Day 0 (pre-injection).
Figure 3 Time-dependent reduction of contraction-induced horizontal skin displacement after injection of BoNT/A. A) Reduction of horizontal skin displacement amplitudes from Day 0 pre-treatment to Day 7 post-treatment of the patient shown in . Again, analyzed grid points of interest ran laterally from the midline, immediately above the eyebrow. B) Reduction of horizontal skin displacement amplitudes of 13 individual patients at the injection site 30 mm lateral to the midline, immediately above the eyebrow (X2, Y2 in , ), normalized to the individual pre-treatment maximum. C) Mean values ± standard deviation of the 13 patients shown in . D) Normalized mean reduction of horizontal skin displacement amplitudes of 13 patients at points of interest 15−45 mm lateral to the midline, immediately above the eyebrow.
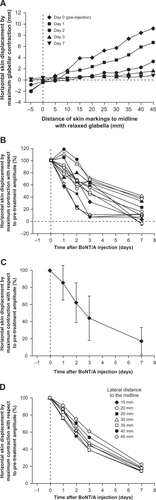
The horizontal skin displacement profiles of 13 individual patients 30 mm lateral to the midline (normalized to pre-injection values) are shown in . Statistical analysis by paired double-sided t-testing revealed that a significant reduction in horizontal displacement was detectable at Day 1 compared with pre-injection data (P = 0.03). The significance of the reduction compared with pre-injection data increased at Days 2, 3, and 7 post-injection (P < 0.0001 for all versus Day 0).
Interindividual variability in the time course of BoNT/A action was observed, as were differences in the final level of blockage of horizontal contraction at Day 7 (). Based on visual assessment, individuals with a slower response to BoNT/A injection also had lower maximum blockage of horizontal contraction compared with individuals who had a rapid reduction in contraction amplitude. Comparing the 6 patients with the least blocking action on Day 7 with the 6 patients with the strongest blocking action on Day 7, the mean (±SD) contraction capability at Day 1 was 98% ± 17% and 79% ± 17%, respectively, of values taken pre-injection (P =0.08 for between-group comparison). At Day 7, mean (±SD) contraction capability was reduced to 32% ± 10% and 4% ± 7%, respectively (P = 0.0003 for between-group comparison).
On average, a 50% reduction in horizontal skin displacement from a relaxed to contracted state at 30 mm lateral to the midline was achieved between 2 and 3 days after BoNT/A injection (). Specifically, relative to Day 0, the maximum horizontal skin displacement 30 mm lateral to the midline showed a time-dependent and highly significant (P < 0.001) reduction to a mean (±SD) of 62% ±23% at Day 2, and a further reduction to 17% ± 16% at Day 7 (). Corresponding values for maximum horizontal skin displacement 15 mm lateral to the midline were 62% ±29% at Day 2 and 15% ±20% at Day 7 (). The blockage kinetics was fastest at 20 and 25 mm, in the middle of the 2 injection points at 15 and 30 mm lateral to the midline, whereas points 40 and 45 mm lateral to the midline showed a slightly less pronounced reduction in horizontal contraction ability ( and ); these differences were not significant.
Table 2 Time course of average reduction of maximum fronto-glabellar contraction elicited skin displacement amplitudes, normalized to Day 0 prior to BoNT/A injection
Safety
Overall BoNT/A injections were well tolerated. The only adverse event reported was a frontal headache in 1 patient at Days 1 and 2 after injection.
Discussion
Since the introduction of different preparations of BoNT/A to aesthetic medicine,Citation1 studies assessing their effectiveness have predominantly relied on semi-quantitative analog scale-derived evaluation tools.Citation2–Citation6 Electromyography, the standard method for evaluation of BoNT/A action on skeletal muscle in neurology, does not allow the analysis of BoNT/A action with a 2-dimensional resolution of a few millimeters, as is frequently required in aesthetic indications. Despite this, electromyography has been proposed for the follow-up of global BoNT/A action on the frontalis muscle.Citation8 Attempts to introduce quantitative image analysis of BoNT/A action in aesthetic settings have, so far, been restricted by their limitation to the analysis of the mobility of existing anatomical structures, such as the evaluation of the eyebrow motion or eyebrow height after BoNT/A injections into the glabellar area.Citation9,Citation10
To overcome some of the limitations of previous evaluation methods, this article describes SDA, a quantitative technique that can be used to evaluate skin movements related to the insertion and contraction of any given mimetic muscle of the face and neck area in any direction. In this study, we were able to effectively demonstrate evaluation of horizontal skin displacement related to fronto-glabellar contraction. One of the major advantages of SDA is that it allows the evaluation of single points of interest, even those remote from anatomical structures such as the eyebrows. The basis for SDA is the appropriate marking of the area to be evaluated with a suitable marking system or pattern. For simplicity in this study, we used a removable grid tattoo. The contraction-related displacements of single points of the fronto-glabellar area were then calculated relative to the coordinates of the medial canthus, which served as the fixed-point origin of the whole geometric system. Due to the characteristics of this evaluation method, even limited vertical or horizontal movements of the patient’s head, which could compromise results with other methods, were eliminated. Furthermore, due to the characteristics of the cosine function, even limited rotation of the head had minimal influence on the calculation of skin displacements. For example, a clockwise rotation of the head of 10° would cause an error of only 1.5% when calculating a horizontal skin displacement using the factor cos(10°). Additionally, the dimensions of any useful marking system, such as the removable grid tattoo with a 5 mm grid used in the current study, allows for the calibration of any contraction-related skin displacement. In this study, it was possible to quantitatively analyze contraction-related skin displacement of the fronto-glabellar area.
In general, the horizontal shortening from relaxed to contracted state along the length of the corrugator muscle was 22% pre-BoNT/A injection, as determined by linear regression analysis. The pre-treatment range of shortening amongst individual patients varied from 13% to 33%, resembling a shortening from one-eighth to one-third of the relaxed corrugator muscle length. Interestingly, the maximum horizontal contraction amplitude above the eyebrow showed a linear correlation to the position measured from the midline up to about 35 mm laterally; horizontal contraction amplitudes then stabilized at 40 and 45 mm lateral to the midline (), suggesting that the average length of the corrugator muscle did not exceed 35 mm.
Close to the midline, evaluation of contraction-induced horizontal skin movements showed some limitations. Contraction effects are superimposed by shifting and folding of excessive skin in a more or less random fashion, finally resulting in deep lines and rhytids in the central glabellar area, for which BoNT/A injection aims to correct. In our study, at the midline, where all horizontal skin movement amplitudes should be zero, measured values ranged from −1.3 to 0.7 mm, reflecting these ‘random’ skin movements. Furthermore, some of the negative skin movement values at the midline might have been caused by asymmetrical use of the corrugator muscles in an individual patient.
In the current study, regardless of age, mass of corrugator muscles, and severity of lines and rhytids, all patients received a fixed dose of 18 units of BoNT/A in the fronto-glabellar area in order to provide standardized conditions with the dosage of the drug used. With this moderate dose of BoNT/A, a full blockage of mimetic muscles of the fronto-glabellar area was not expected in all subjects. Therefore, the study design was able to provide some interesting information in addition to the proof-of-principle of the SDA method. Specifically, the limited dose of BoNT/A in relation to the individual strength of the corrugator muscle may have been the reason that some patients ended up with only about 50% blockage of horizontal contraction 7 days after injection, while others reached 100% blockage of their horizontal fronto-glabellar contraction capability. Interestingly, a slower onset of action of BoNT/A after injection seemed to be related to less pronounced final blockage of contraction. This hypothesis requires confirmation in a specifically designed study. Nevertheless, as displayed in , all patients showed a reduction in contraction-related skin displacement in the horizontal direction 3 days after injection of BoNT/A. At Day 7, all patients reached a stronger level of blockage of contraction amplitudes.
By extrapolating findings from the current trial, SDA could be used in future studies to assess how different doses of BoNT/A affect blockage action or how an increased or decreased number of injection sites could influence blockage of muscle contraction. The method could also be used to assess loss of BoNT/A action weeks and months after injection. Other potentially interesting fields of study could include the analysis of different dilutions of BoNT/A and comparisons between different products, including between different pharmaceutical or cosmetic substances claiming to reduce the activity of mimetic muscles in the face or neck area.
Conclusion
We have successfully demonstrated the use of a new method for assessing skin movements elicited by muscles inserting the overlaying skin. SDA demonstrated high accuracy to within a few millimeters when evaluating BoNT/A-induced blockage of horizontal contraction in the fronto-glabellar area. This method could help to more accurately assess the effects of injected BoNT/A in aesthetic indications in the face or neck area and to overcome some of the limitations of semi-quantitative analog scale-based analyses.
Acknowledgements
Preliminary results from this study were presented at the Munich Educational Conference for Dermatology, 21 July 2008.
The study was partially sponsored by a research grant from Merz Pharmaceuticals GmbH, Frankfurt, Germany.
Disclosure
The author declares no conflicts of interest.
References
- CarruthersJDCarruthersJATreatment of glabellar frown lines with C. botulinum toxin exotoxinJ Dermatol Surg Oncol19921817211740562
- KimEJReeckJBMaasCSA validated scale for hyperkinetic facial linesArch Facial Plast Surg2004625325615262720
- AscherBZakineBKestemontPBaspeyrasMBougaraASantiniJA multicenter, randomized, double-blind, placebo-controlled study of efficacy and safety of 3 doses of botulinum toxin A in the treatment of glabellar linesJ Am Acad Dermatol20045122323315280841
- MonheitGCarruthersABrandtFRandRA randomized, double-blind, placebo-controlled study of botulinum toxin type A for the treatment of glabellar lines: determination of optimal doseDermatol Surg200733S51S5917241415
- CarruthersJDCarruthersJACohenJDilution of botulinum toxin A for the treatment of glabellar rhytids: Does it matter?Dermatol Surg200733S97S10417241422
- HantashBMGladstoneHBA pilot study on the effect of epinephrine on botulinum toxin treatment for periorbital rhytidsDermatol Surg20073346146817430381
- WohlfarthKMüllerCSassinIComesGGrafeSNeurophysiological double-blind trial of a botulinum neurotoxin type A free of complexing proteinsClin Neuropharmacol200730869417414940
- KarsaiSAdrianRHammesSThimmJRaulinCA randomized, double-blind study of the effect of Botox and Dysport/Reloxin on forehead wrinkles and electromyographic actionArch Dermatol20071431447144918025375
- HeckmannMSchön-HupkaGQuantification of the efficacy of botulinum toxin type A by digital image analysisJ Am Acad Dermatol20014550851411568739
- CarruthersACarruthersJEyebrow height after botulinum toxin type A to the glabellaDermatol Surg200733S26S3117241411
- BeneckeRJostWHKnaovskyPRuzickaEComesGGrafeSA new botulinum toxin type A free of complexing proteins for treatment of focal dystoniaNeurology2005641949195115955951
- BlümelJFrevertJSchwaierAComparative antigenicity of three preparations of botulinum neurotoxin type A in the rabbitNeurotox Res20069 Abstract 238