Abstract
Necrolytic acral erythema (NAE) is now considered as a distinct clinical entity. It clinically presents as well demarcated hyperpigmented papules and plaques with thick adherent scales distributed symmetrically over dorsum of feet. It usually develops in patients with Hepatitis C virus (HCV) infection. Cases of NAE have been reported in patients without HCV infection. Hepatic dysfunction resulting in metabolic alterations like hypoalbuminemia, hypoaminoacidemia, hyperglucagonemia and transient zinc deficiency has been proposed as underlying pathogenic mechanism of NAE. Clinically, NAE resembles other necrolytic erythemas like necrolytic migratory erythema (NME), acrodermatitis enteropathica (AE) and pellagra. Better understanding of etiopathogenesis and histopathological features is important to distinguish NAE from other necrolytic erythemas. The disease runs a natural course of exacerbations and remissions. Non-invasive diagnostic tools like dermoscopy can be used in differential diagnosis of NAE. Oral zinc therapy is the most effective treatment of NAE reported in most of the cases irrespective of HCV status or serum zinc levels.
Introduction
Necrolytic acral erythema (NAE) was first reported from Egypt in 1996 by el Darouti and Abu el Ela.Citation1 Since then several cases have been reported predominantly from Egypt. Morphologically it resembles necrolytic erythemas. The understanding of epidemiology, etiology, pathogenesis and management of the disease is based on either large case series from a particular geographical region (Egypt) or from isolated case reports from other parts of the world. There may be possibility of underrecognition of this clinical entity due to lack of knowledge especially in areas with low prevalence of Hepatitis C Virus (HCV) infection.
Epidemiology
NAE is commonly reported in patients with HCV infection. Most of the cases have been reported from Egypt. In a study from the US, all the patients diagnosed with NAE were African Americans.Citation2 Since the first description of the disease in Egypt, case reports are published from other Asian countries like India,Citation3–Citation5 PakistanCitation6 and Canada.Citation7
Gender
The disease affects both the genders equally with males and females constituting 46.2% and 53.8% respectively.Citation8 In a small case series from the US, all the five cases reported were males.Citation2
Age
NAE affects the patients in the age range of 19–58 years with mean of 44±11.3 and median of 50.Citation8 In another case series from Egypt, the age range was 11–76 years with mean of 41.7±11.5 years.Citation9 Similar age distribution has been reported from the US where all the five cases were above 40 years of age.Citation2
Etiopathogenesis
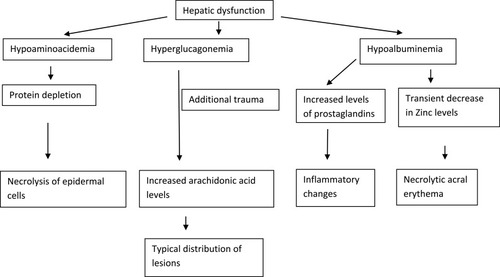
The exact pathogenesis of NAE is not yet conclusively established. The pathogenesis of NAE appears to be multifactorial as several mechanisms have been proposed such as hepatic dysfunction, hypoalbimunemia, hypoglucagonemia, hypoaminoacidemia, zinc deficiency and diabetes with or without an underlying HCV infection. The clinical and histopathological similarities with necrolytic migratory erythema (NME) lead to the hypothesis that NAE may be a variant of NME. However, consistent association of NAE with an HCV infection and typical acral distribution of lesions makes it a distinct entity.Citation10
Hepatitis C Viral Infection
The majority of cases of NAE have been reported from Egypt where the prevalence of HCV infection is high (15–20%).Citation1,Citation9 Among patients with chronic HCV, the prevalence of NAE has been estimated to be 1.7%. In the same study, the majority of patients who did not develop NAE had co-infection with Human Immunodeficiency Virus (HIV).Citation2 Hence, it is unlikely that immunodeficiency or co-infection contribute to the manifestation of NAE.
In patients with HCV, unlike leukocytoclastic vasculitis (LCV), NAE is not associated with high levels (>1 million IU/mL) of HCV viremia. However, in these patients, low and moderate levels of HCV viremia has been reported in 76.9% and 23.1% of patients respectively.Citation8 In studies where the cut off value of 0.5 million IU/mL was used for high HCV viremia, 43.5% of patients with NAE showed high viremia.Citation9 The HCV viral particles have not been demonstrated by PCR from the skin or vascular endothelium of patients with NAE. Similarly, electron microscopy of the lesions failed to demonstrate HCV viral particles in keratinocytes, dermal infiltrate or vascular endothelium. However, clumped tonofilaments on electron microscopy suggests possible viral etiology.Citation9 Hence, apart from viral load, additional factors seem to play role in the pathogenesis of NAE.
Variations in the prevalence of NAE and viral genotype in different regions suggest a role of HCV genotype in the pathogenesis of NAE. In small number of patients with NAE, both genotype 1 and 4 were isolated whereas in LCV only genotype 4 was isolated. However, the role of co-infection with two genotypes in the causation of NAE is not yet established necessitating the need for further evidence. Clinically, NAE and LCV have never been manifested together in patients with HCV infection leading to the hypothesis that certain other factors influence the type of inflammatory response in such patients.Citation8 The absence of NAE in other disorders of the liver suggests that the HCV infection sets up a milieu for the development of NAE in the presence of hepatic dysfunction and other confounding factors like nutritional deficiency, ethnicity and genetic factors.Citation2,Citation8
Metabolic Alterations
The clinical and histopathological similarities of NAE with other necrolytic erythemas such as NME, acrodermatitis enteropathica (AE), pellagra, biotin deficiency and essential fatty acid (EFA) deficiency suggest metabolic alterations. Several cases of NAE have been reported without HCV infection.Citation3–Citation6 It is also pertinent to know that no cases of NAE are reported from Japan, one of the countries with high HCV seroprevalence. The metabolic alterations proposed in the pathogenesis of NAE are hepatic dysfunction, hypoalbuminemia, hypoaminoaciduria and hyperglucagonemia (). In some patients with HCV, the clinical severity of NAE worsened with the increased activity of underlying hepatitis. In addition, the liver transplantation resulted in complete resolution of the lesions of NAE. These findings indicate that NAE is a non-immunologically mediated skin disease.Citation11
Zinc Deficiency
The role of zinc deficiency in the pathogenesis of NAE is based on the case reports demonstrating good response to zinc therapy or low serum zinc levels.Citation12 In the majority of cases the serum zinc levels remains normal. In a study on patients with NAE, diminished zinc levels have been demonstrated in serum, and lesional and perilesional skin.Citation13 There can be low epidermal zinc levels despite normal serum zinc levels.Citation14 A transient decrease in zinc levels can occur in the presence of hypoalbuminemia. Through its effect on epidermal apoptosis, zinc may play a role in necrolysis observed in NAE.Citation10
NAE has also been reported following Hepatitis B vaccinationCitation15 and rivaroxaban treatment.Citation16
Clinical Manifestations
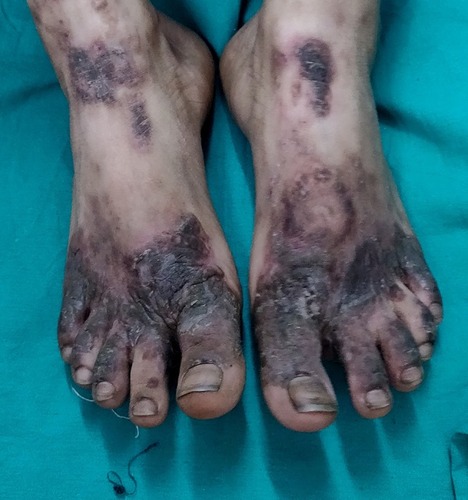
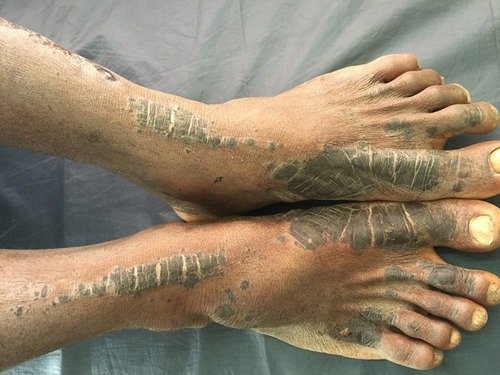
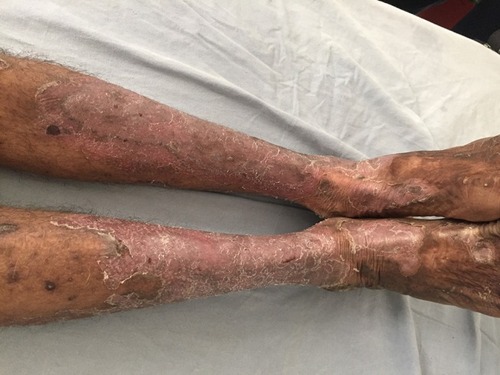
Classically NAE presents as well defined dusky red hyperkeratotic plaques with keratotic border typically distributed over the dorsum of feet and toes. In some cases ankle, legs and knees are also affected. Rarely lesions may develop on hands, elbows, genitalia and buttocks. Involvement of palms, soles and nails, otherwise considered as differentiating features against NAE, has been reported.Citation12 The clinical presentation of NAE can be acute or chronic. The acute lesions present as prominent erythema, flaccid blisters and erosions at the margins. The chronic lesions show hyperkeratotic surface, mild erythema and dark red margin. There may be associated edema. The duration of the disease at presentation may range from 2 to 120 months.Citation8
Clinical Stages
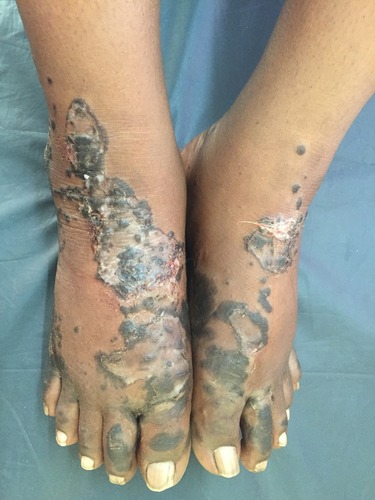
The lesions of NAE evolve in three stages, initial, well developed and late. In the initial stage, scaly erythematous papules/plaques appear with characteristic dusky or eroded centre (). In the well-developed stage, the lesions coalesce to form well demarcated thick hyperpigmented plaque with adherent scales (). There may be occasional pustules. The lesions become more circumscribed, thin and hyperpigmented in late stage ().Citation17 The lesions typically demonstrate a natural course of spontaneous remission and relapse.Citation12
Figure 2 Initial lesions showing well demarcated papules and plaques with dark scales with erosions and edema.
Differential Diagnosis
The differential diagnoses include skin diseases presenting with acral erythemas and psoriasiform plaques. The former group is comprised of AE, pellagra, biotin deficiency and fatty acid deficiency. The psoriasis, erythrokeratoderma, nummular eczema, dermatophytosis and paraneoplastic acrokeratosis are included in the latter group. Typical acral distribution and absence of systemic symptoms help in differential diagnosis of acral erythemas. Dark and verrucous scales, well defined lesions and absence of central clearing differentiate NAE from psoriasis, eczemas and dermatophytosis respectively. In some cases histopathological examination is required to exclude psoriasis. Other differential diagnoses include lichen simplex chronicus and hypertrophic lichen planus (HLP) which can be diagnosed by typical clinical and histopathological features.Citation10,Citation11,Citation18
Laboratory Investigations
The investigations are mainly aimed at detecting the underlying cause and metabolic derangement. Early recognition of the condition is very important for diagnosis and treatment of underlying disease or metabolic alterations, especially HCV infection. The patients may be unaware of their HCV status at first identification of NAE. In >75% of patients with NAE, HCV has been diagnosed for the first time.Citation17 Hence, HCV serology is mandatory in all cases of NAE. Relevant investigation should also be considered to rule out differential diagnosis based on clinical presentation of NAE.
Histopathology
The histopathological features correspond to stage of the disease and site of biopsy. The early lesions from margin show necrosis of upper epidermis with detachment from surrounding by a blister formation. The epidermis shows acanthosis and spongiosis along with superficial dermal infiltrate resembling nummular dermatitis. The well-developed lesions show parakeratosis, prominent papillomatosis, subcorneal pustules, psoriasiform hyperplasia, epidermal pallor and necrotic keratinocytes. The confluence of necrotic keratinocytes can result in cleft formation in the upper epidermis. Foci of epidermal dyskeratosis and epidermal pallor helps in differentiating NAE from psoriasis. The presence of necrosis of keratinocytes along with vacuolar degeneration of some basal cells helps in the diagnosis of NAE.Citation19
Dermoscopy
Dermoscopy is a non-invasive diagnostic method wherein subsurface structures of skin are visualized enabling the treating physician to study the details of lesional morphology.Citation20 Dermoscopic patterns in NAE are not mentioned in the literature. We could do dermoscopic examination in few cases which demonstrated white scales, white globules, brown globules, reddish-brown areas, red dots and yellow globules correlating to hyperkeratosis, acanthosis, epidermal melanin, extravasated of red blood cells, dilated capillaries and dried serous fluid respectively (). The arrangement of basic elements in dermoscopy gives additional clues in the diagnosis and helps in the differentiation of similar conditions.Citation20 NAE showed white and yellow globules and red dots scattered in the centre of the lesion. Brown dots were appreciated at the periphery. Scales were dull white. In psoriasis, red dots which represent tips of tortuous dermal capillaries are arranged in regular and uniform pattern on the erythematous background. Bright white scales are other features in psoriasis.Citation21 In eczema, red dots are arranged in cluster pattern with yellow globules. These yellow globules correspond to dried serous fluid and are referred to as “yellow clod” sign.Citation21 HLP reveals yellow areas, comedo-like openings, Wickham striae and linear vessels.Citation22 Dermoscopy can be used to distinguish NAE, psoriasis, eczema and HLP ().
Figure 5 Dermoscopy of necrolytic acral erythema shows white (black arrows), yellow (red arrows) and brown (yellow arrows) globules. Red dots (green arrows) and dull white scales (black stars) are well appreciated.
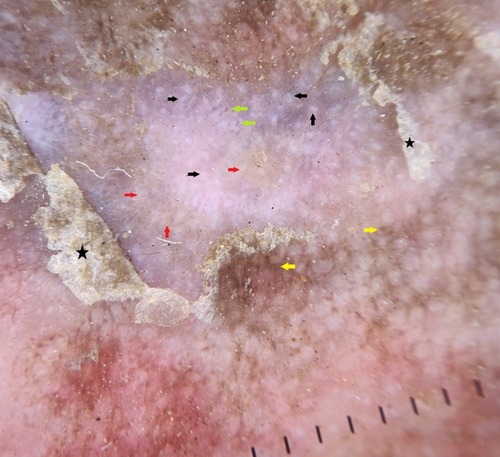
Table 1 Comparative Dermoscopic Patterns in Dermatoses Mimicking Necrolytic Acral Erythema
Biochemical Tests
There are no specific laboratory investigations recommended for diagnosis of NAE. The biochemical tests are used to identify underlying metabolic derangement or to exclude other causes of necrolytic erythemas. No abnormal tests have been consistently associated with NAE. The serum zinc levels are usually normal in patients with NAE, except for a few isolated case reports of low serum zinc levels.Citation3,Citation7,Citation13,Citation14 Amino acid levels and albumin levels may be abnormal in some patients. Normal glucagon levels help in differentiation from NME. Total serum bilirubin and transaminase levels should be done in all cases. In patients with associated HCV, the abnormal liver function tests and abnormal abdominal ultrasonography have been reported in 84.3% and 76.9% of patients with NAE respectively.Citation8
Management
In patients with the HCV infection, antiviral therapy with ribavirin and interferon-alpha have shown improvement in the disease even if there is no change in high viral load. Monotherapy with interferon-alpha has shown a good response. Oral zinc therapy has been the most effective treatment reported. In the absence of zinc deficiency, the clinical response can be attributable to anti-inflammatory, immunomodulatory, antiviral and anti-oxidant effects of zinc. Improvement in efficacy of interferon therapy in patients with HCV has been observed during zinc therapy for NAE.Citation18 Oral zinc, irrespective of serum zinc levels, in the dose of 220mg twice daily for up to 8 weeks was found to be very effective in the complete clearance of the lesions ().Citation3,Citation4,Citation7 Other treatment modalities tried are systemic, intralesional and topical corticosteroids, topical tacrolimus,Citation23 and phototherapy. Topical and intralesional steroids showed a good response in a subset of patients, but in others there was poor response. Combination of topical clobetasol and hand and foot psoralen plus ultraviolet (UV) A therapy and Narrow band UV B therapy have been tried with none of them showing complete resolution of lesions.Citation2
Conclusion
NAE is a distinct clinical entity with characteristic clinical and histopathological features. It is consistently associated with HCV infection and hepatic dysfunction. In this setting, the metabolic alterations involving albumin, amino acids and zinc play a major role in the pathogenesis of the disease. Treatment with antiviral therapy in patients with HCV infections is a mainstay of the treatment. Oral zinc therapy is effective in achieving a complete resolution of the lesions, irrespective of HCV status and/or zinc levels.
Disclosure
The authors report no conflicts of interest in this work.
References
- el Darouti M, Abu el Ela M. Necrolytic acral erythema: a cutaneous marker of viral hepatitis C. Int J Dermatol. 1996;35:252–256. doi:10.1111/j.1365-4362.1996.tb02997.x8786182
- Raphael BA, Dorey-Stein ZL, Lott J, Amorosa V, Re III VL, Kovarik C. Low prevalence of necrolytic acral erythema in patients with chronic hepatitis C virus infection. J Am Acad Dermatol. 2012;67(5):962–968. doi:10.1016/j.jaad.2011.11.96322325461
- Pandit VS, Inamadar AC, Palit A. Seronegative necrolytic acral erythema: a report of two cases and literature review. Indian Dermatol Online J. 2016;7(4):304–307. doi:10.4103/2229-5178.18546427559510
- Panda S, Lahiri K. Seronegative necrolytic acral erythema: a distinct clinical subset? Indian J Dermatol. 2010;55(3):259–261. doi:10.4103/0019-5154.7067621063519
- Nikam BP. Necrolytic acral erythema seronegative for hepatitis C virus-two cases from India treated with oral zinc. Int J Dermatol. 2009;48:1096–1099. doi:10.1111/j.1365-4632.2009.04114.x19775403
- Ilyas S, Cheema SM, Rashid T. Necrolytic acral erythema: a rare entity. J Pak Assoc Dermatol. 2016;26(4):395–398.
- Jakubovic BD, Zipursky JS, Wong N, McCall M, Jakubovic HR, Chien V. Zinc deficiency presenting with necrolytic acral erythema and coma. Am J Med. 2015;128(8):e3–e4. doi:10.1016/j.amjmed.2015.03.022
- El-Darouti MA, Mashaly HM, El-Nabarawy E, Eissa AM, Abdel-Halim MRE, Fawzi MMT. Leukocytoclastic vasculitis and necrolytic acral erythema in patients with hepatitis C infection: do viral load and viral genotype play a role? J Am Acad Dermatol. 2010;63:259–265. doi:10.1016/j.jaad.2009.07.05020462666
- El-Ghandour TM, Sakr MA, El-Sebai H, et al. Necrolytic acral erythema in Egyptian patients with hepatitis C virus infection. J Gastroenterol Hepatol. 2006;21(7):1200–1206. doi:10.1111/jgh.2006.21.issue-716824076
- Nofal AA, Nofal E, Attwa E, et al. Necrolytic acral erythema: a variant of necrolytic migratory erythema or a distinct entity? Int J Dermatol. 2005;44:916–921. doi:10.1111/j.1365-4632.2004.02232.x16336523
- Aanand N, Geria BS, Holcomb KZ MD, MSPH, Scheinfeld NS. Necrolytic acral erythema: a review of literature. Cutis. 2009;83:309–314.19681342
- Bentley D, Andea A, Holzer A, Elewski B. Lack of classic histology should not prevent diagnosis of necrolytic acral erythema. J Am Acad Dermatol. 2009;60(3):504–507. doi:10.1016/j.jaad.2008.08.04618992966
- Moneib HAM, Salem SAM, Darwish MM. Evaluation of zinc level in skin of patients with necrolytic acral erythema. Br J Dermatol. 2010;163(3):476–480. doi:10.1111/j.1365-2133.2010.09820.x20426777
- Najarian DJ, Lefkowitz I, Balfour E, Pappert AS, Rao BK. Zinc deficiency associated with necrolytic acral erythema. J Am Acad Dermatol. 2006;55(5 Suppl):S108–S110. doi:10.1016/j.jaad.2005.09.04417052522
- Pernet C, Guillot B, Araka O, Dereure O, Bessis D. Necrolytic acral erythema following hepatitis B vaccination. Br J Dermatol. 2014;171(5):1255–1256. doi:10.1111/bjd.2014.171.issue-524787551
- Pathania YS, Budania A. Rivaroxaban induced necrolytic acral erythema. Postgrad Med J. 2019;1. doi:10.1136/postgradmedj-2019-136658
- Abdallah MA, Ghozzi MY, Monib HA, et al. Necrolytic acral erythema: a cutaneous sign of hepatitis C virus infection. J Am Acad Dermatol. 2005;53(2):247–251. doi:10.1016/j.jaad.2005.04.04916021118
- Abdallah MA, Hull C, Horn TD. Necrolytic acral erythema: a patient from the United States successfully treated with oral zinc. Arch Dermatol. 2005;141(1):85–87. doi:10.1001/archderm.141.1.8515655150
- Abdallah MA, Ghozzi MY, Monib HA, et al. Histological study of necrolytic acral erythema. J Ark Med Soc. 2004;100(10):354–355.15080276
- Errichetti E, Stinco G. Dermoscopy in general dermatology: a practical overview. Dermatol Ther (Heidelb). 2016;6(4):471–507. doi:10.1007/s13555-016-0141-627613297
- Ankad BS, Beergouder SL. Dermoscopy of inflammatory conditions: the journey so far. EMJ Dermatol. 2017;5(1):98–105.
- Ankad BS, Beergouder SL. Hypertrophic lichen planus versus prurigo nodularis: a dermoscopic perspective. Dermatol Pract Concept. 2016;6(2):3. doi:10.5826/dpc.0602a03
- Manzur A, Siddiqui AH. Necrolytic acral erythema: successful treatment with topical tacrolimus ointment. Int J Dermatol. 2008;47(10):1073‑5. doi:10.1111/ijd.2008.47.issue-10