Abstract
Skin has the natural ability to heal and replace dead cells regulated by a network of complex immune processes. This ability is conferred by the population of resident immune cells that act in coordination with other players to provide a homeostatic environment under constant challenge. Other than providing structure and integrity, the epidermis and dermis also house distinct immune properties. The dermal part is represented by fibroblasts and endothelial cells followed by an array of immune cells which includes dendritic cells (DCs), macrophages, mast cells, NK-cells, neutrophils, basophils, eosinophils, αβ T lymphocytes, B-cells and platelets. On the other hand, the functionally active immune cells in the epidermis comprise keratinocytes, DCs, NKT-cells, γδ T cells and αβ T cells (CD4+ and CD8+). Keratinocytes create a unique microenvironment for the cells of the immune system by promoting immune recognition and cellular differentiation. T lymphocytes exhibit tissue-specific tropism toward the epidermis and the lymphatic drainage system important for their function in immune regulation. This diversity in immune regulators makes the skin a unique organ to overcome pathogenic or foreign invasion. In addition, the highly coordinated molecular events make the skin an attractive model to understand and explore its regenerative potential.
Introduction
Skin is the largest complex organ that provides protection against constantly evolving changes in the environment and pathogenic intrusion.Citation1 The breach of this protective barrier induces fine coordination between cells, molecular factors and matrix remodelers to reestablish and maintain structural and functional integrity.Citation1 The skin’s immune system can be categorized into two parts: the epidermal region as isolated and described by J. StreilinCitation2 and the dermal region with subcutaneous adipose tissue. These two compartments are in close connection with each other further synchronized with the organism’s immune system as a whole. Both possess immune cells from the innate and adaptive immune systems with well-established connections between them. In immunological context, the skin is considered to be a highly diverse yet immunocompetent landscape. It provides the immune system with ideal conditions and an environment to carry out a rapid immune response. Specifically, the integumentary epithelium creates an efficient immunoregulatory microenvironment which forms the basis of the skin’s immune system. In addition to this immune environment, other key players include resident stem cells, with the ability of self-renewal to regulate homeostasis indicative of tissue repair and regeneration.Citation1 For instance, the epithelial stem cells ensure a constant renewal of the epidermis via their regenerative potential. The epidermis then forms an external barrier resembling a filter more than an impenetrable wall. This barrier also houses a rich and diverse microbiota comprising of pathogenic and opportunistic microorganisms, which could be beneficial for the skin microenvironment, according to recent evidence.Citation1 The skin naturally acts a protective barrier against such microorganisms that strive to seize new territories on the host organism. The immune system acts as a deterrent and in the case of microbial aggression intensifies its response.Citation3,Citation4
Infection and stress-related factors activate keratinocytes and immune cells via cascades of cytokine activation.Citation3,Citation4 This activates the proliferation and migration of various immune cell types to the sites of injury or shock. A few examples of such immune responses include the activation of a proinflammatory response (major histompatibilty complex [MHC] and cytokine receptor expression), phagocytosis, and involvement of various immunocompetent cells.Citation3,Citation4 It should be noted that these responses are strong enough to initiate both an innate and adaptive immune response based on the severity of pathogenic invasion or challenge. Immunity defines the ability of multicellular organisms to maintain its macromolecular composition by removing foreign molecules, which in turn provides resistance to infectious agents and neoplastic processes.Citation4 The presented ideas are reflected in one of the latest definitions of immunology which could not have been developed without understanding the role of barrier tissues. Thus, to build an immunological defense system for the body, the existence of a suitable barrier efficient enough to restrict penetration and recognition of foreign invasion is of utmost importance. Importantly, the skin is an ideal candidate that meets these evolutionary requirements. From a pathogen's perspective it is a naturally difficult obstacle to cross due to its dense and durable layer of keratinized epithelium. The skin has many immune cascades in effect at all times, as a result of which it is regarded as one of the emerging model systems to study the immune system.Citation3–Citation5
The dermis
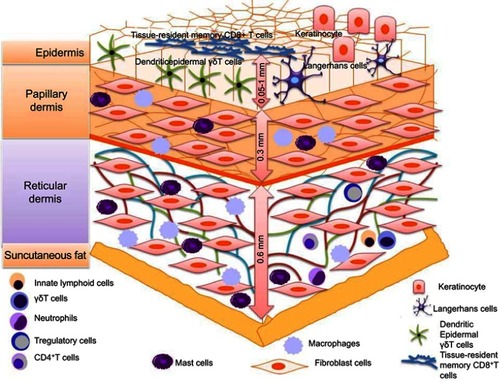
The circulatory system is responsible for uniform distribution of immune cells after their origin from the bone marrow. The population of immune cells comprises of class of T and B lymphocytes including neutrophils, a small number of eosinophils, fibroblasts, dendritic cells (DCs), macrophages, granulocytes, mast cells, basophils, natural killer (NK) cells and natural killer T (NKT) cells. All the aforementioned cells excluding the T- and B-lymphocytes detect and act upon stress signals originating from both foreign and internal stimuli. Pathogenic penetrating microflora are detected by pathogen-associated molecular patterns (PAMPs), and nonpathogenic stress signals are detected via danger-associated molecular patterns (DAMPs). Cells of the innate immune system contain Pathogen Recognizing Receptors (PRR) both on their membranes – Toll-like receptors (TLRs) – and in their cytosols – NOD-like receptors (NLRs). These receptors remain unchanged since the time of birth and hence are associated with innate immunity. All these cells express MHC-I in their dormant state while constitutively expressing MHC-II upon activation. However, in dormancy, induced expression of adhesion molecules on the dermal endothelial cells can trigger proinflammatory response, leading to MHC-II expression in dermal keratinocytes and Langerhans cells (LCs) to recruit leukocytes.Citation5 They also express receptors toward chemokines and cytokines, complement immune system, integrins and other molecules.Citation5–Citation7 The quantity and ability to synthesize certain biologically active molecules differ with each cell and the composition of their receptors. Keeping in mind the diverse properties of various skin immunocytes, in this article,we will discuss the cell populations essential for skin’s immune and regenerative features. The different types of immune cells of epidermis and dermis are presented in .
Figure 1 Different types of immune cells such as Langerhans cells, dendritic epidermal γδT cells (DETC), memory αβT cells (TRM), dendritic cell macrophages, neutrophils, mast cells, T-regulatory cells (T-reg) and CD8+ TEM Cells (Effector-Memory cells) are present in epidermis and dermis.
Fibroblasts
Fibroblasts are immature cells that are highly abundant in the connective tissue. These cells are important in providing a structural basis to the skin. Contemporary history of these cells begins with their discovery by A. Ya. Fridenstein, who observed the ability of cells with a unique morphology to form colonies indicative of their multipotent properties.Citation8 Some of their classical functions include contractility, locomotion, collagen and elastin fiber production and the regulation and degradation of the extracellular matrix.Citation9 Emerging evidence indicates that fibroblasts can express a wide range of functionally active TLRs (from TLR-1 to TLR-9); synthesize antimicrobial peptides (AMPs) (LL-37) and defensins (hBD-1, and hBD-2) and secrete various proinflammatory cytokines (TNFα, INFγ, IL-6, IL-12p70 and IL-10), chemokines (CCL1, CCL2, CCL5, CXCL1, CXCL8, CXCL10 and CX3CL1),and growth factors (granulocyte/macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF), essential for innate immune response against microorganisms.Citation9 Such properties enable these cells to sense pathogens resulting in a coordinated immune response by the recruitment of inflammatory cells.Citation9 Some of the critical immune factors of fibroblasts are presented in .
Figure 2 The microbial defense armor of fibroblasts. (A) Schematic representation of antimicrobial specificity for various Toll-like receptors (TLRs) expressed in the membrane and cytoplasmic organelles of fibroblasts. (B) Secretory factors responsible for elimination and prevention of microbial growth specifically in dermal and epidermal fibroblasts.
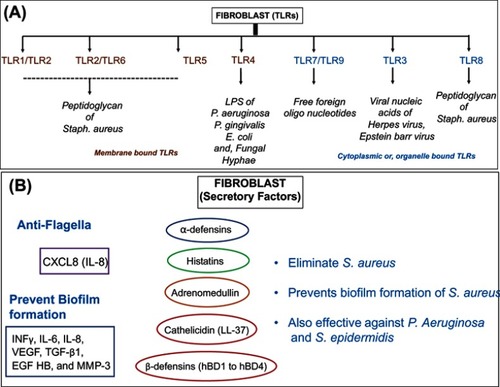
AMPs serve as important constituents of the host innate immune response. They are known effectors against a wide range of pathogens including bacteria, fungi and viruses. In vitro studies establish the antibacterial properties of cathelicidin (LL-37) against S. aureus and mycobacterial species.Citation9 Other studies also indicate the role β-defensin 3 (hBD-3) in the suppression of S. aureus biofilm formation.Citation10 Additional factors also include cytokines that are secreted by the cells of the innate or adaptive immune system. Studies have shown that skin fibroblasts can synthesize proinflammatory cytokines such as INFγ, IL-6 and IL-8 when exposed to biofilm cultures of S. aureus.Citation10 Another class of secretory factors includes chemokines that serve as recruiting factors for cytokines and facilitate the migration of immune cells at the site of immune response. In a study, it was shown that human gingival fibroblasts are capable of producing secretory IL8 in response to S. typhimurium flagellin-induced TLR5 activation.Citation11
Similarly, fibroblasts are also capable of synthesizing growth factors including vascular endothelial growth factor (VEGF-A). VEGF-A is a factor known to promote inflammatory response-induced neovascularization, allowing recruitment of monocytes/macrophages.Citation12 These studies indicate fibroblasts as one of the important factors responsible towards skin's immune defense system. In addition, fibroblasts also secrete bioactive factors into the intracellular matrix of the connective tissue essential for the maintenance of the cellular environment ().Citation13 Along this line of thought, the classical role of the fibroblasts has always been perceived for collagen synthesis and maintenance of extracellular matrix including the formation of scar tissue. However, the possibility towards the differentiation ability of fibroblasts into tissue is an emerging and attractive concept.
Table 1 Components of the intracellular matrix synthesized by dermal fibroblasts in humans
Considering this idea, research has been conducted to study the criterias for hematopoietic multipotent stromal cells (MSCs) differentiation were determined in fibroblasts by studying features including cellular morphology, adhesiveness and expression of cell surface markers (CD44, CD73, CD90, CD105 and CD271). Additionally, these cells possess the ability to undergo osteogenic, chondrogenic and adipogenic differentiation when grown in specialized media in vitro.Citation14 Depending on the availability of interacting cells and membrane affinity, lymphoid cells can also facilitate this process. The properties mentioned are also inherent in dermal MSCs.Citation15 It was found that MSCs have the ability to interact with lymphocytes and form clusters – fibroblast-lymphocytic rosettes (FLR) – in vitro.Citation14 They constitute majority of the hematopoietic stem cells (HSCs) capable of secreting various cytokines into their microenvironment which helps in establishing immunity as a whole.Citation14,Citation16 MSCs found in the skin resemble those in the bone marrow with the exception of a few unique properties.Citation17 Peripheral MSCs primarily have antiproliferative, immunomodulatory and proinflammatory effects.Citation16 The functionality of MSCs is largely in part due to the presence of TLRs.Citation18 MSCs facilitate phagocytosis in macrophages,Citation19 but inhibit the differentiation and activation of classical monocytes.Citation20 They reduce inflammation, accelerate the elimination of bacteria, promote the conversion of pro-inflammatory macrophages M1 to anti-inflammatory M2Citation21 and increase survival during sepsis.Citation22 MSCs have been also observed to induce the differentiation of T-regulatory cells,Citation23 maturation and differentiation of DCsCitation24 and on the other hand can inhibit their migration.Citation25
Recent years have witnessed emerging interest in mechanistic understanding of fibroblast function for the development of novel therapeutic interventions.Citation11 For example, Wnt signaling pathway has been shown to be critical for skin cell differentiation. As a result, Wnt-3a and fibroblast growth factor FGF-9 agonist for Wnt signaling in dermal fibroblasts are sought as therapeutic targets.Citation26 Despite the advances made in the context of fibroblasts in skin healing and regeneration, the field demands further work to fully elucidate the contributions of different dermal fibroblast lineages. Detailed study on the heterogeneity of dermal fibroblasts may yield mechanistic insights into existing therapies and provide cues for developing novel intervention strategies. Thus, we anticipate that the field of regenerative skin biology will witness tremendous advancements, leading to novel therapeutics in the near future.
Dendritic cells
The basic defining properties of DCs are their peculiar branch-like appearance; high MHC-I and MHC-II expression; enhanced active production of cytokines and the ability to capture, process and present antigens. DCs act as critical effectors for T-cell-mediated immune response in the skin. DCs have been shown to produce TNF-alpha- and iNOS-mediated proinflammatory signals in response to bacterial infections. Importantly in psoriasis, dermal DCs expressing CD11c+ surface marker play a major role in the formation of lesions.Citation27
Dermal (myeloid) DCs
Myeloid dermal DCs can exist in the immature state and express various PRRs. After maturing, they migrate toward the lymph node drainage system in the skin where they present the antigen to T cellsCitation28 In the dermis, myeloid DCs are located deeper and primarily in the perivasculature which upon myeloid differentiation give rise to two distinct subsets of DCs: (CD141+, CD103+) and (CD1C+, CD11b+).Citation27 Studies conducted in atopic dermatitis (AD) model have shown the activity of myeloid DCs in immune response by initiating recruitment of TH2 immune cells via secretion of chemokine such as CCL22 and CCL17.Citation27 Due to the divergent immunogenic properties, they are often more efficient than the resident macrophages.Citation27 They exist in an intermediate stage within the blood and constitute up to 0.5% of the total number of immunocompetent cells in it. DCs display their membrane affinity toward lymphocytes in situ by forming rosettes around them.Citation29 As part of the innate immune system, they begin to secrete cytokines and chemokinesCitation30 and accelerate regeneration.Citation31
Plasmacytoid DCs
Plasmacytoid dendritic cells (pDCs) are specialized type I interferon (IFN-α/β)-producing cells. They express intracellular TLRs TLR7 and TLR9 that mediate recognition of viral nucleic acids.Citation32 In the blood, they resemble plasmacytes and are also categorized under lymphoid cells. These cells comprise a large portion of immature DCs in circulation, with the exception of a small amount that also exists in the dermis. It is here that DCs differ in their functional abilities: they produce x10,000 the amount of IFN-1 during a viral infection as compared to other cells.Citation33 pDCs have been observed to infiltrate both murine and human skin wounds to produce type I IFNs via TLR7- and TLR9-dependent recognition of nucleic acids.Citation32 These events have been accounted critical for the induction of early inflammation and reepithelization of injured skin. pDCs significantly secrete IL-6, an inflammatory cytokine indirectly involved in the re-epithelization of skin wounds.Citation34
Monocytes and macrophages
The mononuclear phagocytic system contains macrophages and monocytes, where the former is found in tissues and the latter in circulation.Citation7,Citation35 Due to the wide range of TLRs, mononuclear cells can recognize practically all the major groups of PAMPs. There are two types of resident macrophage populations in the skin such as the Langerhans cells (LC's) found in the epidermis and dermal macrophages found in abundance in the dermis. LCs originate from progenitors derived from the yolk sac during phases of early embryonic development and have the ability to self-renew.Citation35 LCs are defined by their expression of the lectin receptor langerin (CD 207). In addition, LCs express CD11c and CD11b and are positive for F4/80 and MHC-II surface markers. In contrast, dermal macrophages express F4/80 and CD11b cell surface markers without any expression for CD11c or Langerin followed by low levels of MHC-II.Citation36 The contribution of macrophages during wound healing has been well studied; however, the specific repair function of LCs is yet to be determined.Citation36 Studies have shown that LCs undergo activation during events of wound closure that have been implicated in the healing of foot ulcer in diabetic patients.Citation36
Resident macrophages also occur as a result of constitutive migration of monocytes from the blood. Inflammatory macrophages (M1) are formed that migrate as an emergency response toward the site of inflammation.Citation37 These cells are marked by high phagocytic and bactericidal activity along with high levels of cytokine secretion. These properties enable the M1 macrophages to function as effector cells in the skin’s innate immune response. The classical activation of M1 produces pro-inflammatory cytokines thereby activating antigen presentation, phagocytosis, and the production of growth factors essential for wound repair. Alternatively, M2 activation produces antiinflammatory cytokines, primarily IL-10, and induces type 2 helper T cells (Th2 cells).Citation38 In a model of skin repair, macrophages were found to induce IL-4-dependent collagen fibril assembly following injury.Citation39 Absence of IL-4Ra or the IL-4-associated gene Retnla (resistin-like alpha) reduces the levels of lysyl hydroxylase 2 (LH2), an enzyme responsible for persistent profibrotic collagen cross-links, in injured skin.Citation39 Thus, macrophages harness the potential to produce several important wound-healing and profibrotic mediators including regulation of skin collagen fibril synthesis to maintain the three-dimensional structure.
Similarly, the role of monocytes has been well studied in various stages of wound healing utilizing skin injury models in rodent models. Studies have shown that monocyte infiltration occurs in two distinct phases 24 hrs post wounding in skin. Two unique subsets of monocytes have been identified to infiltrate the site of wound formation. The first subset is marked by the expression of CCR-2 and the second subset is characterized by the expression of chemokine receptor CX3CR-1.Citation36 It was shown that CCR-2-expressing monocytes are predominantly recruited to skin wound 48 hrs post wounding using CCR-2/GFP reporter mice. In addition, there is also a reduction in the number of macrophages at the site of wounding. Other than the early influx of CCR-2-expressing monocytes, an influx of CX3CR-1-expressing monocytes is also observed during subsequent stages of wound healing in the skin. Studies performed using excisional punch biopsy of the skin indicate macrophages as the dominant immune cells expressed during tissue formation phase.Citation40 Additionally, macrophage-derived growth factors also induce extracellular matrix(ECM) deposition.Citation36 For instance, macrophages induce VEGF-mediated neoangiogenesis-mediating formation of neoepithelium at the site of wound bed formation as observed in reporter mice.Citation41 Taken together, these findings demonstrate the critical role of macrophages and their interplay with monocytes during early and late stages of wound healing by regulating the synthesis and organization of de novo collagen depositions in the wound.
Neutrophils
Neutrophils are part of the innate immune system, and these cells carry out a variety of functions during the normal wound repair process Citation42,Citation43. They are crucial since they migrate from the blood to the site of inflammation significantly faster than monocytes and are the first to fulfill a protective function. Their effectiveness is also owed to them being able to produce the metabolites needed for a “respiratory burst” within seconds. It is important to note that the chemokines are released by activated keratinocytesCitation35 followed by platelets that attract neutrophils to the site of inflammation. Neutrophils recognize pathogenicity via TLR-1, TLR-2 and TLR-10.Citation42 Secretory factors including G-CSF, IL-17 and IL-23 determine the development, migration and activation of neutrophils, and IL-8 (CXCR1 and CXCR2) acts as the main factors of chemotaxis.Citation5,Citation42 Important receptors for the immune system of the skin which determine the link between neutrophils and the skin are CXCL12 (SDF1) and CXCR4.Citation5,Citation42 Granular neutrophils contain several enzymes that enable them to penetrate tissues and destroy particles within phagosomes.Citation42 Classically, neutrophils have been considered as one of the important mediators for wound repair. According to clinical data, patients with defects in neutrophil trafficking or function exhibit higher risk for developing wound infections and impaired wound healing.Citation2 Studies in animal models have also shown that neutrophils are important for cutaneous wound repair.Citation42 Neutrophils also serve as storehouse for matrix metalloproteases (MMPs) in their granules and secretory vesicles. These MMPs include MMP-2, MMP-8 and MMP-9 whose functions have been widely investigated in the context of wound repair. Studies highlight induced expression of MMP-8 (also known as collagenase-2) mRNA levels and activity in chronic wounds.Citation42 In a study performed using MMP-8-knockout mice, researchers observed a delay in wound closure marked by reduced neutrophil infiltration, suggesting that MMP-8 may also aid in neutrophil trafficking.Citation43
Eosinophils
Eosinophils also reside within the dermis and are a good indicator of allergiesCitation7 TLR-1, TLR-4, TLR-7 and TLR-10 can be found on eosinophils.Citation7,Citation44 The main role of eosinophils is to combat multicellular parasites by undergoing extracellular cytolysis which releases special granules containing a host of proteins: major basic protein, eosinophil cationic protein (ECP), eosinophilic peroxidase and eosinophil-derived neurotoxin (EDN). ECP and EDN have an antiviral effect which activates mast cells and basophils.Citation7,Citation44–Citation46 However, some of these proteins damage normal tissue as well.Citation45,Citation46 Importantly, eosinophils have been shown to stimulate fibroblasts to thereby induce α-smooth muscle actin and collagen secretion in rodent models of fibrosis.Citation47 Presence of eosinophils facilitates the secretion of growth factors that promote keratinocyte migration in vitro, suggesting a possible role in the formation of dermal scars.Citation47
Basophils and mast cells
Another defense against multicellular parasites is pro-inflammatory basophils and mast cells that express TLR-2, TLR-3 and TLR-4.Citation46,Citation48 Basophils belong to the group of granulocytes which constitute rather short-lived effector cells of the innate immune system. These cells can live from a few months to years retaining their proliferative ability. It has been reported that basophils can regulate the entry of eosinophils into the skin layers via induction of VCAM-1 expression on endothelial cells.Citation49 Furthermore, basophils exhibit anti-inflammatory properties in models of UV-B-induced skin.Citation49 Similarly, increased population of basophils have also been found in skin biopsies from patients with various skin disorders, suggesting their role as an inflammation regulator in human skin.Citation49
Mast cells belong to the population of resident immune cells that undergo activation in response to tissue injury, thereby releasing granules into the extracellular space.Citation50,Citation51 Mast cells react immediately at the site of inflammation by secreting histamines, serotonin, heparin and a host of enzymes. They are the main effector cells that mediate hypersensitivity. Mastocytes secrete a number of growth factors (vascular/endothelial, fibroblast and nerve).Citation50 They display a pronounced affinity toward lymphocytes in vitro as seen by rosette formations (MLR).Citation51 Upon activation, mast cells acquire the ability of antigen presentation.Citation50,Citation51 Mast cells have also been found to secrete TGF-β1, including other proteases that promote fibroblast-mediated effects and collagen fibril formation.Citation47 Mast cells also drive the formation of gap junctions with fibroblasts that are essential for the stimulation of cellular differentiation, proliferation and ECM contraction.Citation47
NK lymphocytes
The morphology of NK cells is that of large granular lymphocytes that lack T- and B-lymphocyte markers. NK cells are directed to carry out cytolysis and produce cytokines (primarily IFN) upon contact with tumor cells and cells with viral infections. These cells express many different TLRs. They also possess various receptors that identity and react with molecules sent out by dead or stressed cells (NKG2D-MYC) which enable them to directly affect the process of regeneration. Upon inflammation, NK cells migrate to the skin expressing chemokine receptors that correspond to ligands in keratinocytes.Citation52,Citation53
NKT lymphocytes
NKT lymphocytes are cells of the innate immune system though they develop in the thymus.Citation5,Citation54,Citation55 Unlike the T cells which carry CD4+ or CD8+ receptors, these cells only express an invariant Valpha-14 rearrangement receptor.Citation5,Citation7 Typical molecules associated with NK cells (NK 1.1, NKR-P1, CD56, CD16, KIR and NKG2D) are only found in the periphery. It is the Valpha-14 rearrangement receptor that allows the NKT lymphocytes to recognize glycolipid antigens with respect to antigen-presenting molecule CD1d (MHC – I).Citation54 Among lymphocytes in the blood, NKT constitutes only 1% of the population, and at an even lesser quantity in the dermis. These cells are extremely valuable because other than their cytotoxic function, they are practically the only source of cytokines (primarily IFNγ) in the initial stages of a defense against pathogens.Citation55 NKT cells have been observed to produce cytokines such as IL-4 and IFN-γ that facilitate resident antigen-specific CD8+T-cell and dendritic cell function.Citation5
Platelets
Currently, the functionality of these cells has come from an unexpected and novel direction. Apparently, platelets participate in inducing inflammation, regeneration and the formation of an immune reaction (TLR-2, TLR-4, TLR-7 and TLR-9).Citation56 They produce a majority of the growth factors and other biologically active substances. Data show that granular platelets contain 827 proteins,Citation57 whose secretion creates a platform for interactions between platelets and majority of other immune cells and stromal cells. Platelets activate neutrophils and monocytes via P selectin on their membranes, and their complementary ligand P selectin glycoprotein ligand 1 (PSG-L-1).Citation58 Neutrophils, monocytes, macrophages and endothelial cells are greatly influenced by the following chemokines: CXCL4, CXCL7, β-thromboglobulin, neutrophil-activating peptide (NAP), CCL3 (macrophage inhibitory protein-1α, MIP 1a), CCL5 (RANTES), CXCL1 (GROa) and CXCL5.Citation59 Thrombocidin 1 and 2 realize any microbial threat and growth factors (PDGF, TGF-β, EGF, and VEGF) cause an angiogenic and reparative action as part of the response.Citation60 Effective recruitment of platelets is essential for wound healing.Citation61 The use of platelet-rich autoplasma (PRP) has clinical application in wound healing and tissue regeneration in muscoskeletal injuries and their subsequent complications.Citation62 The introduction of PRP has been successfully used in dermatology, cosmetology, obstetrics, gynecology, cervical diseases, leukoplakia, endocervicitis, cervical erosion and kraurosis of the vulva.Citation62
Epithelial and endothelial cells
Proinflammatory cytokines released by immunocytes of the innate immunity upon exposure to tissue pathogens can induce a reaction from nontraditional cells of the immune system (epithelial and endothelial cells) as they contain PAMP and proinflammatory cytokine receptors. These cells begin to imitate traits of innate immunocytes: adhesion molecules, complimentary Fcγ-receptors, MHC-II molecules, costimulators (CD40, CD80, and CD86) and secretion of proinflammatory cytokines (IL-1, IL-6, TNFα). Although these nontraditional cells have significantly inferior signals in comparison, they are enough to act as APCs to memory cells, αβ T lymphocytes and B-lymphocytes in the skin.Citation63
The epidermis
The epidermis is marked by characteristic features and occupies a distinct area.Citation1 The passage of cells in this compartment is monitored by membrane-bound adhesion molecules that interact with complementary structures found on keratinocytes. An equally important role is played by chemokine receptors secreted by keratinocytes. The specificity of the latter represents the skin as an organ of the immune system. The epidermis is composed of keratinocytes, LCs, β T-cells and αβ T-lymphocytes; among which are present a significant amount of CD4+ and CD8+ lymphocytes with a predominance of memory cells.Citation1 Interesting enough, T lymphocytes comprise 90% of all lymphocytes in the epidermis.
Keratinocytes
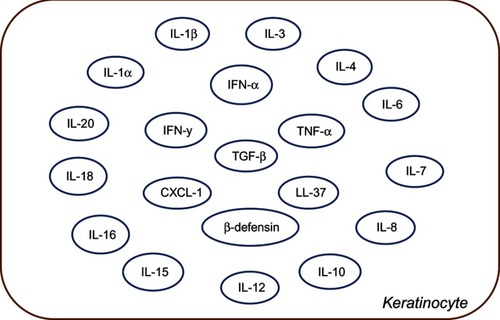
Keratinocytes constitute the structural element of the outer layer of the skin. Other than their role as a structural component, emerging evidence highlight their role in both innate and adaptive immunity.Citation5 Keratinocytes can be rightly called a part of the innate immune system since they occupy a strategic place as the primary physical barrier against pathogens. Keratinocytes together with neutrophils and epithelial cells serve as one of the reservoirs of AMPs that serve as the first line of defense.Citation5 They also express certain pathogen pattern receptors including TLRs. These cells are also capable of secreting immune factors and chemoattractants as depicted in . Prolonged activation of TLRs in the keratinocytes causes the synthesis of IFN1 and the polarization of a Th1 reaction.Citation64
Figure 3 Schematic representation of various cytokines and chemokines secreted by keratinocytes in response to immune activation in skin injury. IFN represents Interferon and IL's represent Interleukin family of cytokines, LL-37 is an important anti-microbial cathelicidin molecule whereas, CXCl, TGF-beta, TNF-alpha and beta-defensins are indicative of predominant chemoattractants.
Keratinocytes can recruit, stimulate and activate multiple cell types in healing process to regain the epidermal structure. Upon epidermal damage, keratinocytes release IL-1, which acts in both autocrine and paracrine cascades to further that activate and facilitate migration and proliferation of keratinocytes to improve injury-induced healing.Citation65 Keratinocytes also secrete VEGF and platelet-derived growth factor (PDGF),Citation65 which are critical for endothelial cell migration and angiogenesis in the wound bed.Citation65 Studies have also shown that keratinocytes can also secrete PDGF to promote fibroblast proliferation and production of extracellular matrix.Citation8,Citation64,Citation65 One can imagine that the synchronous activity of fibroblasts and keratinocytes is important for maintaining skin-tissue homeostasis. It is this cross talk that leads to the recruitment of necessary cellular secretory factors for efficient wound closure.Citation65 Our understanding of the function of keratinocytes has also led to the development of bioengineered products to promote skin-tissue regeneration. Upon topical application, the combination of keratinocyte and fibroblasts aids in secretion of growth factors, cytokines and matrix proteins to promote healing and regeneration. Commercially available therapies include products such as EpiCel, Dermagraft and Apligraf.Citation66
Defective function of some antimicrobial peptides such as cathelicidin and β-defensins has been implicated in increased predisposition to skin infections and psoriatic lesionsCitation5 One of these cathelicidins, LL-37, is also produced by keratinocytes and has an essential role in promoting angiogenesis and wound healing.Citation67 Keratinocytes also express TLRs on their cell surface and on the surface of their endosomal vesicles. Keratinocytes are able to generate proinflammatory cytokines such as IL-1β and IL-18 via inflammasome signaling pathway.Citation68 IL-1 produced by keratinocytes can upregulate expression of intercellular adhesion molecule (ICAM)-1. Upregulation of MHC class II on keratinocytes along with LCs facilitates leukocyte trafficking into the skin, hallmark of skin injury response. In disease conditions, keratinocytes express several chemokine ligands such as CXCL9, CXCL10, CXCL11 and CCL20.Citation69 For instance, ligands such as CXCL1 and CXCL8 signal neutrophils for epidermal infiltration.Citation69 Similarly, studies also show that keratinocytes possess the ability to induce T-cell activation or antigen-specific tolerization.
Langerhans cells
Another type of cells that are in close proximity to the epidermis are the LCs that are related to myeloid cells (histologically they are classified as pigmented epidermal cells). Initially, they were classified by a distinct organelle in their cytoplasm – Birbeck granules.Citation70 LCs are scattered throughout the epidermis which enables them to monitor the penetration of the epidermis by foreign materials. LCs are identified by their expression of langerin (CD207) and CD1a.Citation71 The receptor langerin recognized mannosylated ligands on the surface of a wide range of PAMPs. Upon contact, the processing of an antigen begins with its displacement via exocytosis by forming a complex with CD1a and langerin in Birbeck granules. Presenting antigen is the primary function of DCs, whose effectiveness is far superior to other cells, including macrophages. Epidermal LCs have a few unique properties. They express specific proteinases including MMPs which enable them to penetrate through the basal membrane and reach the lymphatic pathway. Expression of MMP8 in LC inhibits TNFα and IL-1β, and its migration can prevent the inhibition of MMP and antibodies to MMP9 and MMP2.Citation53,Citation72
γδ T cells
The epidermis contains a specific subpopulation of lymphocytes - γδ T cells that contain TCR containing γ and δ chains.Citation73 Usually, these cells are generated in the thymus during embryonic development, but a small number are developed elsewhere and survive in the periphery. After their synthesis, they comprise only about 1% of all cells in the thymus and 5% of the periphery. γδ T cells selectively reside in barrier tissues, particularly the epidermis. In mice, the migration of these cells from the thymus begins on the 14th day of embryogenesis. The tropism of these cells does not vary with species, but certain unique features in the cells do exist. These cells in the epidermis of mice acquire a dendritic morphology (dendritic γδ T cells). The epidermal localization of γδ T cells is mainly due to their chemokine receptor expression, synthesized from keratocyte chemokines. More than 80% of cells express CCR5 which causes their migration to the site of inflammation.Citation73 γδ T cell receptors have a narrow antigen-recognizing repertoire. They have the ability to recognize and respond to antigens without the main histocompatibility complex and costimulating moleculesCitation74 due to the presence of TLR-1 and TLR-3 that recognize PAMPs. Inflammation causes an increased expression of NLR-4. γδ lymphocytes can strengthen anti-infective resistance by being one of the main types of cells that produce IL-17 by migrating to the dermal region postbacterial infection or injury.Citation74,Citation75 They also reduce the activity of delayed-type hypersensitivity effector cells and their production of IFNγ and inhibit the activity of αβ lymphocytes in allergic reactions.Citation76 γδ T cells activate DC and lead to their maturation and cytokine production.Citation77 Due to the presence of NKG2D and NKG2 CD94, γδ lymphocytes are able to recognize stress molecules after which they locally synthesize chemokines, cytokines, growth factors and cytokine effectors including IFN-γ.Citation73–Citation75 These can regulate inflammation, cellular cytotoxicity and wound healingCitation74 γδ lymphocytes interact with keratinocytes and support their survival and functionality which contribute to the regeneration of the epidermis when damaged.Citation78 They are among the first cells to react to skin traumaCitation79 and change their morphology within minutes. They express activation markers (CD69) and produce chemokines attracting other inflammation cells to the matrix. In many ways, the reaction is maintained by mitogens secreted by keratinocytes and the subsequent response of the epithelial cells by ligand expression for γδ lymphocytes.Citation80 Important factors stimulating epithelial regeneration are keratinocyte growth factors: KGF1, KGF2, IRT1 and insulin -ike growth factor (IGF).Citation81 The importance for skin regeneration by γδ lymphocytes indicates that the isolation of prolonged wounds of these cells is anergic and unable to produce IGF1.
αβ T lymphocytes
αβ T lymphocytes appear at the epidermis from circulating blood by the attraction of synthesized keratinocyte chemokines (CTACK) and the intercellular interaction of general adhesives – selectin and integrins – with the endothelial receptors (CD34, GlyCAM-1, ICAM1, ICAM2, VCAM1 and MadCAM1).Citation82 Tropism of classical T lymphocytes to the epidermis in significant quantities is determined by the expression of so-called cutaneous lymphocyte antigen.Citation83 Practically all αβ T lymphocytes belong to memory cells.Citation84
Differences in immune cell types during skin repair
All cells of the immune system take part during the reparative process in the regeneration of skin. Their individual roles change depending on the phase. Damage to the skin causes an inflammatory response. The first reaction is the synthesis of proinflammatory cytokines (IL-1, IL-6, TNFα, IFNγ, etc.), chemokines, receptors and adherent molecules that activate keratinocytes. This is followed by the activation of platelets, endothelial cells, mast cells and NKT cells.Citation85 Within the first few days, chemokines attract a large quantity of neutrophilsCitation42 to the wound, followed by its replacement with phagocytic proinflammatory M1 macrophages.Citation86 The act of inflammation is linked to the effect of proinflammatory cytokines, by the transition of M2 macrophages (anti-inflammatory) to M1 phenotype (proinflammatory),Citation87 mast cells, Th2 and T-regulatory cells (TREG). γδ T cells work in a similar manner by secreting factors that stimulate and maintain keratinocytes (IGF-1 and KGF1 and KGF2 growth factors). αβ and γδ T cells are important for the maintenance and homeostasis of the skin via recognition and clearance of stressed or damaged cells.Citation88 Current studies lack the distinct roles of αβ and γδ cells in skin immunity. However, it is certain that their role in immune mechanisms is conserved.Citation88 γδ T cells can recognize a wide variety of peptide and nonpeptide antigens released as a result of constant changes in the skin, while αβ T cells recognize pathogen-specific peptides or tumors presented by MHC molecules.Citation88
Fibroblasts play one of the main roles in the vivification of damaged skin. In a wounded area, the surface of these cells is attracted to the growth factors released by platelets (PDGFs). However, in such conditions, the fibroblasts proliferate and synthesize matrix molecules that are not very effective. It has been shown that in the presence of immune cells the performance of fibroblasts is greatly enhancedCitation89 At the same time, HSC, MSC and endothelial precursors mobilize from the bone marrow.Citation90 Under the influence of the chemokines, stem cells migrate to the wound site and differentiate and proliferate along with resident elements.Citation91 This is followed by angiogenesis, granulation and epithelialization. Effective remodeling occurs in the event of sufficient intervention of MSC, endothelial precursors and embryonic stem cells. Of great importance in this context are cells synthesizing MMPs and extracellular matrix molecules.
It has been shown that keratinocytes and immune cells are capable of secreting steroidal hormones de novo.Citation83 It is possible that this may be limited only to the inflammatory phase: catecholamineCitation92 and acetylcholine. Anti-inflammatory effects are apparently similar to those of the parasympathetic nervous system and influence acetylcholine receptors (nAchR) which are located on the membranes of immune cells.Citation93,Citation94 It is through these receptors that the synthesis of an extracellular matrix by keratinocytes is facilitated along with factors that influence differentiation. The activation of β-adrenergic receptors also has an influence on the migration and differentiation of keratinocytes.
Conclusion and future remarks
Although the immune system of the skin is a nonstructured diffused lymphoid tissue, it has a distinct spatial and functional organization that is unique to it. Its cellular composition is distinguished by a huge variety of cellular components belonging to both the innate and adaptive immunity. Our current knowledge on the cellular immune repertoire of the skin highlights its importance. In the era of emerging awareness toward cancer diagnosis, autoimmune disorders, aging and vaccine development, the current and emerging knowledge on skin’s immune competence holds greater significance. Immune cells of the skin are broadly categorized based on their prevalence in the two distinct layers. It is not as specific in terms of cellular composition pertaining to the dermis and subcutaneous adipose tissue. Various cells from within circulation accumulate and become activated within this space. Classical T- and B lymphocytes and their subpopulations in their absolute and relative state quantitatively correspond to that in the periphery.Citation5,Citation7 This depends on NK and NKT lymphocytes and circulating myeloid cells: neutrophils, basophils, eosinophils and monocytes.Citation53–Citation55
Progenitors of mast cells, myeloid cells and lymphoid DC travel through the bloodstream and migrate to the dermis where they mature as a result of resident elements. The predominant tissues in the bone marrow are MSCs and fibroblasts. MSCs from hair follicles arising from the neural crest are the closest to this sort judging by their properties. It Is worth a mention to state that the quantity of platelets increases during an immune response due to their special properties. The dermis is the structural and functional backbone of the epidermis. It provides access to cells within circulation which are strictly regulated. These selective cells can practically only be found within this area. This is primarily keratinocytes, LC and γδ T lymphocytes. The last two function as part of the innate as well as the adaptive immunity, which links both the skin compartments with the entire body. This is required to present antigens traveling through the lymphatic path in the dermis followed by the lymphatic follicle. γδ T lymphocytes support keratinocytes and promote their regeneration.
Of the classical lymphocytes, only those with the appropriate tropism are found in the epidermis. This is due to the expression of adhesion molecules in the epidermis, which is attracted to chemokines released by keratinocytes from γδ T lymphocytes. B lymphocytes, on the other hand, are absent. The overwhelming majority of the population are memory cells.
Cells of the innate immune system are activated by PRR that recognizes pathogenic and stress signals (PAMP and DAMP). “Intermediate” cells viz. NKT and γδ T lymphocytes express PRRs as do T-cell receptors, though the latter has a different structure and limited capabilities. Upon activation, many cells express high levels of MHC-I and monocyte chemoattractant protein 1 (MCP1), cytokines and their receptors, chemokines and growth factors. This cytokine network when in contact with the many available cell types triggers the required immune response. Both sections of the immune system participate in the regeneration of the skin and its cellular elements. They are also involved in the process of specialization and its various stages. Keratinocytes, platelets, γδ T lymphocytes and mast cells can induce inflammation by attracting phagocytes against any infection and activate mechanisms to trigger adaptive immunity. Attenuation of inflammation converts the proinflammatory M1-Mø into the anti-inflammatory M2-Mø. It also marks the beginning of the proliferative phase of fibroblasts and stem cells in various regions, both local and in the bone marrow, thereby regulating their immune-cooperative interaction and the process of remodeling multichannel tissue design features.
In-depth understanding on the contribution of skin-resident and skin-infiltrating immune populations bolstered by their cross-talk with other cell types can open avenues toward the development of novel therapeutics. Many questions remain unanswered in this emerging area of skin immunology and skin’s regeneration potential. For instance, the establishment of experimental in vitro human and induced pluoripotent stem cell-based systems will allow assessment of the functional capacity of skin’s immune cell repertoire in the context of immune response and their alterations in skin diseases. Further, use of lineage-tracing concepts in such regenerative experimental model systems will enhance our understanding of molecular events and trigger factors that are responsible for immune cell trafficking to sites of regeneration, post skin injury. Taken together, we conclude that near future discoveries using such innovative strategies will not only help us achieve better therapeutic products for skin-related immune disorders but will also foster ideas toward novel cosmetic formulations and topical applications for improving skin’s regenerative potential.
Disclosure
The authors report no conflicts of interest in this work.
References
- Gonzales KAU, Fuchs E. Skin and its regenerative powers: an alliance between stem cells and their niche. Dev Cell. 2017;43(4):387–401. doi:10.1016/j.devcel.2017.10.00129161590
- Streilein JW. Lymphocyte traffic, T-cell malignancies and the skin. J Invest Dermatol. 1978;71(3):167–171. doi:10.1111/1523-1747.ep1254707129071
- Cogen AL, Nizet V, Gallo RL. Skin microbiota: a source of disease or defence? Br J Dermatol. 2008;158(3):442–455. doi:10.1111/j.1365-2133.2008.08437.x18275522
- Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol. 2011;9(4):244. doi:10.1038/nrmicro253721407241
- Matejuk MA. Skin Immunity. Arch Immunol Ther Exp (Warsz). 2017;66(1):45–54. doi:10.1007/s00005-017-0477-328623375
- de Koning HD, Simon A, Zeeuwen PLJM, Schalkwijk J. Pattern recognition receptors in infectious skin diseases. Microbes Infect. 2012;14(11):881–893. doi:10.1016/j.micinf.2012.03.00422516809
- Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20(1):197–216. doi:10.1146/annurev.immunol.20.083001.08435911861602
- Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4(5):267–274.976387
- Bautista-Hernández LA, Gómez-Olivares JL, Buentello-Volante B, Bautista-de Lucio VM. Fibroblasts: the unknown sentinels eliciting immune responses against microorganisms. Eur J Microbiol Immunol (Bp). 2017:7(3):151–157. Published 2017 Aug 19. doi:10.1556/1886.2017.00009.29034104
- Huang Q, Fei J, Yu HJ, Gou YB, Huang XK. Effects of human β-defensin-3 on biofilm formation-regulating genes dltB and icaA in Staphylococcus aureus. Mol Med Resp. 2014;10:825–831.
- Mahanonda R, SardAIam N, Montreekachon P, et al. IL-8 and IDO expression by human gingival fibroblasts via TLRs. J Immunol. 2007;178(2):1157. doi:10.4049/jimmunol.178.2.1151
- Kirker KR, James GA, Fleckman P, Orelund JE, Stewart PS. Differential effects of planktonic and biofilm MRSA on human fibroblasts. Wound Repair Regen. 2012;20:253–261. doi:10.1111/j.1524-475X.2012.00769.x22332802
- Stappers MHT, Thys Y, Oosting M, et al. TLR1, TLR2, and TLR6 gene polymorphisms are associated with increased susceptibility to complicated skin and skin structure infections. J Infect Dis. 2014;210(2):311–318. doi:10.1093/infdis/jiu08024511099
- Dominici MLBK, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International society for cellular therapy position statement. Cytotherapy. 2006;8(4):315–317. doi:10.1080/1465324060085590516923606
- Janson D, Rietveld M, Mahé C, Saintigny G, El Ghalbzouri A. Differential effect of extracellular matrix derived from papillary and reticular fibroblasts on epidermal development in vitro. Eur J Dermatol. 2017;27(3):237–246. doi:10.1684/ejd.2017.298428524059
- Ma T, Wang X, Jiang D. Immune tolerance of mesenchymal stem cells and induction of skin allograft tolerance. Curr Stem Cell Res Ther. 2017;12(5):409–415. doi:10.2174/1574888x1266617030112274428260519
- Kühbacher A, Henkel H, Stevens P, et al. Dermal fibroblasts play a central role in skin model protection against C. albicans invasion. J Infect Dis. 2017;215. doi:10.1093/infdis/jix153.
- Wang X, Cheng Q, Li L, et al. Toll-like receptors 2 and 4 mediate the capacity of mesenchymal stromal cells to support the proliferation and differentiation of CD34+ cells. Exp Cell Res. 2012;318(3):196–206. doi:10.1016/j.yexcr.2011.11.00122100911
- Jackson MV, Morrison TJ, Doherty DF, et al. Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem Cells. 2016;34(8):2210–2223. doi:10.1002/stem.237227059413
- Rocher BD, Mencalha AL, Gomes BE, Abdelhay E. Mesenchymal stromal cells impair the differentiation of CD14++ CD16− CD64+ classical monocytes into CD14++ CD16+ CD64++ activate monocytes. Cytotherapy. 2012;14(1):12–25. doi:10.3109/14653249.2011.59479221838603
- Abumaree MH, Al Jumah MA, Kalionis B, et al. Human placental mesenchymal stem cells (pMSCs) play a role as immune suppressive cells by shifting macrophage differentiation from inflammatory M1 to anti-inflammatory M2 macrophages. Stem Cell Rev Rep. 2013;9(5):620–641. doi:10.1007/s12015-013-9455-2
- Mei SHJ, Haitsma JJ, Dos Santos CC, et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182(8):1047–1057. PMID: 20558630. doi:10.1164/rccm.201001-0010OC.20558630
- Burr SP, Dazzi F, Garden OA. Mesenchymal stromal cells and regulatory T cells: the Yin and Yang of peripheral tolerance? Immunol Cell Biol. 2013;91(1):12–18. doi:10.1038/icb.2012.6023146942
- Asadi M, Farokhi F, Delirezh N, Ganji Bakhsh M, Nejati V, Golami K. Fibroblast and T cells conditioned media induce maturation dendritic cell and promote T helper immune response. Veterinary research forum : an international quarterly journal 2012;3(2):111–118.
- English K, Barry FP, Mahon BP. Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunol Lett. 2008;115(1):50–58. doi:10.1016/j.imlet.2007.10.00218022251
- Gay D, Kwon O, Zhang Z, et al Fgf9 from dermal γδ T cells induces hair follicle neogenesis after wounding. Nat Med. 2013;19(7):916–923. doi:10.1038/nm.318123727932
- Kashem SW, Haniffa M, Kaplan DH. Antigen-presenting cells in the skin. Annu Rev Immunol. 2017;35:469–499. doi:10.1146/annurev-immunol-051116-05221528226228
- Dos Santos VG, Orfali RL, de Oliveira Titz T, Da Silva Duarte AJ, Sato MN, Aoki V. Evidence of regulatory myeloid dendritic cells and circulating inflammatory epidermal dendritic cells‐like modulated by Toll‐like receptors 2 and 7/8 in adults with atopic dermatitis. Int J Dermatol. 2017;56(6):630–635. doi:10.1111/ijd.1353728083892
- Kyewski BA, Momburg F, Schirrmacher V. Phenotype of stromal cell‐associated thymocytes in situ is compatible with selection of the T cell repertoire at an “immature” stage of thymic T cell differentiation. Eur J Immunol. 1987;17(7):961–967. doi:10.1002/eji.18301707113111861
- Khasawneh A, Baráth S, Medgyesi B, et al. Myeloid but not plasmacytoid blood DCs possess Th1 polarizing and Th1/Th17 recruiting capacity in psoriasis. Immunol Lett. 2017;189:109–113. doi:10.1016/j.imlet.2017.04.00528414181
- Han Z, Chen Y, Zhang Y, et al. MiR‐21/PTEN axis promotes skin wound healing by dendritic cells enhancement. J Cell Biochem. 2017;118(10):3511–3519. doi:10.1002/jcb.2602628374893
- Gregorio J, Meller S, Conrad C, et al. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J Exp Med. 2010;207(13):2921–2930. doi:10.1084/jem.2010110221115688
- Jacquemin C, Rambert J, Guillet S, et al. Heat shock protein 70 potentiates interferon alpha production by plasmacytoid dendritic cells: relevance for cutaneous lupus and vitiligo pathogenesis. Br J Dermatol. 2017;177(5):1367–1375. doi:10.1111/bjd.1555028380264
- Gallucci RM, Sloan DK, Heck JM, Murray AR, O’Dell SJ. Interleukin 6 indirectly induces keratinocyte migration. J Invest Dermatol. 2004;122:764–772. doi:10.1111/j.0022-202X.2004.22323.x15086564
- Hoeffel G, Wang Y, Greter M, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med. 2012;209(6):1167–1181. doi:10.1084/jem.2012034022565823
- Minutti CM, Knipper JA, Allen JE, Zaiss DMW. Tissue-specific contribution of macrophages to wound healing. Semin Cell Dev Biol. 2017;61:3–11. ISSN 1084-9521. doi:10.1016/j.semcdb.2016.08.00627521521
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. doi:10.1016/j.it.2004.09.01515530839
- Gerber JS, Mosser DM. Reversing lipopolysaccharide toxicity by ligating the macrophage Fcγ receptors. J Immunol. 2001;166(11):6861–6868. doi:10.4049/jimmunol.166.11.686111359846
- Knipper JA, Willenborg S, Brinckmann J, et al. Interleukin-4 receptor α signaling in myeloid cells controls collagen fibril assembly in skin repair. Immunity. 2015;43(4):803–816. doi:10.1016/j.immuni.2015.09.00526474656
- Lucas T, Waisman A, Ranjan R, et al. Differential roles of macrophages in diverse phases of skin repair. J.Immunol. 2010;184(7):3964–3977. doi:10.4049/jimmunol.090335620176743
- Willenborg S, Lucas T, vanLoo G, et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood. 2012;120(3):613–625. doi:10.1182/blood-2012-01-40338622577176
- Wilgus TA, Roy S, McDaniel JC. Neutrophils and wound repair: positive actions and negative reactions. Adv Wound Care. 2013;2(7):379–388. doi:10.1089/wound.2012.0383
- Gutiérrez-Fernández, Inada M, Balbin M, et al. Increased inflammation delays wound healing in mice deficient in collagenase-2 (MMP-8). FASEB J. 2017;21(10):2580–2591. doi:10.1096/fj.06-7860com
- Skrzeczynska-Moncznik J, Zabieglo K, Bossowski JP, et al. Eosinophils regulate interferon alpha production in plasmacytoid dendritic cells stimulated with components of neutrophil extracellular traps. Int J Interferon Cytokine Res. 2017;37(3):119–128. doi:10.1089/jir.2016.0036
- Lamback EB, Resende FSS, Lenzi TCR. Eosinophilic fasciitis. An Bras Dermatol. 2016;91(5):57–59. doi:10.1590/abd1806-4841.2016468328300895
- Otsuka A, Kabashima K. Contribution of basophils to cutaneous immune reactions and Th2-mediated allergic responses. Front Immunol. 2015;6:393. doi:10.3389/fimmu.2015.0039326284076
- Larouche J, Sheoran S, Maruyama K, Martino MM. Immune regulation of skin wound healing: mechanisms and novel therapeutic targets. Adv Wound Care. 2018;7(7):209–231. doi:10.1089/wound.2017.0761
- Schwartz C, Eberle JU, Voehringer D. Basophils in inflammation. Eur J Pharmacol. 2016;778:90–95. doi:10.1016/j.ejphar.2015.04.04925959388
- Voehringer D. Recent advances in understanding basophil functions in vivo. F1000Res. 2017;6:1464 Published 2017 Aug 15. doi:10.12688/f1000research.11697.128868143
- Cardamone C, Parente R, Feo GD, Triggiani M. Mast cells as effector cells of innate immunity and regulators of adaptive immunity. Immunol Lett. 2016;178:10–14. doi:10.1016/j.imlet.2016.07.00327393494
- Igawa S, Nardo AD. Skin microbiome and mast cells. Transl Res. 2017;184:68–76. doi:10.1016/j.trsl.2017.03.00328390799
- Bonefeld CM, Geisler C. The role of innate lymphoid cells in healthy and inflamed skin. Immunol Lett. 2016;179:25–28. doi:10.1016/j.imlet.2016.01.00526794088
- Levin C, Bonduelle O, Nuttens C, et al. Critical role for skin-derived migratory DCs and Langerhans cells in TFH and GC responses after intradermal immunization. J Invest Dermatol. 2017;137(9):1905–1913. doi:10.1016/j.jid.2017.04.01628457909
- Godfrey DI, Pellicci DG, Smyth MJ. The elusive NKT cell antigen–is the search over? Science. 2004;306(5702):1687–1689. doi:10.1126/science.110693215576595
- Linsen L, Somers V, Stinissen P. Immunoregulation of autoimmunity by natural killer T cells. Hum Immunol. 2005;66(12):1193–1202. doi:10.1016/j.humimm.2006.02.02016690406
- Koupenova M, Vitseva O, MacKay CR, et al. “Platelet-TLR7 mediates host survival and platelet count during viral infection in the absence of platelet-dependent thrombosis. Blood. 2014; blood-2013. doi:10.1182/blood-2013-11-536003.
- Zufferey A, Schvartz D, Nolli S, Reny J-L, Sanchez J-C, Fontana P. Characterization of the platelet granule proteome: evidence of the presence of MHC1 in alpha-granules. J Proteomics. 2014;101:130–140. doi:10.1016/j.jprot.2014.02.00824549006
- Wagner DD, Frenette PS. The vessel wall and its interactions. Blood. 2008;111(11):5271–5281. doi:10.1182/blood-2008-01-07820418502843
- Hartwig H, Drechsler M, Lievens D, et al. Platelet-derived PF4 reduces neutrophil apoptosis following arterial occlusion. Thromb Haemost. 2014;111(3):562–564. doi:10.1160/TH13-08-0699
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585–601. doi:10.1111/j.1524-475x.2008.00410.x19128254
- Bayer A, Lammel J, Lippross S, et al. Platelet-released growth factors induce psoriasin in keratinocytes: implications for the cutaneous barrier. Ann Anat Anz. 2017;213:25–32. doi:10.1016/j.aanat.2017.04.002
- Chicharro-Alcántara D, Rubio-Zaragoza M, Damiá-Giménez E, et al. Platelet rich plasma: new insights for cutaneous wound healing management. J Funct Biomater. 2018;9(1):10 Published 2018 Jan 18. doi:10.3390/jfb9010010
- Mann ER, Smith KM, Bernardo D, Al-Hassi HO, Knight SC, Hart AL. Review: Skin and the Immune System. J Clin Exp Dermatol Res 2012;S2:003. doi:10.4172/2155-9554.S2-003
- Luger TA, Kock A, Kirnbauer R, Schwarz T, Ansel JC. Keratinocyte‐Derived Interleukin 3. Ann N Y Acad Sci. 1988;548(1):253–261. doi:10.1111/j.1749-6632.1988.tb18813.x2470298
- Wojtowicz AM, Oliveira S, Carlson MW, Zawadzka A, Rousseau CF, Baksh D. The importance of both fibroblasts and keratinocytes in a bilayered living cellular construct used in wound healing. Wound Repair Regen. 2014;22(2):246–255. doi:10.1111/wrr.1215424635175
- Auger FA, Lacroix D, Germain L. Skin substitutes and wound healing. Skin Pharmacol Physiol. 2009;22:94–102. doi:10.1159/00017886819188757
- Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol. 2004;75:39–48. doi:10.1189/jlb.040314712960280
- Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi:10.1146/annurev.immunol.021908.13271519302040
- Albanesi C, Scarponi C, Giustizieri M, Girolomoni G. Keratinocytes in inflammatory skin diseases. Curr Drug Targets Inflamm Allergy. 2005;4(3):329–334. doi:10.2174/156801005402203316101542
- Mc Dermott R, Ziylan U, Spehner D, et al. Birbeck granules are subdomains of endosomal recycling compartment in human epidermal Langerhans cells, which form where Langerin accumulates. Mol Biol Cell. 2002;13(1):317–335. doi:10.1091/mbc.01-06-030011809842
- Bursch LS, Wang L, Igyarto B, et al. Identification of a novel population of Langerin+ dendritic cells. J Exp Med. 2007;204(13):3147–3156. doi:10.1084/jem.2007196618086865
- Johnson LA, Banerji S, Lawrance W, et al. Dendritic cells enter lymph vessels by hyaluronan-mediated docking to the endothelial receptor LYVE-1. Nat Immunol. 2017;18(7):762. doi:10.1038/ni.375028504698
- Fay NS, Larson EC, Jameson JM. Chronic inflammation and γδ T cells. Front Immunol. 2016;7:210. doi:10.3389/fimmu.2016.0021027303404
- MacLeod AS, Havran WL. Functions of skin-resident γδ T cells. Cell Mol Life Sci. 2011;68(14):2399–2408. doi:10.1007/s00018-011-0702-x21560071
- Hartwig H, Drechsler M, Lievens D, et al. Platelet-derived PF4 reduces neutrophil apoptosis following arterial occlusion. Thromb Haemost. 2014;111(3):562–564. doi:10.1160/TH13-08-069924258616
- McMenamin C, Pimm C, McKersey M, Holt PG. Regulation of IgE responses to inhaled antigen in mice by antigen-specific gamma delta T cells. Science. 1994;265(5180):1869–1871. doi:10.1126/science.79164817916481
- Ramírez-Valle F, Gray EE, Cyster JG. Inflammation induces dermal Vγ4+ γδT17 memory-like cells that travel to distant skin and accelerate secondary IL-17–driven responses. Proc Natl Acad Sci. 2015;112(26):8046–8051. doi:10.1073/pnas.150899011226080440
- Sharp LL, Jameson JM, Cauvi G, Havran WL. Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat Immunol. 2005;6(1):73. doi:10.1038/ni115215592472
- Komori HK, Witherden DA, Kelly R, Sendaydiego K, Jameson JM, Teyton L, Havran WL. Cutting edge: dendritic epidermal γδ T cell ligands are rapidly and locally expressed by keratinocytes following cutaneous wounding. J Immunology 2012;188(7):2972–2976. doi:10.4049/jimmunol.1100887
- Xu P, Fu X, Xiao N, et al. Involvements of γδT lymphocytes in acute and chronic skin wound repair. Inflammation. 2017;40(4):1416–1427. doi:10.1007/s10753-017-0585-628540539
- Toulon A, Breton L, Taylor KR, et al. A role for human skin–resident T cells in wound healing. J Exp Med. 2009;206(4):743–750. doi:10.1084/jem.2008178719307328
- Campbell JJ, O’Connell DJ, Wurbel M-A. Cutting edge: chemokine receptor CCR4 is necessary for antigen-driven cutaneous accumulation of CD4 T cells under physiological conditions. J Immunol. 2007;178(6):3358–3362. doi:10.4049/jimmunol.178.6.335817339428
- Slominski AT, Manna PR, Tuckey RC. Cutaneous glucocorticosteroidogenesis: securing local homeostasis and the skin integrity. Exp Dermatol. 2014;23(6):369–374. doi:10.1111/exd.1237624888781
- Fuhlbrigge RC, Kieffer JD, Armerding D, Kupper TS. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature. 1997;389(6654):978. doi:10.1038/401669353122
- Richmond JM, Harris JE. Immunology and skin in health and disease. Cold Spring Harb Perspect Med 2014;4(12):a15339. doi:10.1101/cshperspect.a015339
- Barinova ME, Barinov EF, Sulaieva OM. Functional activity of monocytes and mechanisms of iNOS intracellular regulation during wound process. Fiziol Zh. 2011;57(1):36–44.21516832
- Gilroy D, De Maeyer R. New insights into the resolution of inflammation. Semin Immunol. 2015:27(3):161–168. Academic Press. doi:10.1016/j.smim.2015.05.003.26037968
- Cruz MS, Diamond A, Russell A, Jameson JM. Human αβ and γδ T cells in skin immunity and disease. Front Immunol. 2018;9:1304 Published 2018 Jun 6. doi:10.3389/fimmu.2018.0130429928283
- Supp DM, Wilson-Landy KAILA, Boyce ST. Human dermal microvascular endothelial cells form vascular analogs in cultured skin substitutes after grafting to athymic mice. Faseb J. 2002;16(8):797–804. doi:10.1096/fj.01-0868com12039861
- Nikolsky IS, Serebrovska TV. Role of hypoxia in stem cell development and functioning. Int J Physiol Pathophysiol Pharmacol. 2010;1(1). doi:10.1615/IntJPhysPathophys.v1.i1.90
- Serebrovskaya TV, Nikolsky IS, Nikolska VV, Mallet RT, Ishchuk VA. Intermittent hypoxia mobilizes hematopoietic progenitors and augments cellular and humoral elements of innate immunity in adult men. High Alt Med Biol. 2011;12(3):243–252. doi:10.1089/ham.2010.108621962068
- Grando SA, Pittelkow MR, Schallreuter KU. Adrenergic and cholinergic control in the biology of epidermis: physiological and clinical significance. J Invest Dermatol. 2006;126(9):1948–1965. doi:10.1038/sj.jid.570015116912692
- Pongratz G, Straub RH. Role of peripheral nerve fibres in acute and chronic inflammation in arthritis. Nat Rev Rheumatol. 2013;9(2):117. doi:10.1038/nrrheum.2012.18123147892
- Vinik AI, Strotmeyer ES. Diabetic neuropathy. Pathy’s Principles Pract Geriatric Med. 2012;1:751–767. doi:10.1002/9781119952930.ch65
