Abstract
Rhinophyma is an advanced stage of rosacea affecting the nasal soft tissues and resulting in disruption of the nasal architecture, airway obstruction, and disfigurement of the nasal aesthetic units. Rhinophyma presents with hypertrophy of the nasal soft tissues, erythema, telangiectasias, nodules, and lobules with a bulbous appearance. Significant psychosocial morbidity is associated with the disease. Understanding of this disease has improved and multiple treatment options exist. The article is a review of the literature to evaluate the pathophysiology, clinical presentation, and epidemiology of keywords “rhinophyma” and “rosacea” using an OVID Medline and PubMed search along with a systematic review of outcomes pertaining to treatment of rhinophyma with laser therapy, scalpel excision, and the subunit method using an OVID Medline search. The subunit method has the highest complication and revision rates followed by carbon dioxide laser therapy. Outcomes between carbon dioxide laser and scalpel therapy and electrocautery are equivalent. Scalpel excision is a more cost-effective treatment modality with less post-operative complications; however, it risks poor hemostasis intraoperatively. Patient satisfaction is common post-therapy regardless of the treatment method. Over 89% of patients would recommend undergoing treatment for rhinophyma irrespective of treatment type. Treatment options vary, and choice of treatment can be dependent on practitioner and patients’ treatment goals.
Keywords:
Introduction
Rhinophyma is an advanced stage of rosacea of the nasal soft tissues. The condition results in a progressive disruption of the nasal architecture, airway obstruction, and disfigurement of nasal aesthetic units. Rhinophyma may cause significant psychosocial morbidity,Citation1 and treatment may improve patient self-confidence and psychological well-being.Citation2 Patients may be afraid to go out in public for fear of social stigmatization, as rhinophyma has erroneously been associated with alcoholism. Although there is no cure for rhinophyma, the understanding of the disease has improved, and multiple treatment options exist. The purpose of this article is to evaluate the pathophysiology, clinical presentation, epidemiology, of rhinophyma, and perform a systematic review of its treatments.
Methods
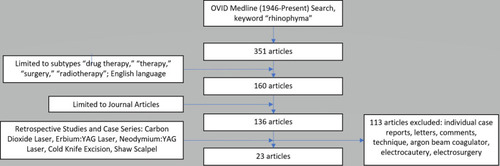
We conducted a review of the literature to evaluate the pathophysiology, clinical presentation, and epidemiology of keyword “rhinophyma” and “rosacea” as an OVID Medline search limited to the years 1946 to present and PubMed. A systematic review of laser therapy, tangential excision, and the subunit method was conducted to evaluate treatment outcomes of rhinophyma. An OVID Medline search limited to 1946 to present was conducted using keyword “rhinophyma,” limited to articles in the English language, journal articles, and subtype categories “drug therapy,” “therapy,” “surgery,” and “radiotherapy.” Individual case reports, letters, comments, technique articles, and articles unavailable for viewing were excluded. Retrospective studies and case series for laser therapy, scalpel excision, and the subunit method were included ().
Epidemiology
The prevalence of rosacea is 5.46% with an estimated range between 0.092% and 2.41% per a 2018 meta-analysis.Citation3 Previous literature estimates the female-to-male ratio to be 3:1;Citation3–Citation5 however, Gether et al showed equal affliction of the disease between males and females.Citation3 In contrast to rosacea, rhinophyma is more common in men. The estimated male-to-female ratio of patients with rhinophyma ranges from 5:1 to 30:1 and is believed to be mediated by increased androgen activity in males.Citation4 Rhinophyma most commonly affects Caucasians between their fifth and seventh decadeCitation4,Citation6,Citation7 and is rare among African American populations and in Asia.Citation8–Citation10
Clinical Presentation
Clinical Presentation
Rosacea presents with flushing (transient erythema), nontransient erythema, papules, pustules, and telangiectasias localized to the central face, including the nose, cheeks, forehead, glabella, and chin.Citation11–Citation13 Burning/stinging, plaques, dry appearance, edema, ocular involvement, and phymatous changes may occur.Citation11–Citation13 A standard grading system created by the National Rosacea Society Expert Committee characterized the disease into four subtypes, including erythematotelangiectactic, papulopustular, phymatous, and ocular.Citation11 This was modified in 2017 as reported by the National Rosacea Society Expert Committee and the global Rosacea Consensus (ROSCO) panel into a classification system based on phenotype rather than fixed subtypes. Persistent centrofacial erythema or phymatous changes are diagnostic features of rosacea.Citation12,Citation13 Major features include flushing/transient centrofacial erythema, inflammatory papules and pustules, and telangiectasias.Citation12,Citation13 Minor features include burning sensation of the skin, stinging sensation of the skin, edema, and dry sensation of the skin.Citation12,Citation13 Ocular manifestations are both major and minor features of rosacea.
The phymatous feature of rosacea is characterized by a benign thickening of skin, surface irregularities, and enlargement with variable presence of patulous, expressive follicles and telangiectasias.Citation11–Citation13 Phymatous disease most commonly presents as rhinophyma (nose); however, it rarely can develop on the chin (gnathophyma), forehead (metophyma), ears (otophyma), and eyes (blepharophyma).Citation14,Citation15
Rhinophyma presents with hypertrophy of the nasal soft tissues, erythema, telangiectasias, nodules, and lobule with a bulbous appearance. Nasal obstruction may occur in severe cases marked by external nasal valve collapse. Rhinophyma affects the lower two-thirds of the nose including the nasal tip, nasal ala and distal dorsum of the nose without appreciable involvement of the nasal sidewalls. Progression of the disease can result in obliteration and blending of the nasal aesthetic subunits. No involvement of the underlying cartilaginous and osseous structures has been identified.
Differential Diagnosis
Rhinophyma is diagnosed based on clinical exam and can be corroborated with concurrent diagnosis of rosacea. Malignant neoplasms may mimic rhinophyma in presentation or may be occult in existing rhinophyma. Underlying malignancy must be excluded. There is an estimated 3%–10% incidence of basal cell carcinoma in patients with rhinophyma;Citation16 however, frequency of malignancy is not reported to be increased in patients with rhinophyma. Conditions reported to occur within rhinophyma include basal cell carcinoma,Citation16–Citation22 cutaneous squamous cell carcinoma,Citation23,Citation24 B-cell neoplasms,Citation25–Citation27 schwannoma,Citation28 cutaneous angiosarcoma,Citation23,Citation29,Citation30 and sebaceous carcinoma.Citation31,Citation32
Grading of Rhinophyma
Several authors propose scaling systems to grade the severity and features of rhinophyma ().Citation15,Citation33,Citation34 Clark et al proposed a classification system based on distribution and degree of involvement of the disease.Citation34 Freeman describes a classification system that grades rhinophyma by degree of severity.Citation35 El-Azhary et al proposed a grading system of minor, moderate, and major rhinophyma based on the degree and presence of hypertrophy and lobules present; this grading system is used most often in treatment studies.Citation33 Wetzig et al developed the Rhinophyma Severity Index (RHISI), which numerically scales the disease based on degree of skin thickening, presence of lobules and fissures, and secondarily presence of prominent asymmetry, cysts, or vessels.Citation36 These grading systems communicate severity of disease but do not guide a particular treatment modality.
Table 1 Classification and Grading of Rhinophyma
Pathophysiology and Histopathology
The exact etiology and pathogenesis of rosacea and rhinophyma is not well understood. Rosacea is believed to be a multifactorial disease. Heat, stress, ultraviolet light, smoking, alcohol, spicy food, and hot beverages are reported as possible exacerbating triggers.Citation37 The role of microorganisms such as Helicobacter pylori through production of vasodilating agents serum gastrin or nitrous oxide may promote transient erythema. Antibody production against collagen VII, elastin, and Demodex folliculorum mite can contribute to the development of papulopustular rosacea.Citation5,Citation37
Phymatous disease is less understood. Rhinophyma is considered an advanced stage and subtype of rosacea characterized by chronic edema, hypervascularity, connective tissue, and sebaceous glandular hypertrophy, and fibrosis.Citation11 Heavy alcohol use was historically considered an underlying cause of rhinophyma but has been debunked. Curnier et al showed no difference in alcohol consumption levels between rhinophyma patients and a control group.Citation18
Studies suggest a pattern of chronic inflammation and fibrosis mediating the development and progression of rhinophyma. The presence on immunohistochemical staining of numerous Factor XIIIa positive fibroblasts and overexpression of TGF-β2 and its receptor intimates the role of fibrosis in its pathogenesis.Citation38–Citation42 Payne et al showed downregulation of TGF-β2 in collagen lattices from rhinophyma tissue using the non-steroidal antiestrogen Tamoxifen.Citation42
Classic (or glandular) and fibrous types are typically described in histopathologic studies. Prominent sebaceous hyperplasia, dilated infundibula with occasional cysts, telangiectasia, presence of perifollicular infiltrates, and occasional Demodex folliculorum infestation may be exhibited histologically.Citation15,Citation39,Citation41 In the fibrous variant of rhinophyma, elastosis, altered architecture of the extracellular matrix, edematous stroma, reduction in pilosebaceous units, thickened dermis, scant presence of perifollicular infiltrates, and absence of elastic tissue in areas of fibrosis are present.Citation15,Citation38,Citation39
Four histological variants of rhinophyma are described by Jansen et al (). The fibrous variant is similar in histology to previous descriptions. The glandular form, similar to the “classic” form, features an enlarged nose with sebaceous hyperplasia, normal to erythematous skin, increased expression of sebum, and infestation with Demodex mites.Citation15 The fibroangiomatous form is characterized by erythema, edema, and presence of pustules.Citation15 The actinic form is characterized by nodular masses of elastic tissue similar to photodamaged skin and histologically with ducts filled with sebum; additionally, there is noted presence of Demodex folliculorum mites, Propionibacterium acnes, and yeast-like organisms.Citation15
Table 2 Variants of Rhinophyma
Treatment of Rhinophyma
Nonsurgical Treatment
Radiotherapy
Radiotherapy was a treatment option predominantly in the 1920s–1930s.Citation19 Plenk reported two cases of rhinophyma with concurrent basal cell carcinoma (BCCa) treated with x-ray therapy with successful control of the BCCas and improvement in their rhinophyma.Citation19 Dosing at 40 Gy in 20 fractions is reported, and doses lower than 40 Gy cause involution of sebaceous glands and subsequent decrease in the number of sebaceous glands and atrophy of pilosebaceous units.Citation43 Given the increased risk of malignancy with repeated exposure to radiation, radiotherapy is no longer a recommended treatment option for rhinophyma.
Enteral and Topical Therapy
The current mainstay of treatment of papulopustular rosacea includes topical therapies such as azelaic acid, ivermectin, and oral therapies such as metronidazole, doxycycline, tamoxifen, and isotretinoin.Citation44,Citation45 Inflamed rhinophyma may be treated with oral doxycycline and isotretinoin (13-cis-retinoic acid); topical therapies are not recommended.Citation44,Citation45
Doxycycline is a tetracycline antibiotic that is considered effective on diseases involving the pilosebaceous units.Citation5 Dosing at 40 mg is found to be superior to placebo and noninferior to a dose of 100 mg for treatment of clinically inflamed rhinophyma and exhibits predominantly anti-inflammatory effects.Citation42 Side effects include photosensitivity and pseudotumor cerebri.
Isotretinoin has successfully improved rhinophyma but recurrence of disease in 1 year can occur.Citation15,Citation44,Citation45 Isotretinoin significantly reduces the volume of papules and pustules and thus sebum production, lymphohistiocytic perivascular infiltration, edema, and number of ectatic vessels.Citation5,Citation46 Dosing of isotretinoin ranges from 0.5 to 1.0 mg/kg. Drug intolerance presents as dry eyes, cheilitis, and facial dermatitis; more adverse reactions include alopecia, epistaxis, myalgias, arthralgias, elevated triglyceride and cholesterol levels, pseudotumor cerebri, hepatotoxicity, and enhancement of depression.Citation5 It is a known teratogen and should not be given to pregnant women.
Tamoxifen, a nonsteroidal anti-estrogen, is shown to downregulate the expression of TGF-β2 and mediate a reduction in fibrosis in vitro in tissue from patients with rhinophyma.Citation42 It may be another option for systemic therapy of rhinophyma; however, in vivo studies are needed to determine its clinical applicability, efficacy, and safety.
Surgical Treatment
Given the limited role of oral therapy for treatment of rhinophyma and low likelihood of spontaneous regression of the disease, surgical removal remains the primary mode of treatment. Indications for surgical treatment are correction of the aesthetic deformity and secondary nasal airway obstruction. Surgical principles include removal of phymatous tissue and preservation of the nasal aesthetic subunits. The pilosebaceous units can promote re-epithelialization.Citation47 Excised tissue should be histologically evaluated to rule out malignancy.
The nasal aesthetic subunits consist of the nasal tip, nasal ala, nasal sidewalls, nasal dorsum, soft triangle, and columella. Preserving these subunits optimizes the aesthetic outcome. The use of local anesthesia facilitates excision of rhinophyma tissue. Depending on surgeon preference and experience, a nasal ring block (anterior ethmoidal nerve, infra-trochlear nerve, and infra-orbital nerve), tumescence, or both are administered using 1% lidocaine-1:100,000 epinephrine.
Patient reported outcomes have been studied using two existing scales by Har-El et al, and Clark and Hanke (). The latter scales are commonly used to evaluate treatment results in a variety of studies. In these scales, a minimal degree of scarring is still considered a “good” result. Lazzeri et al created a patient questionnaire assessing patient satisfaction. This questionnaire is also used in several studies to report outcomes.
Table 3 Post-Operative Outcome Scales
A variety of surgical treatments exist including dermabrasion, electrocautery, electrosurgery/radiofrequency, cryosurgery, cold knife excision, Shaw scalpel, and the subunit method. We highlight outcomes of scalpel excision, laser therapy, and the subunit method.
Laser Therapy
Laser therapy is an ablative approach to rhinophyma treatment that can both debulk and contour the nose. Carbon dioxide (CO2), erbium:YAG (Er:YAG), and neodymium (Nd:YAG) laser therapy is discussed ().
Table 4 Surgical Outcomes by Study
A carbon dioxide laser consists of a coherent, collimated beam that emits invisible light at wavelength of 10,600 µm.Citation48 Water is its target chromophore. A focused mode enables cutting of tissue while a defocused mode facilitates complete absorption of water and tissue loss in a nonspecific fashion. Depth of penetration can extend to the upper layers of the dermis.
The erbium:YAG (Er:YAG) laser is a solid-state laser that emits infrared light at a typical wavelength of 2940 nm, the peak absorption of water. Its shorter wavelength of absorption provides a more specific absorption spectrum and decreased depth of penetration per mass in contrast to the CO2 laser.Citation49 The depth of penetration is estimated to be 10–30 µm per passCitation50,Citation52 or at least equal to or less than the average depth of epidermis. The neodymium:YAG laser is a solid-state laser that emits infrared light at a continuous wave of 1064 nm, a wavelength absorbed by hemoglobin leading to vessel destruction.Citation53 It provides 4–6 mm depth of penetration with a nonspecific spread of thermal energy.Citation54
Among 12 studies, 247 patients with a mean age of 61 years and minor to major disease (minor, n = 67; moderate, n = 64; and major, n = 87) were treated with a carbon dioxide laser in an average of 1.1 sessions (–). A total of 18 patients was treated, with a mean age of 62 years, and a total of 1 patient with minor, 12 with moderate, and five with major rhinophyma using the Er:YAG laser in 1.0 sessions. One study evaluated Nd:YAG laser treatment, five patients underwent treatment with mean age of 61 years, and severity of disease was not reported. Adequate hemostasis was achieved via the carbon dioxide laser or Nd:YAG laser; adjunct methods were required for the Er:YAG laser. Goon et al utilized a combined CO2 and Er:YAG laser, the Er:YAG laser for ablation and the CO2 laser at low settings for its coagulative effects. Similarly, Fincher et al used a dual-mode Er:YAG laser with a similar function as a combined Er:YAG/CO2 laser.
Table 7 Summary of Outcomes by Treatment Type
Post-operatively, for patients who underwent carbon dioxide laser therapy, post-operative erythema was reported to last between 6 and 12 weeks, re-epithelialization occurred in 1.4–6 weeks, recurrence occurred in 1.2% of patients (range 0.0%–25.0%), with a 10.5% complication rate and a 2.0% revision rate. Reported complications of the procedure include formation of a unilateral alar lift or notching, leukoderma, hypertrophic scarring, hypopigmentation, and dilated sebaceous folliclesCitation2,Citation33,Citation48,Citation55,Citation56 (). Patients undergoing Er:YAG and Nd:YAG laser therapy had 0% recurrence, complication, and revision rates. Post-operative erythema was minimal between these techniques, and re-epithelialization occurred within 1–4 weeks in patients undergoing Er:YAG laser.
Table 8 Summary of Complications by Treatment Type
Complete satisfaction regarding the procedure was reported between 77% and 84.1% of patients, and 92%–89.4% of patients would recommend surgeryCitation2,Citation57 (). Eight patients were reported to have poor or unacceptable results and the remainder of patients had good to excellent outcomes (). In Madan et al, patients self-reported an increase in self-confidence post-operatively.Citation2 Lazzeri et al showed excellent self-impression among 89.4% of patients; however, 78.9% of patients felt time back to social life was prolonged.Citation57
Table 5 Surgical Outcomes by Study
Excisional Techniques
Cold Knife and Shaw Scalpel Excision
Tangential excision with secondary healing is a common method of treatment. Excision with a 10-blade scalpel, Weck blade, or disposable razor is performed until adequate debulking of tissue and visualization of the pilosebaceous units is achieved. This is done alone or with adjunct therapies such as dermabrasion and argon beam coagulator.Citation58,Citation59 The Shaw scalpel is a thermal scalpel used for excision of rhinophyma; the scalpel is set between 160°C and 200 °C.
A total of 108 patients underwent cold knife tangential excision among eight studies. Patients had a mean age of 61 years, treated for minor to major rhinophyma, and all required a single session for treatment (–).Citation36,Citation55,Citation57-Citation59 Seven patients with a mean age of 67 years underwent treatment with a Shaw scalpel and all required a single session for treatment. Post-operative erythema was reported in one study and reported to last 6–9 weeks, otherwise no post-operative erythema occurredCitation60 and re-epithelialization was reported to occur between 2 and 6 weeks. There was an overall 10.2% recurrence rate, 3.7% complication rate, and 0.0% revision rate. Complete satisfaction was reported in 78%–85% of patients, with 92.51% of patients recommending the surgery.Citation36,Citation57 Lazzeri et al reported 75% of patients with sufficient time of return to social life and 92.5% of patients reporting excellent self-impression following tangential excision. Overall patients had good to excellent results with only three patients reported to have an unacceptable result in Lazzeri et al. Reported complications were noticeable scarring at the nasal ala, nasal tip, and dorsum; early transient hypopigmentation among African American patients receiving the argon beam coagulator; and depression at the margin of the resection ().
Vural et al describe seven patients (mean age 67 years) who underwent Shaw scalpel excision in a single session.Citation61 Severity of rhinophyma was not reported. Complications occurred in 14.3%; however, there were no recurrences or revisions reported ().
Table 6 Summary of Outcomes by Treatment Type
The Subunit Method
The subunit method is a surgical technique based on utilizing and enhancing the nasal aesthetic subunits to optimize cosmetic and functional outcomes ( and ).Citation62,Citation63 Incisions are made along the junctions of nasal aesthetic subunits. The distal nose is degloved by raising six flaps 2–3 mm based on subunits (two alar, two nasal sidewall, one dorsum, one soft triangle). Phymatous tissue is radically debulked to perichondrium. The skin flaps are re-draped over the nose and quilting sutures obliterate dead space. Excess skin from the “tissue expanded effect” of chronic phymatous growth is removed. Restoration of nasal tip definition, structural support, and external valve function is achieved by placement of interdomal sutures or alar batten grafts as needed. If there is instability of the flap, such as evidence of cobblestoning or pitting, the entire subunit is replaced with full-thickness skin grafts.
Figure 2 Subunit approach for refractory rhinophyma. (A) Intra-operative markings with six subunit based flaps (dorsum, two sidewalls, two alae, tip). (B) Subunit flaps are raised to provide exposure for removal of the phymatous tissue and facilitate correction of nasal support with cartilage grafts. (C) Extensive debulking of phymatous tissue to perichondrium. (D) Excess skin that has been “expanded” by sebaceous growth is trimmed.
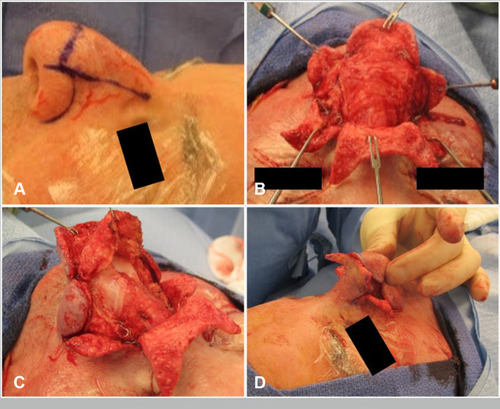
Figure 3 (A) Pre-operative photo of patient with rhinophyma refractory to laser and tangential excision. (B) Intra-operative result after undergoing subunit method. Note incisions are planned at the subunit junctions. (C) Post-operative image with improvement in rhinophyma and nasal definition.
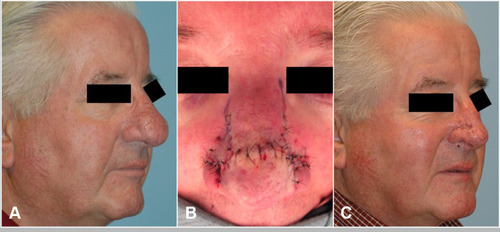
Indications for the subunit method include poor nasal contour following previous partial excision, when secondary healing is contraindicated, or modifications to underlying cartilage is needed to correct external valve collapse.Citation62 Hassanein et al report eight patients (mean age 63 years) who underwent treatment with the subunit method. Four patients had external valve collapse. Four patients received alar batten cartilage grafts, all had interdomal sutures, and one patient required a skin graft.Citation62,Citation63 Both the complication and revision rates were 75%, but only minor revisions under local anesthetic were required and no recurrence of disease was noted.
Post-Operative Management and Care
Post-operative management is dictated by treatment type and practitioner’s preference. The use of antibiotic ointment including mupirocin, bacitracin, polysporin; petrolatum jelly; or petroleum gauze are recommended individually or in combination post-operatively until evidence of re-epithelialization occurs. Following erbium:YAG laser therapy, hemostatic and pressure dressings are often applied, including placement of alginate dressings and an omniderm sheath. Those undergoing neodymium:YAG laser therapy received a xeroform dressing removed on post-operative day two followed by application of bacitracin. We recommend all patients wash their wounds and perform dressing changes at least daily.
Discussion
Several effective management options exist for rhinophyma. The subunit method has the highest complication and revision rates, but this technique is reserved for severe disease and facilitates reconstruction of nasal support (). Carbon dioxide laser treatment has the second highest recurrence rate. Both excisional and laser techniques have similar times to re-epithelialization and rates of post-operative erythema. Patient-reported outcomes and satisfaction is equivalent between patients receiving CO2 laser therapy versus scalpel excision and electrocautery techniques.Citation48,Citation55,Citation57 Patient satisfaction is common post-therapy regardless of the treatment method. Over 89% of patients would recommend undergoing treatment for rhinophyma irrespective of treatment type. Madan et al showed a positive effect on patient psychology and self-confidence after undergoing treatment.Citation2
Table 9 Summary of Treatment Advantages and Disadvantages
Advantages of the carbon dioxide laser include precise decortication, controlled hemostasis, and a relatively bloodless surgical field.Citation2,Citation33,Citation57,Citation64 A relative advantage is its deep tissue penetration through the epidermis and superficial dermis; however, this may result in thermal damage to the underlying dermis and adnexal structures. The procedure is more expensive, has a relatively longer procedure time than other techniques such as scalpel excision or electrosurgery, and is not amenable to obtaining specimens for histopathology.Citation2,Citation33,Citation48,Citation55 Lazzeri et al also showed a relatively longer return time to social life for patients undergoing laser therapy than those undergoing tangential excision.Citation57
The primary advantage of the Er:YAG laser is its limited absorption of water to the epidermis and option for pulsed delivery, which facilitates for a more precise treatment.Citation50 Disadvantages of this technique involve poor intraoperative hemostasis which results in decreased visibility.Citation49–Citation51 Relative to the Er:YAG laser, the Nd:YAG laser is less precise and carries a higher risk of thermal injury.
Scalpel or “cold knife” excision is reported to be a more cost- and time-effective procedure.Citation57 Patients who underwent tangential excision with or without dermabrasion and carbon dioxide laser therapy are equally satisfied.Citation55,Citation57 In contrast to other surgical therapies, cold knife excision allows for a more precise excision of tissue and better preservation of the pilosebaceous units and thus quicker re-epithelialization.Citation36,Citation47,Citation57
Poor hemostasis during the procedure, subsequent poor visualization of the surgical field, unfavorable scarring along the lower aesthetic subunits, and risk of heat injury secondary to use of electrocautery are disadvantages of this technique.Citation36,Citation47,Citation57 Electrocautery is commonly used to improve hemostasis during the procedure. Use of alginate dressing and heated soaked abdominal cloths are reported alternatives to electrocautery for hemostasis.Citation36,Citation58
The Shaw scalpel carries a similar profile of benefits as a cold excision with the added benefit of integrated hemostasis in the medium but increased risk of thermal injury and poor post-operative scarring.
The subunit method is a unique approach to the treatment of rhinophyma. Several benefits are described with this method. Scars are hidden at the borders of each nasal aesthetic subunit. Scar contraction along these areas enhance the natural contour lines. It achieves maximal removal of phymatous tissue, nasal tip enhancement, and framework restoration of the nose. Nonetheless, it is technically demanding, and most patients will require a second-stage procedure. This revision rate may be mediated by an inherently poor wound healing environment given an increased bacterial load and diseased tissue in rhinophyma.Citation63
Choice of treatment does not appear to be dependent on severity of disease but rather is surgeon-/practitioner-dependent. Laser therapy, scalpel excision (Shaw and cold knife), and the subunit method require a more advanced and niche skillset that is entirely surgeon-dependent. Minor to major rhinophyma, per the el-Azhary scale, can be adequately treated with laser and excisional techniques; however, the subunit method is more advantageous for the indications documented and as a combined method for patients with underlying functional nasal obstruction.
Our review is limited by the quality of data available for rhinophyma treatment outcomes. There are currently no randomized controlled trials evaluating treatment outcomes for rhinophyma, and available studies are limited to retrospective studies, case series, individual case reports, and associated technique articles and commentary. Reporting of quantitative and qualitative outcomes between studies is not standardized. This limits our ability to provide a rigorous statistical analysis of outcomes. There are no studies dedicated to evaluating the quality of life and psychosocial outcomes after rhinophyma treatment. General patient satisfaction is evaluated by most studies without extensive exploration into the individual elements comprising patient quality of life. Further studies are needed to prospectively evaluate treatment of rhinophyma as well as detail patient satisfaction and psychosocial outcomes after treatment.
Conclusion
Management of rhinophyma is complex because of a range of severity and multiple available treatment modalities. Rhinophyma is a severe form of rosacea and is graded with the RHISI classification. The management options are variably invasive and effective depending on the severity of the disease and the goals of treatment. Excisional and laser techniques are widely used. The subunit surgical approach is reserved for the most severe rhinophyma exhibiting functional nasal problems and facilitates enhancing support and structure. Patients receiving treatment universally report both cosmetic and functional improvements post-operation.
Consent Statement
The patient pictured in the figure provided written, informed consent specifically for publication of the photographs and the case details.
Disclosure
The authors report no conflicts of interest in this work.
References
- Smith AE. Correction of advanced rhinophyma by means of plastic reconstructive surgery: A new technique. Am J Surgery. 1958;96(6):792–801. doi:10.1016/0002-9610(58)91002-X
- Madan V, Ferguson JE, August PJ. Carbon dioxide laser treatment of rhinophyma: a review of 124 patients. Br J Dermatol. 2009;161(4):814–818. doi:10.1111/j.1365-2133.2009.09317.x19624541
- Gether L, Overgaard LK, Egeberg A, Thyssen JP. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179(2):282–289. doi:10.1111/bjd.1648129478264
- Rohrich RJ, Griffin JR, Adams WP Jr. Rhinophyma: review and update. Plast Reconstr Surg. 2002;110(3):860–869. (). doi:10.1097/01.PRS.0000019919.70133.BF12172152
- Rebora A. The management of rosacea. Am J Clin Dermatol. 2002;3(7):489–496. doi:10.2165/00128071-200203070-0000512180896
- Fisher WJ. Rhinophyma: its surgical treatment. Plast Reconstr Surg. 1970;45(5):466–470. doi:10.1097/00006534-197005000-000094245205
- Wiemer DR. Rhinophyma. Clin Plast Surg. 1987;14(2):357–365. doi:10.1016/S0094-1298(20)30606-42953521
- Furukawa M, Kanetou K, Hamada T. Rhinophyma in Japan. Int J Dermatol. 1994;33(1):35–37. doi:10.1111/j.1365-4362.1994.tb01490.x8112937
- Khoo CTK, Saad MN. Rhinophyma in a negro: case report. Br J Plast Surg. 1980;33(2):161–163. doi:10.1016/0007-1226(80)90005-36446339
- Kraeva E, Ho D, Jagdeo J. Successful treatment of rhinophyma with fractionated Carbon Dioxide (CO2) laser in an african-american man: case report and review of literature of fractionated CO2 laser treatment of rhinophyma. J Drugs Dermatol. 2016;15(11):1465–1468.28095565
- Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50(6):907–912. doi:10.1016/j.jaad.2004.01.04815153893
- Tan J, Almeida LM, Bewley A, et al. Updating the diagnosis, classification and assessment of rosacea: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176(2):431–438. doi:10.1111/bjd.1512227718519
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78(1):148–155. doi:10.1016/j.jaad.2017.08.03729089180
- Rebora A. Rosacea. J Investigative Dermatol. 1987;88(3,Supplement):56–60. doi:10.1038/jid.1987.11
- Jansen T, Plewig G. Clinical and histological variants of rhinophyma, including nonsurgical treatment modalities. Facial Plast Surg. 1998;14(4):241–253. doi:10.1055/s-2008-106445611816064
- Acker DW, Helwig EB. Rhinophyma with carcinoma. Arch Dermatol. 1967;95(3):250–254. doi:10.1001/archderm.1967.016003300080024225333
- McKenna DJ, McKenna K. Basal cell carcinoma lurking within gross rhinophyma. Clin Exp Dermatol. 2006;31(1):173–174. doi:10.1111/j.1365-2230.2005.01929.x16309536
- Curnier A, Choudhary S. Rhinophyma: dispelling the myths. Plast Reconstr Surg. 2004;114(2):351–354. doi:10.1097/01.PRS.0000131875.67987.6915277798
- Plenk HP. Rhinophyma, associated with carcinoma, treated successfully with radiation. Plast Reconstr Surg. 1995;95(3):559–562. doi:10.1097/00006534-199503000-000207870783
- Silvis NG, Zachary CB. Occult basal-cell carcinoma within rhinophyma. Clin Exp Dermatol. 1990;15(4):282–284. doi:10.1111/j.1365-2230.1990.tb02090.x2208777
- Keefe M, Wakeel RA, McBride DI. Basal cell carcinoma mimicking rhinophyma. Case report and literature review. Arch Dermatol. 1988;124(7):1077–1079. doi:10.1001/archderm.1988.016700700650212968781
- Rees TD. Bascal cell carcinoma in association with rhinophyma. Plast Reconstr Surg. 1955;16(4):283–287. doi:10.1097/00006534-195510000-00005
- Gallardo MA, Bosch RJ, Vidal L, et al. Angiosarcoma arising on rhinophyma. Eur J Dermatol. 2000;10(7):555–558.11056431
- Broadbent NR, Cort DF. Squamous carcinoma in longstanding rhinophyma. Br J Plast Surg. 1977;30(4):308–309. doi:10.1016/0007-1226(77)90126-6145255
- Shatkin S Jr, Shatkin M, Smith K, Beland LE, Oppenheimer AJ. Diffuse large B-cell lymphoma occurring with rhinophyma: a case report. Cureus. 2018;10(4):e2536.29946504
- Barzilai A, Feuerman H, Quaglino P, et al. Cutaneous B-cell neoplasms mimicking granulomatous rosacea or rhinophyma. Arch Dermatol. 2012;148(7):824–831. doi:10.1001/archdermatol.2011.357522508769
- Ogden S, Coulson IH. B-cell lymphoma mimicking rhinophyma. Clin Exp Dermatol. 2008;33(2):213–214. doi:10.1111/j.1365-2230.2007.02476.x18257841
- Geyton T, Henderson AH, Morris J, McDonald S. Nasal tip schwannoma mimicking rhinophyma. BMJ Case Rep. 2017;2017.
- Duzgun S, Pekdemir I, Yilanci S, Bali YY, Singin S, Tapan M. A cutaneous angiosarcoma arising from the rhinophyma. Kulak Burun Bogaz Ihtis Derg. 2013;23(6):344–347. doi:10.5606/kbbihtisas.2013.3555624283810
- Traaholt L, Eeg Larsen T. Rhinophyma and angiosarcoma of the nose. A case report. Scand J Plast Reconstr Surg. 1978;12(1):81–83. doi:10.3109/02844317809010485149363
- Hoffmann M, Braun-Falco M. Rhinophyma-like sebaceous carcinoma. J Eur Acad Dermatol Venereol. 2009;23(10):1216–1218. doi:10.1111/j.1468-3083.2009.03127.x19368609
- Motley RJ, Douglas-Jones AF, Holt PJ. Sebaceous carcinoma: an unusual cause of a rapidly enlarging rhinophyma. Br J Dermatol. 1991;124(3):283–284. doi:10.1111/j.1365-2133.1991.tb00575.x2018736
- el-Azhary RA, Roenigk RK, Wang TD. Spectrum of results after treatment of rhinophyma with the carbon dioxide laser. Mayo Clin Proc. 1991;66(9):899–905. doi:10.1016/S0025-6196(12)61576-61921499
- Clark DP, Hanke CW. Electrosurgical treatment of rhinophyma. J Am Acad Dermatol. 1990;22(5 Pt 1):831–837. doi:10.1016/0190-9622(90)70115-X2140840
- Freeman BS. Reconstructive rhinoplasty for rhinophyma. Plast Reconstr Surg. 1970;46(3):265–270. doi:10.1097/00006534-197009000-000104247302
- Wetzig T, Averbeck M, Simon JC, Kendler M. New rhinophyma severity index and mid-term results following shave excision of rhinophyma. Dermatology. 2013;227(1):31–36. doi:10.1159/00035155624008235
- van Zuuren EJ. Rosacea. N Engl J Med. 2017;377(18):1754–1764. doi:10.1056/NEJMcp150663029091565
- Tope WD, Sangueza OP. Rhinophyma’s fibrous variant. Am J Dermatopathol. 1994;16(3):307–310. doi:10.1097/00000372-199406000-000147943640
- Aloi F, Tomasini C, Soro E, Pippione M. The clinicopathologic spectrum of rhinophyma. J Am Acad Dermatol. 2000;42(3):468–472. doi:10.1016/S0190-9622(00)90220-210688718
- Schuurmann M, Wetzig T, Wickenhauser C, Ziepert M, Kreuz M, Ziemer M. Histopathology of rhinophyma - a clinical-histopathologic correlation. J Cutan Pathol. 2015;42(8):527–535. doi:10.1111/cup.1251825950712
- Pu LL, Smith PD, Payne WG, et al. Overexpression of transforming growth factor beta-2 and its receptor in rhinophyma: an alternative mechanism of pathobiology. Ann Plast Surg. 2000;45(5):515–519. doi:10.1097/00000637-200045050-0000811092361
- Payne WG, Ko F, Anspaugh S, Wheeler CK, Wright TE, Robson MC. Down-regulating causes of fibrosis with tamoxifen: a possible cellular/molecular approach to treat rhinophyma. Ann Plast Surg. 2006;56(3):301–305. doi:10.1097/01.sap.0000199155.73000.2f16508362
- Sadick H, Goepel B, Bersch C, Goessler U, Hoermann K, Riedel F. Rhinophyma: diagnosis and treatment options for a disfiguring tumor of the nose. Ann Plast Surg. 2008;61(1):114–120. doi:10.1097/SAP.0b013e31815f12d218580161
- Schaller M, Almeida LM, Bewley A, et al. Rosacea treatment update: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176(2):465–471. doi:10.1111/bjd.1517327861741
- Tuzun Y, Wolf R, Kutlubay Z, Karakus O, Engin B. Rosacea and rhinophyma. Clin Dermatol. 2014;32(1):35–46. doi:10.1016/j.clindermatol.2013.05.02424314376
- Gupta AK, Chaudhry MM. Rosacea and its management: an overview. J Eur Acad Dermatol Venereol. 2005;19(3):273–285. doi:10.1111/j.1468-3083.2005.01216.x15857452
- Redett RJ, Manson PN, Goldberg N, Girotto J, Spence RJ. Methods and results of rhinophyma treatment. Plast Reconstr Surg. 2001;107(5):1115–1123. doi:10.1097/00006534-200104150-0000411373550
- Greenbaum SS, Krull EA, Watnick K. Comparison of CO2 laser and electrosurgery in the treatment of rhinophyma. J Am Acad Dermatol. 1988;18(2 Pt 1):363–368. doi:10.1016/S0190-9622(88)70053-52964461
- Fincher EF, Gladstone HB. Use of a dual-mode erbium: yAGlaser for the surgical correction of rhinophyma. Arch Facial Plast Surg. 2004;6(4):267–271. doi:10.1001/archfaci.6.4.26715262723
- Goon PKY, Dalal M, Peart FC. The gold standard for decortication of rhinophyma: combined erbium-YAG/CO2 laser. Aesthetic Plast Surg. 2004;28(6):456–460. doi:10.1007/s00266-004-0012-x15625593
- Orenstein A, Haik J, Tamir J, et al. Treatment of rhinophyma with Er: yAGlaser. Lasers Surg Med. 2001;29(3):230–235. doi:10.1002/lsm.111211573224
- Beran SJ, Rohrich RJ, Clark PJ. The potential role of the erbium: YAG laser in skin rejuvenation. Aesthetic Surgery J. 1998;18(2):147–149. doi:10.1016/S1090-820X(98)80015-0
- Hofmann MA, Lehmann P. Physical modalities for the treatment of rosacea. J Dtsch Dermatol Ges. 2016;14(Suppl 6):38–43.
- Wenig BL, Weingarten RT. Excision of rhinophyma with Nd: yAGlaser: a new technique. Laryngoscope. 1993;103(1 Pt 1):101–103. doi:10.1288/00005537-199301000-000208421411
- Har-El G, Shapshay SM, Bohigian RK, Krespi YP, Lucente FE. The treatment of rhinophyma. ‘Cold’ vs laser techniques. Arch Otolaryngol Head Neck Surg. 1993;119(6):628–631. doi:10.1001/archotol.1993.018801800420078499092
- Karim Ali M, Streitmann MJ. Excision of rhinophyma with the carbon dioxide laser: a ten-year experience. Ann Otol Rhinol Laryngol. 1997;106(11):952–955. doi:10.1177/0003489497106011119373086
- Lazzeri D, Larcher L, Huemer GM, et al. Surgical correction of rhinophyma: comparison of two methods in a 15-year-long experience. J Cranio-Maxillofacial Surgery. 2013;41(5):429–436. doi:10.1016/j.jcms.2012.11.009
- Curnier A, Choudhary S. Triple approach to rhinophyma. Ann Plast Surg. 2002;49(2):211–214. doi:10.1097/00000637-200208000-0001712187352
- Stucker FJ, Lian T, Sanders K. The AbCs of rhinophyma management. Am J Rhinol. 2003;17(1):45–49. doi:10.1177/19458924030170010812693655
- Kilty S, Brownrigg P. Surgical treatment of rhinophyma. J Otolaryngol Head Neck Surg. 2008;37(2):269–272.19128625
- Vural E, Royer MC, Kokoska MS. Sculpting resection of rhinophyma using the shaw scalpel. Arch Facial Plast Surg. 2009;11(4):263–266. doi:10.1001/archfacial.2009.3419620533
- Hassanein AH, Caterson EJ, Erdmann-Sager J, Pribaz JJ. The subunit method: A novel excisional approach for rhinophyma. J Am Acad Dermatol. 2016;74(6):1276–1278. doi:10.1016/j.jaad.2016.01.00427185440
- Hassanein AH, Vyas RM, Erdmann-Sager J, Caterson EJ, Pribaz JJ. Management of rhinophyma: outcomes study of the subunit method. J Craniofac Surg. 2017;28(3):e247e250. doi:10.1097/SCS.000000000000346728468207
- Bassi A, Campolmi P, Dindelli M, et al. Laser surgery in rhinophyma. G Ital Dermatol Venereol. 2016;151(1):9–16.25236318
- Shapshay SM, Strong MS, Anastasi GW, Vaughan CW. Removal of rhinophyma with the carbon dioxide laser: a preliminary report. Arch Otolaryngol. 1980;106(5):257–259. doi:10.1001/archotol.1980.007902900090046445187
- Wheeland RG, Bailin PL, Ratz JL. Combined carbon dioxide laser excision and vaporization in the treatment of rhinophyma. J Dermatol Surg Oncol. 1987;13(2):172–177. doi:10.1111/j.1524-4725.1987.tb00515.x2948980
- Bohigian RK, Shapshay SM, Hybels RL. Management of rhinophyma with carbon dioxide laser: lahey Clinic experience. Lasers Surg Med. 1988;8(4):397–401. doi:10.1002/lsm.19000804102971846
- Cravo M, Miguel Canelas M, Carlos Cardoso J, Vieira R, Figueiredo A. Combined carbon dioxide laser and bipolar electrocoagulation: another option to treat rhinophyma. J Dermatolog Treat. 2009;20(3):146–148. doi:10.1080/0954663080251265319365784
- Karge HJ, Konz B. Surgical methods in the treatment of rhinophyma. J Dermatol Surg. 1975;1(3):31–32. doi:10.1111/j.1524-4725.1975.tb00091.x131134
- Dolezal R, Schultz RC. Early treatment of rhinophyma–a neglected entity? Ann Plast Surg. 1983;11(5):393–396. doi:10.1097/00000637-198311000-000066228181
- Bogetti P, Boltri M, Spagnoli G, Dolcet M. Surgical treatment of rhinophyma: a comparison of techniques. Aesthetic Plast Surg. 2002;26(1):57–60. doi:10.1007/s00266-001-0039-111891601