Abstract
Introduction: Dermoscopy is a low-cost examination performed by a dermatologist and good for the diagnosis of pigmented lesions. However, dermoscopy diagnosis of lentigo maligna (LM) and lentigo maligna melanoma (LMM) is still questionable. The objective of this study was to evaluate whether dermoscopy is an effective diagnostic method to diagnose LM/LMM from other pigmented skin lesions, and to identify which are the most frequent dermoscopic criteria associated with LM/LMM
Methods: For this systematic review and meta-analysis, we used the following descriptors: dermoscopy, lentigo maligna, lentigo maligna melanoma, histopathology; and the following databases to search for articles: Cochrane Collaboration, MEDLINE; PMC (PubMed Central) - NIH (National Institutes of Health), EMBASE (The Excerpt Medical Database), and SCISEARCH, from inception to March 30, 2018. The evaluation of studies was performed using the QUADAS (Quality Assessment of Diagnostic Accuracy Studies)-2 tool. The PRISMA (Preferred Reporting Itens for Systematic Reviews and Meta-Analyses) and MOOSE (Meta-analysis Of Observational Studies in Epidemiology) guidelines were followed for data extraction. Also, we extracted from each study the dermoscopic criteria most commonly found in the lesions of LM/LMM.
Results: This systematic review included 15 articles for qualitative analysis (a total of 2,012 lesions evaluated) and 7 for meta-analysis. In the bivariate model the mean sensitivity was 0.824 and the mean specificity was 0.835. The area under the curve was 0.889. Rhomboid structures, pseudonetwork, and homogeneous areas were the most frequent dermoscopic criteria associated with LM/LMM.
Conclusion: These findings suggest that dermoscopy has good accuracy in the diagnosis of LM/LMM.
Video abstract

Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Lentigo maligna melanoma (LMM) accounts for 10% of the malignant melanomas and describes when lentigo maligna (LM) acquires a vertical invasive phase.Citation1 LM is considered an in situ melanoma, and in its evolution of atypical melanocytes that invade the dermis, it is characterized as LMM. LM occurs in chronically sun-damaged skin, has slow growth, prevalent in patients aged over 40 years and has a malignant progression rate of approximately 5–20% of the total.Citation2
The gold standard for the diagnosis of LM and LMM should be made using biopsy with distinct histopathological characteristics. These include a pagetoid appearance of melanocytes, melanocyte atypia, nonuniform pigmentation/distribution of melanocytes, and increased melanocyte density in a background of extensive photodamage.Citation2
Dermoscopy is a low-cost examination performed by a dermatologist that is the most useful technique to study melanocytic skin lesions in the differentiation of benign lesions from malignant lesions. It is the most cost-effective examination that can direct the dermatologist to the best site for biopsy. It can also be very useful in the demarcation of lesion limits such as those of LM/LMM and to follow-up and perform cure control of these lesions.Citation1
The LM/LMM diagnosis based on dermoscopy is ambiguous. For example, asymmetric pigmented follicular openings, hyperpigmentation of the follicle rim, light brown pseudonetworks, light rhomboidal structures, light streaks, and peripheral gray dots could be observed in actinic keratosis and in LM/LMM.Citation2
The importance of establishing dermoscopic criteria for the diagnosis of LM/LMM is due to the correct selection of treatment, to minimize the damage of invasive procedures and reduce the associated morbidity and costs of unnecessary surgeries. In some cases, dermoscopy may be a cheap screening test for other noninvasive diagnostic techniques, such as confocal dermoscopy, optical coherence tomography, and high-frequency ultrasonography.Citation3
Information from systematic reviews of diagnostic tests is important to determine the appropriate and effective use of diagnostic tests in clinical practice and to develop information necessary to determine the directions of future research in diagnostic medicine.Citation4
The aim of this study was to evaluate whether dermoscopy is an effective diagnostic method to differentiate LM and LMM from other pigmented skin lesions and to identify which are the most frequent dermoscopic criteria associated with LM/LMM.
Methods
The following question was asked to evaluate the role of dermoscopy in the diagnosis of lentigo maligna and lentigo maligna melanoma on dermatological examination: What is the efficacy of dermoscopy in the diagnosis of lentigo maligna and lentigo maligna melanoma compared to histopathology?
Data source and search strategy
To address the issue, we conducted detailed and automated research using the following databases to search for articles, without any restrictions regarding the date, language or any other parameter: Cochrane Collaboration; MEDLINE; PMC (PubMed Central) - NIH (National Institutes of Health); EMBASE (The Excerpt Medical Database); SCISEARCH from inception of these databases to March 30, 2018.
The search strategy of the articles was as follows: “Lentigo“ OR “Hutchinson’s Melanotic Freckle“ OR Lentigos OR Lentigines OR Lentiginosis OR Lentiginoses OR (Freckle, Hutchinson’s Melanotic) OR (Hutchinson Melanotic Freckle) OR (Hutchinsons Melanotic Freckle) OR (Melanotic Freckle, Hutchinson’s) OR (Melanotic Freckle) OR (Lentigo, Malignant) OR (Lentigos, Malignant) OR (Malignant Lentigo) OR (Malignant Lentigos) OR (Freckle, Melanotic) OR (Freckles, Melanotic) OR (Melanotic Freckles) OR (Lentigo Maligna) AND ”Dermoscopy” OR Dermoscopies OR Dermatoscopy OR Dermatoscopies OR (Skin Surface Microscopy) OR (Microscopies, Skin Surface) OR (Microscopy, Skin Surface) OR (Skin Surface Microscopies) OR (Surface Microscopies, Skin) OR (Surface Microscopy, Skin) OR (Epiluminescence Microscopy) OR (Epiluminescence Microscopies) OR (Microscopies, Epiluminescence) OR (Microscopy, Epiluminescence) AND ”Biopsy” OR Biopsies AND ‘Histopathology’.
Eligibility criteria and study selection
The studies with a grade of recommendation A or B were selected according to the document “Levels of Evidence 1” of the CEBM (Center for Evidence-based Medicine),Citation5 and the C or D studies listed in this document were excluded.
Quality assessment of included studies
The evaluation of the studies was performed using the QUADAS (Quality Assessment of Diagnostic Accuracy Studies)-2 tool, recommended for systematic reviews of diagnostic accuracy by the Agency for Healthcare Research and Quality, Cochrane Collaboration, and UK National Institute for Health and Clinical Excellence.Citation6
Data extraction
Two authors (MOLC and MAP) independently assessed the titles and abstracts of all studies identified in the electronic search. In the event of disagreement, a consensus was reached. From this action, a collection of studies that affirmatively answered our question was created that was evaluated in their entirety by the authors.
From each study were extracted the tests used to evaluate the lesions, which lesions were evaluated and their location and the number of cases evaluated. The general sensitivity and specificity of dermoscopy were extracted for meta-analysis.
Additionally, we extracted from each study the dermoscopic criteria most commonly found in the lesions of LM/LMM.
Definitions
The definitions for sensitivity, specificity, likelihood ratio (LR), likelihood ratio for a positive test, likelihood ratio for a negative test, and diagnostic odds ratio (DOR) can be found in the article by Šimundić.Citation7
Meta-analysis
The heterogeneity among the included studies was assessed using the Q test for statistical significance and the I-square test to quantify heterogeneity, where p<0.1 is statistically significant and I-square >25% shows significant heterogeneity. The corresponding 95% confidence intervals (CI) were also estimated.
The receiver operating characteristic (ROC) curve was used to summarize the results of the studies. The area under the curve (AUC), which summarizes the diagnostic performance, was calculated. A perfect test has an AUC close to 1, and poor tests have AUCs close to 0.5. The index Q*, defined by the point where the sensitivity and specificity are equal, is the nearest point of the ideal in the upper left corner of the ROC curve (specificity =0, sensitivity =1), and the standard errors of AUC [SE (AUC)] and Q * [SE (Q *)] were also calculated. The statistical tests were performed using Meta-Disc software.Citation8
The bivariate model of Reitsma et alCitation9 was used to enhance the understanding of the heterogeneity of results between studies and the correlation within studies. For the bivariate analysis, we used the Software R v. 3.5.3 with the package MADA (Meta-Analysis of Diagnostic Accuracy) v. 0.5.8.
All statistical tests were performed with a significance level of 5%.
Results
In the databases searched for the period from 1996 to 2018, 226 articles were found, from which 15 were included for qualitative analysis among a total of 2,012 lesions evaluated.
shows the search strategy of the articles based on the PRISMA (Preferred Reporting Itens for Systematic Reviews and Meta-Analyses)Citation10 and MOOSE (Meta-analysis Of Observational Studies in Epidemiology)Citation11 guidelines. summarizes the characteristics of each study.
Table 1 General data of the studies
Regarding the quality evaluation of the 15 studies, only 2 studiesCitation15,Citation23 presented an uncertain/high risk of bias in relation to the index test (dermoscopy); most of the included studies had a low risk of bias with respect to the index test and patient choice and flow and time ().
Table 2 Quality assessment of the included studies based on the QUADAS (Quality Assessment of Diagnostic Accuracy Studies)-2 tool
Of the 15 included studies comparing dermoscopy and histopathology, from 7 studies,Citation12,Citation15,Citation17,Citation21,Citation24–Citation26 it was possible to extract data on the general specificity and sensitivity of dermoscopy. These seven studies were included in the quantitative analysis (meta-analysis).
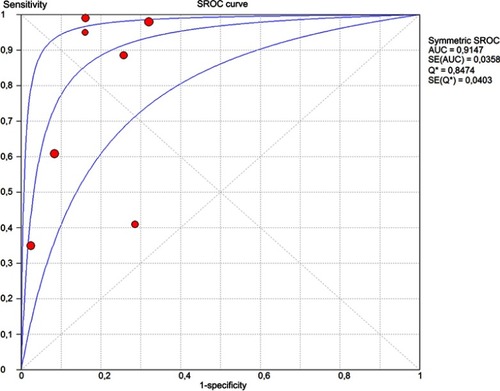
The random-effects model was used for the combination of specificity and sensitivity. Cochran’s Q statistic for heterogeneity showed the heterogeneity between the studies, both for specificity (p<0.001) and sensitivity (p<0.001), and that heterogeneity is large for specificity [I-square (I2)=91.2%] and for sensitivity (I2=96.3%). The pooled sensitivity was 0.71 (95% CI: 0.67–0.76), and the pooled specificity was 0.81 (95% CI: 0.78–0.84) (). The I-square value for the positive likelihood ratio (LR) was 84.1%, that for the negative LR was 94.4% and that for the diagnostic odds ratio (DOR) was 86.9%. The pooled positive LR was 4.352 (95% CI: 2.734–6.925), the pooled negative LR was 0.248 (95% CI: 0.122–0.503), and the pooled DOR was 28.47 (95% CI: 7.859–102.68). The accuracy of the test, as measured by the area under the curve (AUC), was 0.9147 (SE=0.0358) ().
Figure 2 Diagram of the meta-analysis for the sensitivity and specificity of dermoscopy.
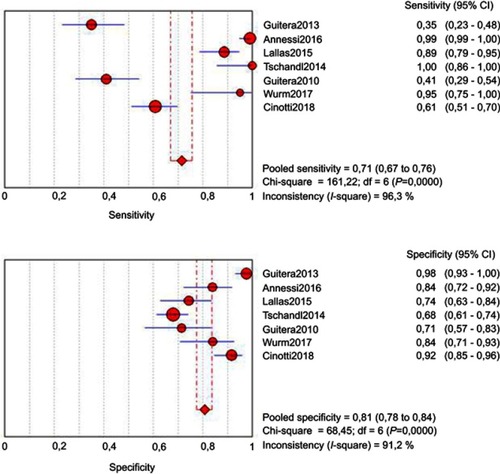
Figure 3 Estimated SROC (Summary Receiver Operating Characteristics) curve and original data points for dermoscopy compared with histopathology.
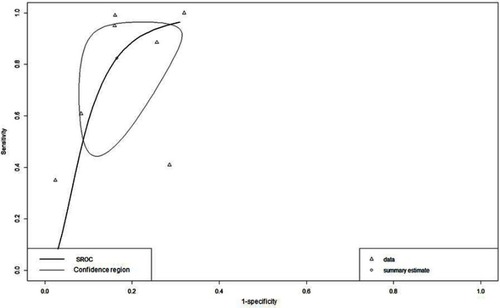
In the bivariate model, mean sensitivity was 0.824 (95% CI: 0.531–0.951), mean specificity was 0.835 (95% CI: 0.720–0.909), and mean DOR was 30.400 (95% CI: 6.880–92.400). The AUC was 0.889 and the partial AUC (restricted to observed false-positive results and normalized) was 0.712 ().
Figure 4 Estimated SROC (Summary Receiver Operating Characteristics) curve (Bivariate model) and the corresponding 95% confidence ellipse.
Dermoscopic criteria
It was possible to extract the incidence of the dermoscopic criteria used for the diagnosis of LM/LMM from eleven included studies. The most frequent criteria in each study are described in .
Table 3 Dermoscopic criteria occurring in 50% or more of the LM/LMM lesions in eleven selected studies
The common dermoscopic criteria most observed in all studies were rhomboidal structures, described in four studiesCitation12,Citation14,Citation19,Citation22 (56–75% of the cases presented the criteria); pseudonetwork, described in three studiesCitation14,Citation15,Citation18 (71–87.5% of the cases presented the criteria); homogeneous areas, described in three studiesCitation18,Citation19,Citation23 (54.5–75% of the cases presented the criteria); black points, in two articlesCitation19,Citation22 (86.4–100% of the cases); blue-gray dots and globules, in two articlesCitation19,Citation22 (60.8–95.8% of the cases); brown color in two articlesCitation12,Citation19 (72.7–90% of the cases); and gray circles, in two articlesCitation12,Citation19 (54.2–56% of the cases).
Two studiesCitation12,Citation15 analyzed the specificity, sensitivity, and relative risk of the main dermoscopic criteria found. In the study by Tschandl et al,Citation15 the most sensitive dermoscopic criteria were any gray structure (95.8% CI: 78.8–99.3%), with a specificity of 30.6% (CI: 24.5–37.2%), dot vessels, with a higher specificity (98.1% CI: 95.3–99.5%) and a sensitivity of 8.3% (CI: 1.3–27.0%), and circle in a circle/double circle, with a specificity of 98.1% (CI: 95.3–99.5%) and sensitivity of 4.2% (CI: 0.7–21.2%). In the study of Lallas et al,Citation12 the dermoscopic criteria that presented the highest relative risk were not evident follicles (6.33; CI: 3.06–12.98) and gray circles (5.9; CI: 2.76–12.65).
Discussion
In this meta-analysis, dermoscopy showed a good accuracy in the diagnosis of LM/LMM, demonstrated by AUC between 0.8 and 0.9 in the bivariate model. Also rhomboid structures, followed by pseudonetwork and homogeneous areas were the most observed dermoscopic criteria in LM/LMM in the studies analyzed.
The effectiveness of the use of dermoscopy depends on the professional conducting the procedure because the criteria used for the diagnosis vary among several authors, as well as the interpretation of these criteria during the examination. Therefore, studies that described the dermoscopic analysis without revision by another dermatologist were considered to harbor a risk of uncertain/high bias in relation to the index test (dermoscopy). Only two studiesCitation15,Citation23 presented an uncertain/high risk of bias in relation to the index test (dermoscopy).
Among the included studies, we observed that only one differentiated between the LMM and LMM dermoscopic criteria,Citation23 suggesting a tendency to study such diseases as the same entity or perhaps because of the difficulty of dermoscopic differentiation of both using the existing criteria.
Among the seven studiesCitation12,Citation15,Citation17,Citation21,Citation24–Citation26 that described the general sensitivity and specificity, dermoscopy for LM/LMM showed a pooled sensitivity of 0.71 and pooled specificity of 0.81. The pooled positive LR was 4.352, the pooled negative LR was 0.248, and the pooled DOR was 28.407. Good diagnostic tests have positive LRs greater than 10 and negative LRs less than 0.1.Citation7 However, diagnostic tests with AUC between 0.9 and 1.0 have excellent diagnostic accuracy;Citation7 in our study, the AUC was 0.9147. Although the pooled positive LR and the pooled DOR did not show favorable indices for dermatoscopy, the pooled sensitivity, the pooled specificity, the pooled negative LR, and AUC favor that dermoscopy is a good test for the diagnosis of LM/LMM.
But the studies included in this meta-analysis present some general limitations in its design, such as the heterogeneity of lesions evaluated, indistinct evaluation between LM and LMM, a small number of cases evaluated in some studies, retrospective studies, and great heterogeneity of the specificity and sensitivity among the studies included in the meta-analysis. Because of this, we performed a bivariate analysis.
In the bivariate model, that estimates the amount of between-study variability in both sensitivity and specificity and evaluates more adequately studies with a lot of heterogeneity,Citation9 the mean sensitivity of 0.824, a mean specificity of 0.835, and mean DOR of 30.400. Also, the AUC was 0.889 and the partial AUC was 0.712. Diagnostic tests with AUC between 0.8 and 0.9 have very good diagnostic accuracy and tests with AUC between 0.7 and 0.8 have good diagnostic accuracy.Citation7 These data show that dermoscopy has a good accuracy for the diagnosis of LM/LMM.
Currently, there are classic criteria used in dermoscopy, but several authors have tried to elucidate the diagnoses of these lesions by improving pre-existing standardized criteriaCitation25 and sometimes introducing new criteria, such as those by Pralong et al,Citation16 who introduced four new criteria (darkening in dermoscopic examination, target-like pattern, red rhomboidal structures, and increased vascular network density) and Tschandl et alCitation15 (four-dot clods, double circle, incomplete circles, and edge in bite).
Some authors have described that if asymmetric pigmented follicular openings, dark rhomboidal structures, slate-gray areas, and slate-gray dots/globules/pepper pattern are found in one lesion, they have high sensitivity and specificity for a diagnosis of LMM.Citation22,Citation27
In the assessment of the dermoscopic criteria, which were identified in more than 50% of the biopsied LM/LMM lesions, rhomboidal structures were the dermoscopic criteria that appeared in more studies (four), with an incidence ranging from 56% to 75% of the cases, followed by a pseudonetwork (incidence between 71% and 87.5% of the cases), and homogeneous areas (incidence between 54.5% and 75% of the cases), identified in three articles. Thus, we suggest that the identification of these criteria for dermoscopy favors the diagnosis of LM/LMM.
Although any gray structure was the most sensitive dermoscopic criteria and dot vessels and circle in a circle/double circle were more specific, the specificity and sensitivity of the dermoscopic criteria were only evaluated in two articles,Citation12,Citation15 with a few cases studied that only evaluated LM, preventing adequate extrapolation of these findings to all lentiginous lesions.
This systematic review offers evidence of previously used criteria to diagnose LM and LMM, as described by Stolz et alCitation27 (hyperpigmented follicular opening, annular-granular pattern, pigmented rhomboid structures, and obliterated hair follicles) as well as new criteria used by the authors that can be considered in the evaluation of these lesions by the prevalence of appearance in the studies, such as homogeneous areas, black spots, dots and bluish-gray globules, brown color, and gray circle.
We have to consider that only seven studiesCitation12,Citation15,Citation17,Citation21,Citation24–Citation26 presented sensitivity and specificity assessment for dermoscopy in the diagnosis of LM/LMM, and only twoCitation12,Citation15 evaluated the sensitivity and specificity of dermoscopic criteria. So, further studies evaluating the sensitivity and specificity of the dermoscopic criteria for LM/LMM may contribute to the improvement of the dermoscopic diagnosis of these lesions.
Conclusion
On the basis of this study, dermoscopy has good accuracy in the diagnosis of LM/LMM compared to histopathology and can be used for define the optimal biopsy site or even for setting the total removal of the lesion. Also the identification of rhomboidal structures, pseudonetwork, and homogeneous areas are the criteria that seem most favor the diagnosis of LM/LMM.
Acknowledgment
The authors would like to thank Mrs Jakeline MQ Ortega, from the Library Division of the Universidade do Oeste Paulista, for her assistance during the initial selection of articles.
Disclosure
The authors report no conflicts of interest in this work.
References
- Edwards SJ, Osei-Assibey G, Patalay R, Wakefield V, Karner C. Diagnostic accuracy of reflectance confocal microscopy using VivaScope for detecting and monitoring skin lesions: a systematic review. Clin Exp Dermatol. 2017;42:266–275. doi:10.1111/ced.1305528218469
- Juhász MLW, Marmur ES. Reviewing challenges in the diagnosis and treatment of lentigo maligna and lentigo-maligna melanoma. Rare Cancers Ther. 2015;3:133–145. doi:10.1007/s40487-015-0012-927182482
- Kasprzak JM, Xu YG. Diagnosis and management of lentigo maligna: a review. Drugs Context. 2015;4:212281. doi:10.7573/dic.21228126082796
- Gatsonis C, Paliwal P. Meta-analysis of diagnostic and screening test accuracy evaluations: methodologic primer. AJR. 2006;187:271–281. doi:10.2214/AJR.06.022616861527
- OCEBM Levels of Evidence Working Group. The Oxford Levels of Evidence 2. Oxford Centre for Evidence-Based Medicine; 2016 Available from: https://www.cebm.net/index.aspx?o=5653. Accessed April 17, 2017.
- Whiting PF, Rutjes AW, Westwood ME, et al; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi:10.7326/0003-4819-155-8-201110180-0000922007046
- Šimundić AM. Measures of diagnostic accuracy: basic definitions. EJIFCC. 2009;19:203–211.27683318
- Zamora J, Abraira V, Muriel A, Khan KS, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31. doi:10.1186/1471-2288-6-3116836745
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005; 58(10):982–990. doi:10.1016/j.jclinepi.2005.02.02216168343
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. doi:10.1371/journal.pmed100009719621072
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi:10.1001/jama.283.15.200810789670
- Lallas A, Tschandl P, Kyrgidis A, et al. Dermoscopic clues to differentiate facial lentigo maligna from pigmented actinic keratosis. Br J Dermatol. 2016;174:1079–1085. doi:10.1111/bjd.1435526784739
- Jaimes N, Marghoob AA, Rabinovitz H, et al. Clinical and dermoscopic characteristics of melanomas on nonfacial chronically sun-damagedskin. J Am Acad Dermatol. 2015;72:1027–1035. doi:10.1016/j.jaad.2015.02.111725824275
- Gomez-Martin I, Moreno S, Andrades-Lopez E, et al. Histopathologic and immunohistochemical correlates of confocal descriptors in pigmented facial macules on photodamaged skin. JAMA Dermatol. 2017;153:771–780. doi:10.1001/jamadermatol.2017.132328564685
- Tschandl P, Rosendahl C, Kittler H. Dermatoscopy of flat pigmented facial lesions. J Eur Acad Dermatol Venereol. 2015;29:120–127. doi:10.1111/jdv.1248324661420
- Pralong P, Bathelier E, Dalle S, Poulalhon N, Debarbieux S, Thomas L. Dermoscopy of lentigo maligna melanoma: report of 125 cases. Br J Dermatol. 2012;167:280–287. doi:10.1111/j.1365-2133.2012.10932.x22404578
- Guitera P, Moloney FJ, Menzies SW, et al. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149:692–698. doi:10.1001/jamadermatol.2013.230123553208
- Goncharova Y, Attia EAS, Souid K, Vasilenko IV. Dermoscopic features of facial pigmented skin lesions. ISRN Dermatol. 2013;2013:546813. doi:10.1155/2013/54681323431466
- Sahin MT, Öztürkcan S, Ermertcan AT, Günes AT. A comparison of dermoscopic features among lentigo senilis/initial seborrheic keratosis, seborrheic keratosis, lentigo maligna and lentigo maligna melanoma on the face. J Dermatol. 2004;31:884–889.15729860
- Ciudad-Blanco C, Avilés-Izquierdo JA, Lázaro-Ochaita P, Suárez-Fernández R. Dermoscopic findings for the early detection of melanoma: an analysis of 200 cases. Actas Dermosifiliogr. 2014;105:683–693. doi:10.1016/j.ad.2014.01.00824704190
- Annessi G, Bono R, Abeni D. Correlation between digital epiluminescence microscopy parameters and histopathological changes in lentigo maligna and solar lentigo: A dermoscopic index for the diagnosis of lentigo maligna. J Am Acad Dermatol. 2017;76:234–243. doi:10.1016/j.jaad.2016.08.03228341252
- AkayBN, Kocyigit P, Heper AO, Erdem C. Dermatoscopy of flat pigmented facial lesions: diagnostic challenge between pigmented actinic keratosis and lentigo maligna. Br J Dermatol. 2010;163:1212–1217. doi:10.1111/j.1365-2133.2010.10025.x21083845
- Schiffner R, Schiffner-Rohe J, Vogt T, et al. Improvement of early recognition of lentigo maligna using dermatoscopy. J Am Acad Dermatol. 2000;42:25–32.
- Guitera P, Pellacani G, Crotty KA, et al. The impact of in vivo reflectance confocal microscopy onthe diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. J Invest Dermatol. 2010;130:2080–2091. doi:10.1038/jid.2010.8420393481
- Wurm E, Pellacani G, Longo C, et al. The value of reflectance confocal microscopy in diagnosis of flat pigmented facial lesions: a prospective study. J Eur Acad Dermatol Venereol. 2017;31:1349–1354. doi:10.1111/jdv.1417128214381
- Cinotti E, Labeille B, Debarbieux S, et al. Dermoscopy vs. reflectance confocal microscopy for the diagnosis of lentigo maligna. J Eur Acad Dermatol Venereol. 2018;32:1284–1291. doi:10.1111/jdv.14791
- Stolz W, Schaffner R, Burgdorf WH. Dermatoscopy for facial pigmented skin lesions. Clin Dermatol. 2002;20:276–278.12074867

