Abstract
Many therapeutic modalities have been used to treat alopecia areata, with variable efficacy and safety profiles. Unfortunately, none of these agents is curative or preventive. Also, many of these therapeutic agents have not been subjected to randomized, controlled trials, and, except for topical immunotherapy, there are few published studies on long-term outcomes. The treatment plan is designed according to the patient’s age and extent of disease. In this paper, the therapeutic agents are organized according to their efficacy and safety profiles into first-line, second-line, and third-line options.
Introduction
Alopecia areata is a common, nonscarring, autoimmune disease that can affect any hair- bearing area. Alopecia areata is a lymphocyte cell-mediated inflammatory type of hair loss, but its pathogenesis is not fully understood. The disease can present as a single, well demarcated patch of hair loss, multiple patches, or extensive hair loss in a form of total loss of scalp hair (alopecia totalis) or loss of entire scalp and body hair (alopecia universalis). A number of treatments can induce hair regrowth in alopecia areata but do not change the course of the disease. Treatment is more effective in patchy alopecia areata than in alopecia totalis/alopecia universalis. Therapy for alopecia areata should be tailored in light of severity of the condition and the patient’s age. This review discusses the therapeutic options and management strategies for alopecia areata.
First-line therapies
Intralesional corticosteroids
Several studies have shown the efficacy of intralesional corticosteroid injections. Abell and Munro reported hair regrowth in 71% of patients with subtotal alopecia areata treated by triamcinolone acetonide injections and in 7% of a placebo group.Citation1 For limited scalp alopecia areata, intralesional corticosteroid therapy is considered as the drug of choice by many experts. The most widely used agent is triamcinolone acetonide. Different concentrations of triamcinolone acetonide are used, in the range of 2.5–10 mg/mL, but 5 mg/mL is the preferred concentration for the scalp and face. A maximum volume of 3 mL on the scalp in one visit is recommended. Corticosteroid is injected into the deep dermis level or just beneath the dermis in the upper subcutis. The injections can be repeated at 4–6 weekly intervals. The use of mesotherapy multi-injectors with 5–7 needles is an alternative approach to decrease injection pain and to make the procedure more homogenous.Citation2 Side effects include skin atrophy and telangiectasia which can be minimized by the use of smaller volumes and avoiding superficial injections. To alleviate injection pain, topical anesthetic may be applied 30–60 minutes before the treatment. Although the effect of a single intralesional corticosteroid injection has been observed to persist for up to 9 months,Citation3 reported relapse rates were 29% in limited alopecia areata and 72% in alopecia totalis during a 3-month follow-up period.Citation1
Topical corticosteroids
Many forms of topical corticosteroids have been prescribed for alopecia areata, including creams, gels, ointments, lotions, and foams. Sixty-one percent of patients using 0.1% betamethasone valerate foam achieved more than 75% hair regrowth in comparison with 27% in the 0.05% betamethasone dipropionate lotion group.Citation4 Topical corticosteroids are far less effective in alopecia totalis and alopecia universalis.Citation5 A highly potent topical corticosteroid under occlusion is the preferred method when using topical corticosteroids. Folliculitis is a common side effect to topical corticosteroids. Telangiectasia and atrophy may develop rarely. The reported relapse rate is 37%–63%.Citation5,Citation6
Minoxidil
In a placebo-controlled, double-blind study, hair regrowth was observed in 63.6% and 35.7% of the minoxidil-treated and placebo groups, respectively.Citation7 However, only 27% of the minoxidil-treated patients showed cosmetically acceptable hair regrowth. In another study, hair regrowth was achieved in 38% and 81% of patients treated with 1% and 5% topical minoxidil, respectively.Citation8 Most studies have shown no beneficial effect of topical minoxidil in alopecia totalis and alopecia universalis.Citation9 Minoxidil 5% solution or foam is frequently used with other therapeutic agents as an adjuvant therapy. The adverse effects of topical minoxidil include contact dermatitis and facial hypertrichosis.
Anthralin
A few controlled trials have assessed the efficacy of topical anthralin in the treatment of alopecia areata. In an open study, a cosmetic response was seen in 25% of patients with severe alopecia areata treated using 0.5%–1.0% anthralin cream.Citation10 In another trial, combination therapy of 5% minoxidil and 0.5% anthralin was used to treat 51 patients with severe alopecia areata; only 11% of patients achieved cosmetically acceptable hair regrowth.Citation11 Anthralin needs to be applied in a high enough concentration (0.5%–1%) and sufficiently frequently (daily) to produce a mild irritant reaction in order to be effective. Severe irritation and staining of skin and clothes are some of the possible adverse events with anthralin.
Topical immunotherapy
Topical sensitizers that have been used in the treatment of alopecia areata include diphenylcyclopropenone, squaric acid dibutylester (SADBE), and dinitrochlorobenzene. Dinitrochlorobenzene is no longer used because it was shown to be mutagenic in the Ames test.Citation12 Diphenylcyclopropenone is the topical sensitizer of choice. SADBE is expensive and not stable in acetone.Citation13 Diphenylcyclopropenone is lightsensitive and should be protected from light.Citation14
Initially the patient is sensitized using a 2% solution of diphenylcyclopropenone applied to a 4 × 4 cm area of the scalp. After two weeks, 0.001% diphenylcyclopropenone solution is applied to the same half of the scalp. The diphenylcyclopropenone concentration is increased gradually every week until mild dermatitis is observed.Citation15 The solution should be on the scalp for 48 hours. The scalp should be protected from the sun during this time. Once hair regrowth is obtained on the treated half of the scalp, both sides are treated. Both sides of the scalp can be treated from the start also. Diphenylcyclopropenone is applied on a weekly basis by a trained nurse. If there is no response after 6 months of treatment, diphenylcyclopropenone can be discontinued. SADBE may be tried in poor responders to diphenylcyclopropenone or in those who do not develop a sensitization to 2% diphenylcyclopropenone. SADBE is applied once or twice per week.Citation16,Citation17 The adverse effects to topical sensitizers include cervical lymphadenopathy, a severe eczematous reaction, urticaria, and postinflammatory pigment changes.Citation18,Citation19
The response rate of alopecia totalis/alopecia universalis patients to diphenylcyclopropenone was 17.4% in the largest reported diphenylcyclopropenone study, whereas the cumulative patient response was 77%.Citation20 Several negative prognostic factors in the treatment of alopecia areata with diphenylcyclopropenone have been suggested, including long duration of disease, alopecia totalis/alopecia universalis, nail changes, atopy, and family history of alopecia areata.Citation20–Citation22 Recurrence of alopecia areata after achieving significant hair regrowth developed in 62.6% of patients.Citation20 In a retrospective study of 121 patients with extensive alopecia areata, fexofenadine hydrochloride has been shown to enhance the efficacy of topical immunotherapy.Citation23
The mechanism of action of topical sensitizers could be due to perifollicular lymphocyte apoptosis,Citation24 changes in the peribulbar CD4/CD8 lymphocyte ratio,Citation25,Citation26 and antigenic competition.Citation27
Prostaglandin analogs
Eyelash hypertrichosis is a common adverse effect to the use of these antiglaucoma eye drops.Citation28–Citation30 Some case series did not show an effect in the treatment of eyelashes in patients with alopecia areata.Citation31,Citation32 In a nonrandomized, controlled study of latanoprost (a prostaglandin F2α analog) eye drops in patients with alopecia universalis, acceptable results (total and moderate hair regrowth) were achieved in 45% of patients.Citation33 In another retrospective trial, 0.03% bimatoprost eye drops were used once a day for one year. Complete regrowth of the eyelashes was noted in 24.3% of patients and moderate growth in 18.9% of treated subjects.Citation34 Relapses were observed in 17.5% of the patients, mainly in the slight response group.Citation33
Topical retinoids
In a comparative study of topical tretinoin 0.05%, topical betamethasone dipropionate lotion, and dithranol paste 0.25%, a good response has been seen in 55% of patients treated with topical tretinoin in comparison with 70% and 35% in the topical steroid and dithranol groups, respectively. Citation35 Although the mechanism for its action in alopecia areata is not completely understood, the associated tretinoin-induced dermatitis might contribute to regrowth in alopecia areata. Larger, double-blind, placebo-controlled trials are needed.
Bexarotene
In a randomized bilateral half-head study, hair regrowth of at least 50% on treated sites was noticed in only 26% of patients treated with 1% bexarotene gel.Citation36 Mild irritation is a common side effect.
Capsaicin
In a nonblinded randomized study, 9.5% of patients with alopecia areata showed cosmetically acceptable hair regrowth after 12 weeks of applying capsaicin ointment.Citation37
Second-line therapies
Sulfasalazine
Sulfasalazine is a combination of sulfapyridine and 5-aminosalicylic acid linked by a diazo bond. Sulfasalazine has both immunomodulatory and immunosuppressive actions that include suppression of T cell proliferation and reducing the synthesis of cytokines, including interleukin (IL) 6, 1, and 12, tumor necrosis factor alpha, and antibody production.Citation38 Sulfasalazine has been used safely as a long-term treatment of various inflammatory and autoimmune diseases, including inflammatory bowel disease and rheumatoid arthritis. Several case reports and case series showed good hair regrowth with sulfasalazine in the treatment of alopecia areata.
In an uncontrolled prospective trial of sulfasalazine in 39 patients with persistent alopecia areata, hair regrowth of more than 60% was achieved in 25.6% of patients. A moderate response was seen in 30.7% of patients.Citation39 Also, in another uncontrolled open-label study, complete hair regrowth was reported in 27.3% of subjects.Citation40 Sulfasalazine was started at 500 mg twice daily for one month, 1 g twice daily for one month, and then 1 g three times daily.Citation41 Side effects to sulfasalazine include gastrointestinal distress, dizziness, and headache.Citation39 Gastrointestinal symptoms can be minimized by using enteric-coated tablets, taking the medication with food, and starting at lower doses. Initially, patients should have a complete blood count, liver function tests, creatinine, and glucose-6-phosphate dehydrogenase level measurement. Complete blood counts and liver function tests should be performed at 2–4-week intervals during the first three months of therapy. The reported relapse rates are 22.7%–45.5%.Citation39,Citation40
Photochemotherapy
The success rate for oral and topical psoralen plus ultraviolet A (PUVA) ranged from 15% to more than 70%.Citation42,Citation43 PUVAturban is a method of administering a dilute psoralen solution (8-methoxypsoralen 0.0001%) selectively to the scalp for 20 minutes using a cotton towel as a turban. The patient’s scalp is then exposed to ultraviolet A radiation.Citation44 Treatment sessions are performed two or three times per week. PUVA- turban has been shown to be effective in about 70% of treated patients.Citation44,Citation45 During a follow-up period of 15 months after PUVA-turban therapy, recurrences of alopecia areata were observed in 26% of responders.Citation44 PUVA-turban therapy lacks the systemic side effects of oral PUVA and can be considered as alternative therapy for patients with alopecia areata.
Excimer laser
In a treatment of 42 alopecia areata patches with the 308 nm excimer laser, hair regrowth was observed in 41.5% of treated areas.Citation46 Hair regrowth was noticed to begin to appear during the second month of therapy. No regrowth of hair was noted on the control patches. Laser therapy was administered twice a week for a maximum of 24 sessions. Apart from erythema at the treated sites, there were no significant adverse effects. Relapses of alopecia areata were observed in two patients with patchy alopecia areata of the scalp who had shown complete regrowth earlier. Also, the use of excimer laser in children with alopecia areata has been reported to have a good success rate.Citation47
Fractional photothermolysis laser
Good hair regrowth was achieved with fractional Er: Glass laser in a single case report.Citation48 Randomized controlled trials in a larger number of patients are required to confirm the efficacy of this modality of treatment.
Third-line therapies
Systemic corticosteroids
Systemic corticosteroids are one of the commonly prescribed therapies in patients with extensive alopecia areata. Various forms of corticosteroids have been used in different regimens. In one study, a once-monthly oral pulse of 300 mg prednisone induced a complete response in 41% of patients.Citation49 A similar effect has been reported in a placebo-controlled trial of oral prednisolone 200 mg once weekly in the treatment of extensive alopecia areata.Citation50 The relapse rate was 25%, and side effects of the therapy were noted in 55% of patients.Citation50 In a comparative trial, the response rate was better in patients treated with intramuscular triamcinolone acetonide 40 mg once monthly than in those treated with oral dexamethasone 0.5 mg/day.Citation51 In the same study, impairment of adrenocortical reserve was seen in 23% of the intramuscular triamcinolone acetonide group and in 7% of patients treated with oral prednisolone pulse therapy of 80 mg for 3 consecutive days once every 3 months. In a study of 139 patients treated with pulse corticosteroid therapy, a good response was achieved in 59.4% of patients with recent- onset disease (duration of alopecia areata up to 6 months) in comparison with 15.8% of subjects who had had alopecia areata for more than 6 months.Citation52 Alopecia totalis and alopecia universalis are far less responsive to this therapy than patchy alopecia areata.Citation53 The use of systemic corticosteroids is limited by their side effects (hyperglycemia, weight gain, hypertension, adrenal suppression, dysmenorrhea, immunosuppression, and acneiform eruption)Citation50,Citation54 and the high relapse rate (14%–100%).Citation51,Citation55,Citation56
Methotrexate
In a long-term follow-up study of methotrexate in 33 patients with alopecia areata, complete hair regrowth was achieved in 57% and 63% of patients who used methotrexate alone or with low doses of oral corticosteroids (prednisone 10–20 mg/day), respectively.Citation57 Thirty percent of patients had partial hair regrowth. The weekly dosages of methotrexate were 15–25 mg. The onset of hair regrowth was seen after a median delay of three months. Recurrences of alopecia areata after a decrease of the methotrexate dose or after stopping treatment were observed in 57% (8/14 cases) of responders. In a retrospective trial of methotrexate in 14 children with alopecia areata, approximately one third of patients experienced a clinically relevant therapeutic response.Citation58 The mean age of the patients was 14.7 (range 8–18) years. Adverse effects to methotrexate include persistent nausea, transient elevation of hepatic enzymes, and leucopenia.
Cyclosporine
The success rate with oral cyclosporine is 25%–76.6%.Citation59,Citation60 A recent study showed that a good response to oral cyclosporine can be predicted if the serum level of IL 18 is elevated and the level of soluble IL 2 receptor is low.Citation61 The use of oral cyclosporine in patients with alopecia areata is not generally favored due to its adverse event profile (nephrotoxicity, immune suppression, and hypertension) and a high relapse rate (up to 100%).Citation62 Also, alopecia areata incidence has been reported in several organ transplant patients receiving cyclosporine.Citation63–Citation66 Although hypertrichosis is a documented side effect of oral cyclosporine,Citation67 a good response has not been achieved by using topical cyclosporine in humans.Citation68,Citation69
Azathioprine
Azathioprine, a thiopurine analog immunosuppressive drug, has been used to treat a vast array of autoimmune diseases. It inhibits DNA synthesis and thus decreases proliferation of cells, especially T and B lymphocytes. Azathioprine also decreases the number of Langerhans cells and other antigen-presenting cells in the skin. In a recent pilot study of 20 patients treated with azathioprine 2 mg/kg/day as monotherapy, mean hair regrowth was 52.3% ± 38.4%.Citation70 These results need to be confirmed in large-scale, randomized, controlled studies.
Biologics
Although tumor necrosis factor alpha is implicated in the pathogenesis of alopecia areata, there are several reported cases that have shown either development of alopecia areata or complete failure to respond to different tumor necrosis factor alpha inhibitors, including adalimumab,Citation71,Citation74 infliximab,Citation75,Citation76 and etanercept.Citation77–Citation79 In a prospective trial of 17 patients with alopecia areata, Strober et al concluded that etanercept does not effectively treat moderate to severe alopecia areata.Citation80 Also, in a placebo-controlled study, Price et al showed that efalizumab, an anti-CD11a antibody, is not effective in the treatment of alopecia areata.Citation81 Some clinical trials are ongoing to evaluate the efficacy of the newer biologic therapies in the treatment of alopecia areata.
Psychological support
Alopecia areata is considered to be an example of a psychosomatic disorder, leading to dramatic and devastating emotions which can negatively impact patient self-esteem, body image, and self-confidence.Citation82
One important step that should not be overlooked during the course of management of alopecia areata is offering psychological support to foster increased self-esteem and adaptation to this disease. Helping patients with alopecia areata cope with depression and an unpredictable disease like alopecia areata can be achieved by several ways, including education of the patient about the nature of disease, psychotherapy, hypnotherapy,Citation83 antidepressants,Citation84,Citation85 and support groups. Hypnotherapy may significantly improve depression, anxiety, and quality of life, but not hair regrowth.Citation86 Patients with extensive disease may wear scalp prostheses, such as wigs, hairpieces, or other scalp coverings.
Other therapies
Other therapeutic agents have been tried, with some degree of success. These modalities include aromatherapy,Citation87 a combination of topical garlic gel and betamethasone valerate cream,Citation88 topical azelic acid,Citation89 oral zinc supplementation,Citation90–Citation92 topical onion juice,Citation93 a simvastatin-ezetimibe combination,Citation94,Citation95 inosiplex,Citation96–Citation98 and intralesional injections of candida antigen.Citation99 These treatment modalities need to be confirmed in large-scale, double-blind, placebo-controlled trials.
There are other modalities of therapy that have not shown good efficacy. These agents include imiquimod,Citation100,Citation101 topical calcineurin inhibitors,Citation102–Citation106 botulinum toxin type A,Citation107 topical tri-iodothyronine ointment,Citation108 photodynamic therapy,Citation109–Citation111 and topical 5-fluorounacil.Citation112
Management plan
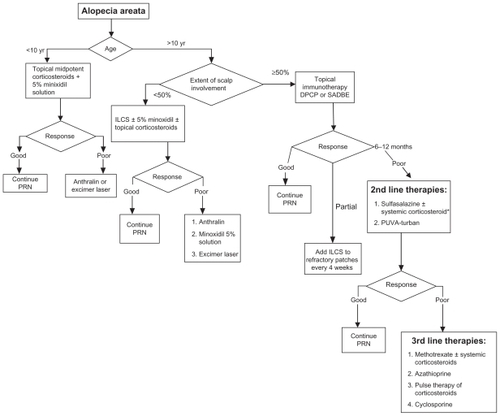
Treatment options should be selected according to patient age and extent of disease. For patients younger than 10 years, a combination of 5% minoxidil solution twice daily with midpotent corticosteroids should be tried first. If there is no good improvement after 6 months, short-contact anthralin is considered as second-line therapy. Excimer laser can be used, particularly in patchy alopecia areata.
For patients older than 10 years of age with alopecia areata involving less than 50% of the scalp, intralesional triamcinolone acetonide injection (5 mg/cc) is the recommended option for treatment. If there is no good response after 6 months, other options can be tried, including potent topical corticosteroids under occlusion at night, 5% topical minoxidil twice a day, short-contact anthralin, and excimer laser.
If alopecia areata involves more than 50% of the scalp, topical immunotherapy with diphenylcyclopropenone is the first therapeutic option recommended by many experts in hair diseases. Intralesional injections of triamcinolone acetonide are used to treat persistent alopecic patches.
For patients who respond poorly to diphenylcyclopropenone and those who cannot use it, second-line therapies can be used. Several reviews of alopecia areata therapy suggest topical minoxidil and topical corticosteroidsCitation113–Citation116 but, as discussed earlier, the yield of these topical agents in the treatment of extensive alopecia areata is limited. Therefore, we suggest that patients with extensive resistant disease can use sulfasalazine with or without systemic corticosteroids. Systemic steroids are used as bridge therapy until the sulfasalazine takes effect. Treatment with sulfasalazine is generally well tolerated and characterized by a lower incidence of serious side effects in comparison with other systemic therapies like corticosteroids and methotrexate. The other second-line therapy is PUVA-turban. It is a well tolerated therapy with minimal local phototoxic side effects and without the systemic side effects of PUVA. These options are selected based on a balance between the efficacy and safety of these therapeutic agents.
If these therapies fail or are not tolerated, third-line therapeutic options can be discussed with patients in terms of the expected outcome of therapy and possible side effects. These agents include methotrexate with or without a systemic corticosteroid, azathioprine, cyclosporine, and pulse therapy of corticosteroids. While using these drugs, close monitoring of patients is important to avoid possible side effects. A summary of an alopecia areata treatment plan is shown as an algorithmic approach in .
Disclosure
The author reports no conflicts of interest in this work.
References
- AbellEMunroDDIntralesional treatment of alopecia areata with triamcinolone acetonide by jet injectorBr J Dermatol197388155594686543
- FerrandoJMoreno-AriasGAMulti-injection plate for intralesional corticosteroid treatment of patchy alopecia areataDermatol Surg200026769069110886282
- OrentreichNSturmHMWeidmanAIPelzigALocal injection of steroids and hair regrowth in alopeciasArch Dermatol19608289490213731135
- MancusoGBalducciACasadioCEfficacy of betamethasone valerate foam formulation in comparison with betamethasone dipropionate lotion in the treatment of mild-to-moderate alopecia areata: a multicenter, prospective, randomized, controlled, investigator-blinded trialInt J Dermatol200342757257512839615
- TostiAPiracciniBMPazzagliaMVincenziCClobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalisJ Am Acad Dermatol2003491969812833016
- PascherFKurtinSAndradeRAssay of 0.2 percent fluocinolone acetonide cream for alopecia areata and totalis. Efficacy and side effects including histologic study of the ensuing localized acneform responseDermatologica197014131932024250339
- PriceVHDouble-blind, placebo-controlled evaluation of topical minoxidil in extensive alopecia areataJ Am Acad Dermatol1987163 Pt 27307363549809
- Fiedler-WeissVCTopical minoxidil solution (1% and 5%) in the treatment of alopecia areataJ Am Acad Dermatol1987163 Pt 27457483549811
- PriceVHTopical minoxidil (3%) in extensive alopecia areata, including long-term efficacyJ Am Acad Dermatol1987163 Pt 27377443549810
- Fiedler-WeissVCBuysCMEvaluation of anthralin in the treatment of alopecia areataArch Dermatol198712311149114933314718
- FiedlerVCWendrowASzpunarGJMetzlerCDeVillezRLTreatment-resistant alopecia areata. Response to combination therapy with minoxidil plus anthralinArch Dermatol199012667567592140670
- StrobelRRohrbornGMutagenic and cell transforming activities of 1-chlor-2,4-dinitrobenzene (DNCB) and squaric-acid-dibutylester (SADBE)Arch Toxicol19804543073147004402
- WilkersonMGHenkinJWilkinJKSmithRGSquaric acid and esters: analysis for contaminants and stability in solventsJ Am Acad Dermatol1985132 Pt 12292344044949
- WilkersonMGHenkinJWilkinJKDiphenylcyclopropenone: examination for potential contaminants, mechanisms of sensitization, and photochemical stabilityJ Am Acad Dermatol1984115 Pt 18028076239880
- OrecchiaGPerfettiLAlopecia areata and topical sensitizers: allergic response is necessary but irritation is notBr J Dermatol199112455092039735
- OrecchiaGMalagoliPSantagostinoLTreatment of severe alopecia areata with squaric acid dibutylester in pediatric patientsPediatr Dermatol199411165688170854
- Dall’oglioFNascaMRMusumeciMLTopical immunomodulator therapy with squaric acid dibutylester (SADBE) is effective treatment for severe alopecia areata (AA): results of an open-label, paired-comparison, clinical trialJ Dermatolog Treat2005161101415897160
- AghaeiSTopical immunotherapy of severe alopecia areata with diphenylcyclopropenone (DPCP): experience in an Iranian populationBMC Dermatol20055615918897
- FrancomanoMSeidenariSUrticaria after topical immunotherapy with diphenylcyclopropenoneContact Dermatitis200247531031112534538
- WisemanMCShapiroJMacDonaldNLuiHPredictive model for immunotherapy of alopecia areata with diphencyproneArch Dermatol200113781063106811493099
- van der SteenPHvan BaarHMHappleRBoezemanJBPerretCMPrognostic factors in the treatment of alopecia areata with diphenylcyclopropenoneJ Am Acad Dermatol1991242 Pt 12272302007667
- WeiseKKretzschmarLJohnSMHammHTopical immunotherapy in alopecia areata: anamnestic and clinical criteria of prognostic significanceDermatology199619221291338829494
- InuiSNakajimaTTodaNItamiSFexofenadine hydrochloride enhances the efficacy of contact immunotherapy for extensive alopecia areata: retrospective analysis of 121 casesJ Dermatol200936632332719500180
- HerbstVZollerMKisslingSWenzelEStutzNFreyschmidt-PaulPDiphenylcyclopropenone treatment of alopecia areata induces apoptosis of perifollicular lymphocytesEur J Dermatol200616553754217101475
- HappleRKleinHMMacherETopical immunotherapy changes the composition of the peribulbar infiltrate in alopecia areataArch Dermatol Res198627832142182873796
- WasylyszynTKozlowskiWZabielskiSLChanges in distribution pattern of CD8 lymphocytes in the scalp in alopecia areata during treatment with diphencyproneArch Dermatol Res20072995–623123717530266
- HappleRAntigenic competition as a therapeutic concept for alopecia areataArch Dermatol Res198026711091146446265
- BeardenWAndersonRTrichiasis associated with prostaglandin analog useOphthal Plast Reconstr Surg2004204320322
- HartJShafranovGHypertrichosis of vellus hairs of the malar region after unilateral treatment with bimatoprostAm J Ophthalmol2004137475675715059720
- TostiAPazzagliaMVoudourisSTostiGHypertrichosis of the eyelashes caused by bimatoprostJ Am Acad Dermatol200451Suppl 5S149S15015577756
- RoseboroughILeeHChwalekJStamperRLPriceVHLack of efficacy of topical latanoprost and bimatoprost ophthalmic solutions in promoting eyelash growth in patients with alopecia areataJ Am Acad Dermatol200960470570619293023
- RossEKBolducCLuiHShapiroJLack of efficacy of topical latanoprost in the treatment of eyebrow alopecia areataJ Am Acad Dermatol20055361095109616310083
- Coronel-PerezIMRodriguez-ReyEMCamacho-MartinezFMLatanoprost in the treatment of eyelash alopecia in alopecia areata universalisJ Eur Acad Dermatol Venereol201024448148520028444
- VilaOTCamacho MartinezFMBimatoprost in the treatment of eyelash universalis alopecia areataInt J Trichology201022868821712909
- DasSGhoramiRCChatterjeeTBanerjeeGComparative assessment of topical steroids, topical tretenoin (0.05%) and dithranol paste in alopecia areataIndian J Dermatol201055214814920606883
- TalpurRVuJBassettRStevensVDuvicMPhase I/II randomized bilateral half-head comparison of topical bexarotene 1% gel for alopecia areataJ Am Acad Dermatol2009614592.e591e59919682769
- EhsaniAHToosiSSeirafiHCapsaicin vs clobetasol for the treatment of localized alopecia areataJ Eur Acad Dermatol Venereol200923121451145319281605
- RanganathVKFurstDEDisease-modifying antirheumatic drug use in the elderly rheumatoid arthritis patientRheum Dis Clin North Am200733119721717367700
- RashidiTMahdAATreatment of persistent alopecia areata with sulfasalazineInt J Dermatol200847885085218717871
- AghaeiSAn uncontrolled, open label study of sulfasalazine in severe alopecia areataIndian J Dermatol Venereol Leprol200874661161319171984
- EllisCNBrownMFVoorheesJJSulfasalazine for alopecia areataJ Am Acad Dermatol200246454154411907504
- TaylorCRHawkJLPUVA treatment of alopecia areata partialis, totalis and universalis: audit of 10 years’ experience at St John’s Institute of DermatologyBr J Dermatol199513369149188547044
- MohamedZBhouriAJallouliAFazaaBKamounMRMokhtarIAlopecia areata treatment with a phototoxic dose of UVA and topical 8-methoxypsoralenJ Eur Acad Dermatol Venereol200519555255516164707
- Broniarczyk-DylaGWawrzycka-KaflikADubla-BernerMPrusinska- BratosMEffects of psoralen-UV-A-Turban in alopecia areataSkinmed200652646816603835
- Behrens-WilliamsSCLeiterUSchienerRWeidmannMPeterRUKerscherMThe PUVA-turban as a new option of applying a dilute psoralen solution selectively to the scalp of patients with alopecia areataJ Am Acad Dermatol200144224825211174382
- Al-MutairiN308-nm excimer laser for the treatment of alopecia areataDermatol Surg200733121483148718076615
- Al-MutairiN308-nm excimer laser for the treatment of alopecia areata in childrenPediatr Dermatol200926554755019840308
- YooKHKimMNKimBJKimCWTreatment of alopecia areata with fractional photothermolysis laserInt J Dermatol201049784584719627384
- Ait OurhrouiMHassamBKhoudriITreatment of alopecia areata with prednisone in a once-monthly oral pulseAnn Dermatol Venereol20101378–9514518 French20804894
- KarBRHandaSDograSKumarBPlacebo-controlled oral pulse prednisolone therapy in alopecia areataJ Am Acad Dermatol200552228729015692475
- KurosawaMNakagawaSMizuashiMA comparison of the efficacy, relapse rate and side effects among three modalities of systemic corticosteroid therapy for alopecia areataDermatology2006212436136516707886
- NakajimaTInuiSItamiSPulse corticosteroid therapy for alopecia areata: study of 139 patientsDermatology2007215432032417911990
- FriedliALabartheMPEngelhardtEFeldmannRSalomonDSauratJHPulse methylprednisolone therapy for severe alopecia areata: an open prospective study of 45 patientsJ Am Acad Dermatol1998394 Pt 15976029777767
- LesterRSKnowlesSRShearNHThe risks of systemic corticosteroid useDermatol Clin19981622772889589201
- SharmaVKGuptaSTwice weekly 5 mg dexamethasone oral pulse in the treatment of extensive alopecia areataJ Dermatol199926956256510535249
- LuggenPHunzikerTHigh-dose intravenous corticosteroid pulse therapy in alopecia areata: own experience compared with the literatureJ Dtsch Dermatol Ges200865375378 German18205838
- ChartauxEJolyPLong-term follow-up of the efficacy of methotrexate alone or in combination with low doses of oral corticosteroids in the treatment of alopecia areata totalis or universalisAnn Dermatol Venereol20101378–9507513 French20804893
- RoyerMBodemerCVabresPEfficacy and tolerability of methotrexate in severe childhood alopecia areataBr J DermatolApril 252011 [Epub ahead of print]
- ShapiroJLuiHTronVHoVSystemic cyclosporine and low-dose prednisone in the treatment of chronic severe alopecia areata: a clinical and immunopathologic evaluationJ Am Acad Dermatol19973611141178996277
- KimBJMinSUParkKYCombination therapy of cyclosporine and methylprednisolone on severe alopecia areataJ Dermatolog Treat200819421622018608727
- LeeDHongSKParkSWSerum levels of IL-18 and sIL-2R in patients with alopecia areata receiving combined therapy with oral cyclosporine and steroidsExp Dermatol201019214514719758343
- GuptaAKEllisCNCooperKDOral cyclosporine for the treatment of alopecia areata. A clinical and immunohistochemical analysisJ Am Acad Dermatol1990222 Pt 12422502138175
- PhillipsMAGravesJENunleyJRAlopecia areata presenting in 2 kidney-pancreas transplant recipients taking cyclosporineJ Am Acad Dermatol2005535 Suppl 1S252S25516227102
- Dyall-SmithDAlopecia areata in a renal transplant recipient on cyclosporinAustralas J Dermatol19963742262278961599
- CerottiniJPPanizzonRGde ViraghPAMultifocal alopecia areata during systemic cyclosporine A therapyDermatology1999198441541710490301
- DaviesMGBowersPWAlopecia areata arising in patients receiving cyclosporin immunosuppressionBr J Dermatol199513258358367772501
- SternthalMBMurphySJGeorgeJKornbluthALichtigerSPresentDHAdverse events associated with the use of cyclosporine in patients with inflammatory bowel diseaseAm J Gastroenterol2008103493794318177449
- GilharAPillarTEtzioniATopical cyclosporin A in alopecia areataActa Derm Venereol19896932522532566234
- MauduitGLenversPBarthelemyHThivoletJTreatment of severe alopecia areata with topical applications of cyclosporin AAnn Dermatol Venereol19871144507510 French3619297
- FarshiSMansouriPSafarFKhiabanlooSRCould azathioprine be considered as a therapeutic alternative in the treatment of alopecia areata? a pilot studyInt J Dermatol201049101188119320883409
- Garcia BartelsNLeeHHWormMBurmesterGRSterryWBlume- PeytaviUDevelopment of alopecia areata universalis in a patient receiving adalimumabArch Dermatol2006142121654165517179003
- KirshenCKanigsbergNAlopecia areata following adalimumabJ Cutan Med Surg2009131485019298772
- ChavesYDuarteGBen-SaidBTebibJBerardFNicolasJFAlopecia areata universalis during treatment of rheumatoid arthritis with anti- TNF-alpha antibody (adalimumab)Dermatology2008217438018849606
- PelivaniNHassanASBraathenLRHungerREYawalkarNAlopecia areata universalis elicited during treatment with adalimumabDermatology2008216432032318230980
- EttefaghLNedorostSMirmiraniPAlopecia areata in a patient using infliximab: new insights into the role of tumor necrosis factor on human hair folliclesArch Dermatol20041408101215313825
- FabreCDereureOWorsening alopecia areata and de novo occurrence of multiple halo nevi in a patient receiving infliximabDermatology2008216218518618216487
- PostenWSwanJRecurrence of alopecia areata in a patient receiving etanercept injectionsArch Dermatol2005141675976015967923
- AbramovitsWLosornioMFailure of two TNF-alpha blockers to influence the course of alopecia areataSkinmed20065417718116855408
- PanYRaoNAAlopecia areata during etanercept therapyOcul Immunol Inflamm200917212712919412875
- StroberBESiuKAlexisAFEtanercept does not effectively treat moderate to severe alopecia areata: an open-label studyJ Am Acad Dermatol20055261082108415928633
- PriceVHHordinskyMKOlsenEASubcutaneous efalizumab is not effective in the treatment of alopecia areataJ Am Acad Dermatol200858339540218280336
- Ruiz-DobladoSCarrizosaAGarcia-HernandezMJAlopecia areata: psychiatric comorbidity and adjustment to illnessInt J Dermatol200342643443712786868
- WillemsenRVanderlindenJDeconinckARoseeuwDHypnotherapeutic management of alopecia areataJ Am Acad Dermatol200655223323716844504
- CiprianiRPeriniGIRampinelliSParoxetine in alopecia areataInt J Dermatol200140960060111737460
- PeriniGZaraMCiprianiRImipramine in alopecia areata. A double-blind, placebo-controlled studyPsychother Psychosom1994613–41951988066157
- WillemsenRHaentjensPRoseeuwDVanderlindenJHypnosis in refractory alopecia areata significantly improves depression, anxiety, and life quality but not hair regrowthJ Am Acad Dermatol201062351751820159323
- HayICJamiesonMOrmerodADRandomized trial of aromatherapy. Successful treatment for alopecia areataArch Dermatol199813411134913529828867
- HajheydariZJamshidiMAkbariJMohammadpourRCombination of topical garlic gel and betamethasone valerate cream in the treatment of localized alopecia areata: a double-blind randomized controlled studyIndian J Dermatol Venereol Leprol2007731293217314444
- SasmazSAricanOComparison of azelaic acid and anthralin for the therapy of patchy alopecia areata: a pilot studyAm J Clin Dermatol20056640340616343028
- ParkHKimCWKimSSParkCWThe therapeutic effect and the changed serum zinc level after zinc supplementation in patients with alopecia areata who had a low serum zinc levelAnn Dermatol200921214214620523772
- BhatYJManzoorSKhanARQayoomSTrace element levels in alopecia areataIndian J Dermatol Venereol Leprol2009751293119172027
- LutzGKreyselHWSelective changes in lymphocytic differentiation antigens in the peripheral blood of patients with alopecia areata treated with oral zincZ Hautkr1990652132134 German1971470
- SharquieKEAl-ObaidiHKOnion juice (Allium cepa L.), a new topical treatment for alopecia areataJ Dermatol200229634334612126069
- RobinsDNCase reports: alopecia universalis: hair growth following initiation of simvastatin and ezetimibe therapyJ Drugs Dermatol20076994694717941369
- AliAMartinJM4thHair growth in patients alopecia areata totalis after treatment with simvastatin and ezetimibeJ Drugs Dermatol201091626420120427
- GalbraithGMThiersBHJensenJHoehlerFA randomized double-blind study of inosiplex (isoprinosine) therapy in patients with alopecia totalisJ Am Acad Dermatol1987165 Pt 19779832438319
- GeorgalaSKatoulisACBefonAGeorgalaKStavropoulosPGInosiplex for treatment of alopecia areata: a randomized placebocontrolled studyActa Derm Venereol200686542242416955187
- LowyMLedoux-CorbusierMAchtenGWybranJClinical and immunologic response to isoprinosine in alopecia areata and alopecia universalis: association with autoantibodiesJ Am Acad Dermatol1985121 Pt 178842579988
- RosenbergEWSkinnerRBJrImmunotherapy of alopecia areata with intralesional Candida antigenPediatr Dermatol200623329916780487
- D’OvidioRClaudatusJDi PrimaTIneffectiveness of imiquimod therapy for alopecia totalis/universalisJ Eur Acad Dermatol Venereol200216441641712224709
- KocETuncaMAkarAKurumluZLack of efficacy of topical imiquimod in the treatment of patchy alopecia areataInt J Dermatol200847101088108918986369
- RigopoulosDGregoriouSKorfitisCLack of response of alopecia areata to pimecrolimus creamClin Exp Dermatol200732445645717537231
- FeldmannKAKunteCWollenbergAWolfeHIs topical tacrolimus effective in alopecia areata universalis?Br J Dermatol200214751031103212410729
- ParkSWKimJWWangHYTopical tacrolimus (FK506): treatment failure in four cases of alopecia universalisActa Derm Venereol200282538738812430746
- PriceVHWilleyAChenBKTopical tacrolimus in alopecia areataJ Am Acad Dermatol200552113813915627095
- ThiersBHTopical tacrolimus: treatment failure in a patient with alopecia areataArch Dermatol2000136112410632221
- ChoHRLewBLLewHSimWYTreatment effects of intradermal botulinum toxin type A injection on alopecia areataDermatol Surg201036Suppl 42175218121134049
- NasiriSHaghpanahVTaheriEHeshmatRLarijaniBSaeediMHair regrowth with topical triiodothyronine ointment in patients with alopecia areata: a double-blind, randomized pilot clinical trial of efficacyJ Eur Acad Dermatol Venereol2011Apr 27 [Epub ahead of print]
- YooKHLeeJWLiKKimBJKimMNPhotodynamic therapy with methyl 5-aminolevulinate acid might be ineffective in recalcitrant alopecia totalis regardless of using a microneedle roller to increase skin penetrationDermatol Surg201036561862220384755
- Fernandez-GuarinoMHartoAGarcia-MoralesIPerez-GarciaBArrazolaJMJaenPFailure to treat alopecia areata with photodynamic therapyClin Exp Dermatol200833558558718355356
- BissonnetteRShapiroJZengHMcLeanDILuiHTopical photodynamic therapy with 5-aminolaevulinic acid does not induce hair regrowth in patients with extensive alopecia areataBr J Dermatol200014351032103511069515
- KaplanALOlsenEATopical 5-fluorouracil is ineffective in the treatment of extensive alopecia areataJ Am Acad Dermatol200450694194315153898
- WassermanDGuzman-SanchezDAScottKMcMichaelAAlopecia areataInt J Dermatol200746212113117269961
- MadaniSShapiroJAlopecia areata updateJ Am Acad Dermatol200042454956610727299
- AlkhalifahAAlsantaliAWangEMcElweeKJShapiroJAlopecia areata update: part II. TreatmentJ Am Acad Dermatol201062219120220115946
- RossEKShapiroJManagement of hair lossDermatol Clin200523222724315837153