Abstract
Background
VYC-12L is a hyaluronic acid (HA) injectable gel designed to treat fine cutaneous lines and improve skin quality attributes such as hydration and elasticity.
Objective
Expert consensus was sought on VYC-12L injection technique and primary treatment target areas.
Methods
A multinational group of aesthetic medicine clinicians (n = 128) attended product training and each identified ~10 patients for VYC-12L. After treating their first and last patients, the clinicians completed a survey on preferred injection methodology/technique, including injection angle, volume, and spacing. An expert panel (n = 12) discussed survey results and their clinical experiences to obtain consensus on VYC-12L technique and appropriate treatment areas.
Results
Recommendations included micro-depot injections of VYC-12L into the deep dermis with a 32G ½ inch needle inserted at <45º to the skin, spaced 0.5‒1.0 cm apart, with 0.01‒0.05 mL volume per injection (full-face total volume: ~2 mL). Recommended primary treatment areas were the malar, perioral, neck, and décolletage regions. Injection techniques for different treatment areas/demographic characteristics were similar, with some variability in treatment approach. Patient selection criteria, pre- and post-treatment guidelines, and managing patient expectations are important components of treatment.
Conclusion
These consensus recommendations may assist clinicians in optimizing the treatment of fine lines with VYC-12L.
Introduction
The topographic appearance of facial skin can influence judgments of age, physical health and fitness, and psychological factors such as self-esteem and well-being.Citation1–Citation4 Skin smoothness, hydration, firmness, and texture are just a few of the attributes that contribute to overall skin appearance or skin quality.Citation5–Citation9 Poor skin quality results from a variety of intrinsic and extrinsic factors, such as age- and hormone-related structural changes and environmental stressors (eg, ultraviolet, infrared A, and ozone exposure); effects on the skin may include roughness, surface irregularities, and wrinkling.Citation6,Citation10–Citation15 Minimally invasive cosmetic procedures to improve skin appearance, such as chemical peels, laser skin resurfacing, and microdermabrasion, have increased in popularity over the last 2 decades, exceeding 3.1 million procedures in the United States in 2017.Citation16,Citation17
VYC-12 with lidocaine (VYC-12L [Juvéderm Volite]; Allergan plc, Dublin, Ireland) is a crosslinked HA injectable gel that was developed using the Vycross technology platform (Allergan plc, Dublin, Ireland) and was designed to treat superficial cutaneous depressions such as fine lines and to improve skin quality attributes such as hydration and elasticity.Citation18 A prospective, single-center, single-arm study in 131 subjects with moderate to severe cheek skin roughness at baseline showed that a single treatment with VYC-12 without lidocaine was safe and effective for reducing skin roughness and fine lines up to 6 months and improving skin hydration up to 9 months.Citation19 Most subjects (76.4–90.8%) reported significant improvements in satisfaction with skin at all time points through month 9 on the FACE‐Q Satisfaction With Skin scale, a validated patient-reported outcome measure, and more than 80% said they would recommend the treatment to a friend.Citation20 A prospective, open-label, 2-center study also demonstrated that VYC-12 improved skin quality and texture for up to 6 months, based on both objective and subjective analysis methods, in 40 women aged 35 to 60 years presenting with facial lines, low skin hydration or brightness, and signs of chrono- or photoaging.Citation21 This article provides consensus-based guidance and direction on VYC-12L injection technique, ideal treatment areas, and pre- and post-treatment management based on clinical experiences of aesthetic medicine clinicians and the author group.
Consensus Methodology
Experience Phase
A multinational group of 128 European aesthetic medicine clinicians participated in an initiative to gather real-world clinical experience on the use of VYC-12L. After attending a product training event, participants identified approximately 10 patient candidates for VYC-12L treatment. The clinicians each completed a survey after treating their first and last patients. The clinicians were asked to indicate their preferred injection methodology, including technique, depth and angle of injection, volume per injection, and spacing between injection sites; primary target areas of treatment; and pre- and post-injection advice. Survey questions and results from the experience phase are summarized in the Supplemental Table.
Consensus Phase
An expert panel of 12 international advisors convened in Paris, France, in January 2017 and reviewed the results of the survey (Supplemental Table). The advisors were selected and invited by Allergan to participate in this initiative based on their positive reputation in the aesthetic community and extensive clinical practice and/or clinical trial experience with VYC-12L. During the meeting, the advisors discussed their individual experiences using VYC-12L and established consensus on general injection technique; area-specific recommendations for the forehead, malar, perioral, neck, hands, and décolletage regions; and pre- and post-treatment management.
Consensus Recommendations
Injection Technique
Key recommendations for VYC-12L injection technique were to use micro-depot injections, inject into the deep dermis at an angle less than 45 degrees from the skin surface, and space injections approximately 0.5 to 1.0 cm apart ().Citation22–Citation25 The depth and spacing of the injection may be individualized to the patient and the treatment area based on the severity level of the skin lines, roughness, and photodamage.
Table 1 Expert Panel Consensus on VYC-12L Injection Technique
Anatomical Region
The malar, perioral, neck, and décolletage regions were identified as primary target areas for VYC-12L treatment with area-specific injection techniques based on skin characteristics in the respective regions ().
Table 2 Area-Specific Recommendations for VYC-12L Injection Technique
The panel did not identify the forehead as a primary indication for injection in otherwise treatment-naïve patients. Forehead skin has different sebaceous properties than the other areas identified for treatment; specifically, greater sebum production in the forehead results in increased skin surface hydration and a better skin appearance. Further, patient concerns about the forehead generally relate to dynamic lines, typically addressed with a neuromodulator.Citation22,Citation26,Citation27 However, clinical data have demonstrated the efficacy and safety of VYC-12 without lidocaine for the treatment of forehead lines, and there is clinical practice experience in this facial area.
The dorsum of the hands was not considered a primary target for VYC-12L treatment; visible or protruding tendons or veins in the hands would be best addressed by other HA-based products with greater lift capacity,Citation28 such as VYC-17.5L (Juvéderm Vollure XC/Juvéderm Volift XC; Allergan plc, Dublin, Ireland). Based on the clinical experience of some members of the panel, however, VYC-12L may be used to improve the appearance of the skin on the hands without producing an excessive volumizing effect.
Pre- and Post-treatment Management
Specific recommendations for both patients and clinicians to observe before and after treatment with VYC-12L ()Citation29 are largely consistent with pre- and post-treatment guidance for other HA-based dermal fillers.Citation23,Citation30,Citation31 Specific training on the use of VYC-12L is essential in order to avoid potential side effects associated with HA dermal fillers.
Table 3 Pre- and Post-treatment Recommendations
Demographic-Based Treatment Considerations
Improvements in skin appearance with VYC-12L treatment are expected regardless of age, skin phototype, and ethnicity ().Citation32–Citation36 However, there are specific considerations for treatment with VYC-12L in each group. For example, older individuals whose skin is highly damaged may require more volume or closely spaced injections compared with younger individuals. Further, VYC-12L would not typically be the first aesthetic treatment for older patients, who may have previously received neuromodulating or volumizing treatments, or may have undergone cosmetic procedures that involve greater downtime, such as laser skin resurfacing.Citation37 Therefore, older patients (or patients with a previous history of aesthetic and/or cosmetic procedures) should be advised that VYC-12L involves minimal downtime in addition to other distinguishing treatment considerations.
Table 4 Demographic-Based Treatment Considerations
Patient Selection
A broad range of patients are appropriate for VYC-12L treatment ().Citation18,Citation38 Younger patients who have sun damage or concerns about the texture or lackluster appearance of their skin may be good candidates for VYC-12L as an early or initial aesthetic treatment. Based on personal observation, the panel commented that skin with low-grade acne scars also responds well to VYC-12L.
Table 5 Recommendations for Selecting Patients
Patient Expectations
In setting appropriate treatment expectations, patients may be counseled to expect visible results approximately 5 to 10 days after treatment, rather than immediately, and to experience effects for about 4 to 6 months. Increased, prolonged internal cutaneous moisturization was identified as a major driver of patient satisfaction with VYC-12L, and it was recommended that patient management and communication efforts emphasize such tangible effects more than the instant visibility of results. Clinicians might communicate anticipated results to patients using words such as luminosity, radiance, glow, and freshness, which may be easier for patients to visualize than improved skin quality. Clinicians may also share with patients that improvements in horizontal neck lines and décolletage skin may be particularly satisfying, as these results, in the opinion of the panel, may last the longest. Patients should also be made aware that some skin characteristics, such as uneven pigment and large pores, will not improve significantly.
Representative images of results before and after treatment with VYC-12L are presented in –. These figures demonstrate the effectiveness of VYC-12L for fine lines and skin appearance at 1-month post-treatment in patients who had no prior experience with the product.
Figure 1 Representative images of facial area (A) before treatment and (B) 1 month after injection of VYC-12L into cheek, jawline, and perioral area, 1.0 mL per side. Images courtesy of Jesper Thulesen, MD.
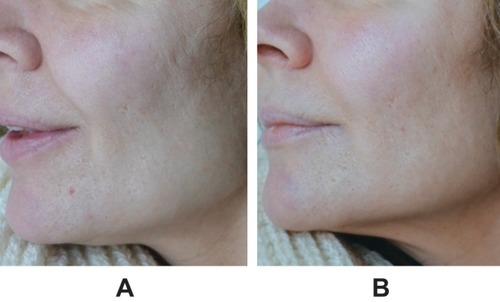
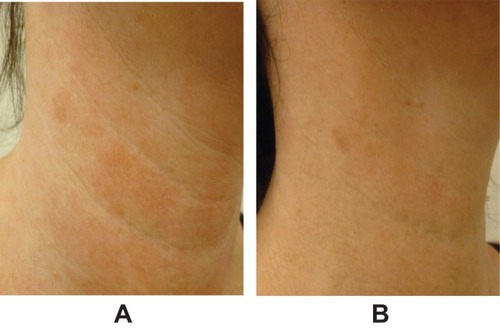
Figure 2 Representative images of décolletage (A) before treatment and (B) 1 month after 1.5-mL injection of VYC-12L. Images courtesy of Patricia Ogilvie, MD.
Role of VYC-12L in an Aesthetic Treatment Plan
Treatment with VYC-12L may be performed in the same session as injection of neurotoxins or other facial rejuvenation procedures. For example, a study conducted in 45 patients aged 35 to 52 years demonstrated high patient satisfaction and multiple skin quality and texture improvements, including smoothness, brightness, hydration, elasticity, and tightness, when VYC-12L was combined with microinjections of onabotulinumtoxinA and fractional resurfacing laser treatments.Citation39 However, the initial treatment with VYC-12L in the face may be best administered without facial volumizing treatments in the same session. This approach will allow the injector to gain experience with VYC-12L and its distinct effects on a patient’s skin independent of any changes in skin appearance resulting directly from the volumizer. It may be appropriate to combine VYC-12L and volumizing treatment in the face once clinical experience has been gained and clear expectations of the results of this combination can be determined. The use of VYC-12L in the neck or décolletage during the same session as a facial volumizing treatment is supported, because these treatment areas are distant from the face and results are more likely to be complementary.
Conclusions
These consensus recommendations provide important information to guide clinicians in the use of VYC-12L. They support clinicians in optimizing aesthetic outcomes for patients who are seeking enhancement of their skin appearance, whether because of extrinsic effects, such as photodamage, or intrinsic changes, such as skin aging. The expert panel concluded that VYC-12L is especially appropriate for treatment of the malar, perioral, neck, and décolletage regions using the same general injection technique, with some modifications based on region-specific skin and patient characteristics. Patients may expect skin appearance improvements with VYC-12L regardless of their age, sex, or ethnicity. VYC-12L can be part of a comprehensive aesthetic treatment plan for patients of any age.
VYC-12L has several features that differentiate it from other treatments intended to improve the appearance of the skin. For example, topical retinoids,Citation40,Citation41 photodynamic therapy,Citation42 chemical peels,Citation8,Citation43 laser skin resurfacing,Citation37 and nonablative skin rejuvenation devicesCitation44 are applied to the surface of the skin, whereas VYC-12L is injected intradermally, with the objective being long-term improvements in skin appearance.Citation18 Treatment with injectable HA-based fillers has been shown to temporarily improve attributes of skin quality, by hydrating the dermis and stabilizing the structure of the extracellular matrix–supporting fibroblasts.Citation45,Citation46
Unlike targeted volumizing treatments, VYC-12L treatment involves covering the whole topographical area with evenly spaced intradermal injections followed by targeted injections to correct lines, depressions, and other modalities of concern in the skin. VYC-12L also requires strategic and systematic intradermal injections, unlike mesotherapy, which consists of random microinjections of noncrosslinked HA, vitamins, minerals, and amino acids into the superficial part of the dermis.Citation27,Citation47,Citation48
Setting patient expectations is an important component of treatment with VYC-12L. VYC-12L is a reasonable option for patients who want more subtle improvements in the appearance of their skin. To this end, physician-patient communication is key; patient education should be focused on the palpable, rather than instantly visible, effects of the treatment (eg, increased, prolonged moisturization). As with other aesthetic treatment options, high-quality, standardized, and reproducible medical photography showing patients’ skin before and after treatment may be useful in helping patients to detect and appreciate the effects of treatment with VYC-12L.
Prior Presentation
A poster based on this paper was presented at the 13th Aesthetic and Anti-aging Medicine European Congress, September 15−17, 2017, Monte Carlo, Monaco. The poster’s abstract was made available to meeting attendees in the congress’ on-site abstract book.
Disclosure
P Ogilvie is a consultant, trainer, and principal investigator for Allergan plc, participated in an Allergan Juvéderm Volite advisory board, and received research funding for the clinical registration trial of VYC-12L. M Cavallini, J Chantrey, V Figueiredo, I Heydenrych, E Langeland, M Safa, D Sykianakis, J Thulesen, and A Wetter are investigators for Allergan plc and have participated in an Allergan Juvéderm Volite advisory board. J Chantrey received honorarium from Allergan, during the conduct of the study; honorarium for teaching from Allergan, outside the submitted work. V Figueiredo and E Langeland report personal fees, non-financial support from Allergan, outside the submitted work. I Heydenrych reports personal fees from Allergan, during the conduct of the study; personal fees, non-financial support from Allergan, personal fees from SkinCeuticals, outside the submitted work. C Leys is an investigator for Allergan plc, participated in an Allergan Juvéderm Volite advisory board, and received research funding for the clinical registration trial of VYC-12L. J Thulesen is a consultant for Allergan plc, participated in an Allergan Juvéderm Volite advisory board, and is a faculty member of the Allergan Medical Institute. The authors report no other conflicts of interest in this work.
Additional information
Funding
References
- Lai M, Oruc I, Barton JJ. The role of skin texture and facial shape in representations of age and identity. Cortex. 2013;49(1):252–265.22055429
- Tsankova E, Kappas A. Facial skin smoothness as an indicator of perceived trustworthiness and related traits. Perception. 2016;45(4):400–408. doi:10.1177/030100661561674826621963
- Sutherland CA, Rowley LE, Amoaku UT, et al. Personality judgments from everyday images of faces. Front Psychol. 2015;6:1616. doi:10.3389/fpsyg.2015.0161626579008
- Jones AL, Kramer RS, Ward R. Signals of personality and health: the contributions of facial shape, skin texture, and viewing angle. J Exp Psychol Hum Percept Perform. 2012;38(6):1353–1361. doi:10.1037/a002707822288693
- Donofrio L, Carruthers A, Hardas B, et al. Development and validation of a photonumeric scale for evaluation of facial skin texture. Dermatol Surg. 2016;42(suppl 1):S219–S226. doi:10.1097/DSS.000000000000085227661744
- Trojahn C, Dobos G, Lichterfeld A, Blume-Peytavi U, Kottner J. Characterizing facial skin ageing in humans: disentangling extrinsic from intrinsic biological phenomena. BioMed Res Int. 2015;2015:318586. doi:10.1155/2015/31858625767806
- Nkengne A, Bertin C. Aging and facial changes–documenting clinical signs, part 1: clinical changes of the aging face. Skinmed. 2013;11(5):281–286.24340467
- Berardesca E, Cameli N, Primavera G, Carrera M. Clinical and instrumental evaluation of skin improvement after treatment with a new 50% pyruvic acid peel. Dermatol Surg. 2006;32(4):526–531. doi:10.1111/j.1524-4725.2006.32106.x16681660
- Carruthers J, Donofrio L, Hardas B, et al. Development and validation of a photonumeric scale for evaluation of facial fine lines. Dermatol Surg. 2016;42(suppl 1):S227–S234. doi:10.1097/DSS.000000000000084727661745
- Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337(20):1419–1428. doi:10.1056/NEJM1997111333720039358139
- Callaghan TM, Wilhelm KP. A review of ageing and an examination of clinical methods in the assessment of ageing skin. Part 2: clinical perspectives and clinical methods in the evaluation of ageing skin. Int J Cosmet Sci. 2008;30(5):323–332. doi:10.1111/j.1468-2494.2008.00455.x18822037
- Fisher GJ, Varani J, Voorhees JJ. Looking older: fibroblast collapse and therapeutic implications. Arch Dermatol. 2008;144(5):666–672. doi:10.1001/archderm.144.5.66618490597
- Le Louarn C, Buthiau D, Buis J. Structural aging: the facial recurve concept. Aesthetic Plast Surg. 2007;31(3):213–218. doi:10.1007/s00266-006-0024-917380358
- Schroeder P, Krutmann J. Infared A-induced skin aging In: Farage MA, Miller KW, Maibach HI, editors. Textbook of Aging Skin. Berlin, Germany: Springer, Berlin, Heidelberg; 2010:421–425.
- Valacchi G, Sticozzi C, Pecorelli A, Cervellati F, Cervellati C, Maioli E. Cutaneous responses to environmental stressors. Ann N Y Acad Sci. 2012;1271:75–81. doi:10.1111/j.1749-6632.2012.06724.x23050967
- Plastic surgery statistics report 2016; 2017 Available from: https://d2wirczt3b6wjm.cloudfront.net/News/Statistics/2016/plastic-surgery-statistics-full-report-2016.pdf. Accessed 615, 2017.
- American Society of Plastic Surgeons. 2017 Plastic Surgery Statistics Report. 2018 Available from: https://www.plasticsurgery.org/documents/News/Statistics/2017/plastic-surgery-statistics-full-report-2017.pdf. Accessed 627, 2018.
- Juvederm Volite B [direction for use]. Pringy, France: Allergan; 2017.
- Niforos F, Ogilvie P, Cavallini M, et al. VYC-12 injectable gel is safe and effective for improvement of facial skin topography: a prospective study. Clin Cosmet Investig Dermatol. 2019;12:791–798. doi:10.2147/CCID.S216222
- Ogilvie P, Safa M, Chantrey J, et al. Improvements in satisfaction with skin after treatment of facial fine lines with VYC-12 injectable gel: patient-reported outcomes from a prospective study. J Cosmet Dermatol. 2019. doi:10.1111/jocd.13129
- Cavallini M, Papagni M, Ryder TJ, Patalano M. Skin quality improvement with VYC-12, a new injectable hyaluronic acid: objective results using digital analysis. Dermatol Surg. 2019;45:1598–1604. doi:10.1097/DSS.000000000000193230893167
- de Maio M, Swift A, Signorini M, Fagien S. Facial assessment and injection guide for botulinum toxin and injectable hyaluronic acid fillers: focus on the upper face. Plast Reconstr Surg. 2017;140(2):265e–276e. doi:10.1097/PRS.0000000000003544
- Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295–316. doi:10.2147/CCID.S50546
- Ha RY, Nojima K, Adams WP Jr., Brown SA. Analysis of facial skin thickness: defining the relative thickness index. Plast Reconstr Surg. 2005;115(6):1769–1773. doi:10.1097/01.PRS.0000161682.63535.9B15861089
- Bilac C, Sahin MT, Ozturkcan S. Chronic actinic damage of facial skin. Clin Dermatol. 2014;32(6):752–762. doi:10.1016/j.clindermatol.2014.02.01425441468
- Tagami H. Location-related differences in structure and function of the stratum corneum with special emphasis on those of the facial skin. Int J Cosmet Sci. 2008;30(6):413–434. doi:10.1111/ics.2008.30.issue-619099543
- Andre P. New trends in face rejuvenation by hyaluronic acid injections. J Cosmet Dermatol. 2008;7(4):251–258. doi:10.1111/jcd.2008.7.issue-419146600
- Bass LS. Injectable filler techniques for facial rejuvenation, volumization, and augmentation. Facial Plast Surg Clin North Am. 2015;23(4):479–488. doi:10.1016/j.fsc.2015.07.00426505544
- Gazzola R, Pasini L, Cavallini M. Herpes virus outbreaks after dermal hyaluronic acid filler injections. Aesthet Surg J. 2012;32(6):770–772. doi:10.1177/1090820X1245229322859551
- Signorini M, Liew S, Sundaram H, et al. Global aesthetics consensus: avoidance and management of complications from hyaluronic acid fillers—evidence- and opinion-based review and consensus recommendations. Plast Reconstr Surg. 2016;137(6):961e–971e. doi:10.1097/PRS.0000000000002184
- Urdiales-Galvez F, Delgado NE, Figueiredo V, et al. Preventing the complications associated with the use of dermal fillers in facial aesthetic procedures: an expert group consensus report. Aesthetic Plast Surg. 2017;41(3):667–677. doi:10.1007/s00266-017-0798-y28411354
- Keaney TC. Aging in the male face: intrinsic and extrinsic factors. Dermatol Surg. 2016;42(7):797–803. doi:10.1097/DSS.000000000000050527196541
- Vashi NA, de Castro Maymone MB, Kundu RV. Aging differences in ethnic skin. J Clin Aesthet Dermatol. 2016;9(1):31–38.
- Lee BM, Han DG, Choi WS. Rejuvenating effects of facial hydrofilling using Restylane Vital. Arch Plast Surg. 2015;42(3):282–287. doi:10.5999/aps.2015.42.3.28226015882
- Roh NK, Kim MJ, Lee YW, Choe YB, Ahn KJ. A split-face study of the effects of a stabilized hyaluronic acid-based gel of nonanimal origin for facial skin rejuvenation using a stamp-type multineedle injector: a randomized clinical trial. Plast Reconstr Surg. 2016;137(3):809–816. doi:10.1097/01.prs.0000480686.68275.6026910661
- Seok J, Hong JY, Choi SY, Park KY, Kim BJ. A potential relationship between skin hydration and stamp-type microneedle intradermal hyaluronic acid injection in middle-aged male face. J Cosmet Dermatol. 2016;15(4):578–582. doi:10.1111/jocd.2016.15.issue-427401775
- Rhie JW, Shim JS, Choi WS. A pilot study of skin resurfacing using the 2,790-nm erbium:YSGG laser system. Arch Plast Surg. 2015;42(1):52–58. doi:10.5999/aps.2015.42.1.5225606490
- De Boulle K, Heydenrych I. Patient factors influencing dermal filler complications: prevention, assessment, and treatment. Clin Cosmet Investig Dermatol. 2015;8:205–214. doi:10.2147/CCID.S80446
- Bertossi D, Giampaoli G, Lucchese A, et al. The skin rejuvenation associated treatment-Fraxel laser, Microbotox, and low G prime hyaluronic acid: preliminary results. Lasers Med Sci. 2019;34(7):1449–1455. doi:10.1007/s10103-019-02738-z30762198
- Ho ET, Trookman NS, Sperber BR, et al. A randomized, double-blind, controlled comparative trial of the anti-aging properties of non-prescription tri-retinol 1.1% vs. prescription tretinoin 0.025%. J Drugs Dermatol. 2012;11(1):64–69.22206079
- Ogden S, Samuel M, Griffiths CE. A review of tazarotene in the treatment of photodamaged skin. Clin Interv Aging. 2008;3(1):71–76. doi:10.2147/CIA.S110118488880
- Karrer S, Kohl E, Feise K, et al. Photodynamic therapy for skin rejuvenation: review and summary of the literature–results of a consensus conference of an expert group for aesthetic photodynamic therapy. J Dtsch Dermatol Ges. 2013;11(2):137–148.23190505
- Ghersetich I, Brazzini B, Peris K, Cotellessa C, Manunta T, Lotti T. Pyruvic acid peels for the treatment of photoaging. Dermatol Surg. 2004;30(1):32–36. doi:10.1111/j.1524-4725.2004.30002.x14692923
- Abraham MT, Vic RE. Current concepts in nonablative radiofrequency rejuvenation of the lower face and neck. Facial Plast Surg. 2005;21(1):65–73. doi:10.1055/s-2005-87176515988658
- Landau M, Fagien S. Science of hyaluronic acid beyond filling: fibroblasts and their response to the extracellular matrix. Plast Reconstr Surg. 2015;136(5 Suppl):188s–195s. doi:10.1097/PRS.000000000000182326441098
- Streker M, Reuther T, Krueger N, Kerscher M. Stabilized hyaluronic acid-based gel of non-animal origin for skin rejuvenation: face, hand, and decolletage. J Drugs Dermatol. 2013;12(9):990–994.24002145
- Savoia A, Landi S, Baldi A. A new minimally invasive mesotherapy technique for facial rejuvenation. Dermatol Ther (Heidelb). 2013;3(1):83–93. doi:10.1007/s13555-012-0018-223888258
- Baspeyras M, Rouvrais C, Liegard L, et al. Clinical and biometrological efficacy of a hyaluronic acid-based mesotherapy product: a randomised controlled study. Arch Dermatol Res. 2013;305(8):673–682. doi:10.1007/s00403-013-1360-723715889