Abstract
Background
Colloidal oatmeal is a natural ingredient used in the formulation of a range of personal care products for relief of skin dryness and itchiness. It is also used as an adjunctive product in atopic dermatitis. The safety of personal care products used on vulnerable skin is of particular importance and the risk of developing further skin irritations and/or allergies should be minimized.
Methods
In a series of studies, we tested the safety of personal care products containing oatmeal (creams, cleansers, lotions) by assessing their irritant/allergenic potential on repeat insult patch testing, in safety-in-use and ocular studies using subjects with nonsensitive and sensitive skin. We also tested the skin moisturizing and repair properties of an oatmeal-containing skin care product for dry skin.
Results
We found that oatmeal-containing personal care products had very low irritant potential as well as a very low allergenic sensitization potential. Low-level reactions were documented in 1.0% of subjects during the induction phase of repeat insult patch testing; one of 2291 subjects developed a persistent but doubtful low-level reaction involving edema during the challenge phase in repeat insult patch testing. No allergies were reported by 80 subjects after patch testing after in-use application. Sustained skin moisturizing was documented in subjects with dry skin that lasted up to 2 weeks after product discontinuation.
Conclusion
Our results demonstrate that colloidal oatmeal is a safe and effective ingredient in personal care products. No allergies were reported by consumers of 445,820 products sold during a 3-year period.
Introduction
Colloidal oatmeal is a natural product derived from oat grains (Avena sativa) that have been ground into a very fine powder, with a complex chemical composition including polysaccharides, lipids, proteins, flavonoids, minerals, and vitamins.Citation1 Colloidal oatmeal is appreciated for its moisturizing, cleansing, antioxidative, and anti-inflammatory properties, which are conferred by its chemical heterogeneity. A variety of oatmeal-containing personal care products are available, including bath products, shampoos, moisturizers, and shaving foams, for the protection and alleviation of, eg, rashes and dry skin, and for cleansing and moisturizing.Citation2 Used as adjunctive therapy in infants with moderate-to-severe atopic dermatitis, it can help to reduce the need for high-potency topical corticosteroids.Citation3 The anti-irritant effects of colloidal oatmeal appear to be mediated by avenanthramides, which inhibit immune-dependent skin inflammation.Citation4
Epidemiological studies have shown that a high number of individuals suffer from sensitive skin, with a prevalence of 51%–52% in women and 38% in men.Citation5,Citation6 Symptoms of cosmetic-induced skin discomfort, such as burning, stinging, and itching, are reported more commonly by individuals with sensitive skin than by those who consider themselves nonsensitive.Citation6 Fragrances and preservatives are the most frequently identified allergenic sensitizers in cosmetic dermatitis.Citation7 The inclusion of food proteins in personal care products is controversial in terms of whether topical application of oatmeal-containing products induces percutaneous sensitization in subjects with atopic dermatitis and to what extent.Citation8–Citation10
Therefore, it is of the utmost importance that personal care products, in particular those intended to treat sensitive skin or to help in the treatment of atopic dermatitis, do not aggravate existing skin conditions and that the risk of allergic reactions is minimized by excluding sensitizing ingredients. We sought to determine the irritant and allergenic potential of a range of personal care products containing colloidal oatmeal as an active ingredient after repeated applications in human subjects including those with sensitive skin or a history of atopic dermatitis. We also assessed the efficacy of an oatmeal-containing cream in relieving skin dryness.
Safety assessment
Repeat insult patch tests
Twelve independent studies were performed at two centers in two countries (10 studies in the US, and two studies in Romania) between February 2000 and May 2009. The tests were conducted under the supervision of a dermatologist who participated in the evaluation of irritation/allergic reactions to the test materials. Each panel comprised 114–245 male and female volunteers who gave their written informed consent before enrolment. Subjects with dermatological or other medical or physical conditions precluding topical application of the test material, were excluded, along with pregnant and nursing women. The study centers used different protocols. For the induction period, a series of nine induction patchings were performed over a period of 3 weeks.
The materials tested were 12 skin care products containing oatmeal as the active ingredient. These comprised three lotions, two face creams, one serum product, two cleansing lotions, one exfoliating cleanser, two baby products (one cream and one cleanser), and one hand cream.
At the US site, an occlusive or semiocclusive patch containing 0.2 g of test material was applied to the left side of the back where it remained for 24 hours. Subjects were instructed to keep the patch as dry as possible and to remove it after 24 hours. No test material was applied for the following 24 (on weekdays) or 48 hours (on weekends), after which evaluation for potential dermal reactions was undertaken. Reactions after patching were scored according to a modified version of the International Contact Dermatitis Research Group scoring systemCitation11 (see ).
Table 1 Scoring methods used for evaluation of irritation/allergic skin reaction
At the Romanian site, patches containing 25 μL of the test material were applied to the back in a semiocclusive manner and removed after 48 hours. The dermal response during this period was scored using a five-point scale (see ). The rest period comprised 2 weeks without application of the test material. During the challenge period, patches were prepared and fixed in the same manner as in the induction period, but on the right side of the back (ie, a virgin site).
The patches were removed after 24 hours at the US study center, and scoring of skin reactions was performed in the same manner as before at 24, 48, 72, and 96 hours after patching. At the Romanian study center, the patches remained in place for 48 hours and skin reactions were scored 30 minutes and 24 and 48 hours after patch removal using the International Contact Dermatitis Research Group scoring system.
Safety-in-use tests
Twelve independent studies were performed at four study centers in four countries (seven studies in the UK, two in Poland, two in Germany, and one in Bulgaria) between April 2006 and August 2009. Subjects with a range of self-reported skin types and sensitivities were recruited. In the UK studies, the test materials were applied for 7, 10 or 28 days and skin reactions were evaluated using self-assessment questionnaires (adverse reaction, yes/no, severity slight, moderate, severe). The studies conducted in Poland involved a 3-week application period, with ophthalmological assessment by slit-lamp and evaluation of subjective functional signs before and after use on day 21 and subjective self-assessment patient questionnaires regarding functional/physical signs. The Bulgarian study involved 3 weeks of application, with dermatological evaluation (detection of allergy, irritation, dryness, discomfort, pimples) and subject self-assessment questionnaires (adverse reaction, yes/no). The studies in Germany included a 4-week application period in adults and children followed by occlusive patch testing in the adult participants. Reactions during the application period and after patch testing were evaluated by a dermatologist-allergologist at 24, 48, and 72 hours after patching. The following test materials were used: shower and bath oil, cream, moisturizing oil, shower gel, night cream, conditioning shampoo, body lotion, liquid hand wash, face and eye cleansing lotion (two products), facial exfoliating cleanser, intimate wash, and baby milk.
Efficacy assessment
An open prospective study was performed to assess the effect of an oatmeal-containing body cream on various aspects of dry skin. Fifty female subjects aged 20–67 years were enrolled. All subjects were of Caucasian origin and had dry to very dry skin of phototype I–IV. The study duration was 6 weeks, which included 4 weeks of study product application followed by 2 weeks without product application. Subjects were instructed to avoid use of other skin care products, with the exception of cleansers, for 3 days before starting the study, as well as for 2 weeks following the study period. The study product was to be applied once a day in the morning on the leg (external part of the calf) and on the inner forearm according to the subjects’ usual application habits. Assessments were performed at baseline, and on days 1, 14, and 28, and 2 weeks after the last application (day 42). Skin hydration (moisture content in the upper epidermis) was assessed using a Corneometer® CM 825 (Courage + Khazaka Electronic GmbH, Germany). The mean of three consecutive measurements was calculated. The desquamation index and the surface area of dead epithelial cells were assessed using adhesive disc stripping (D-squame®; CuDerm Corporation, Dallas, TX) with subsequent digital image analysis.
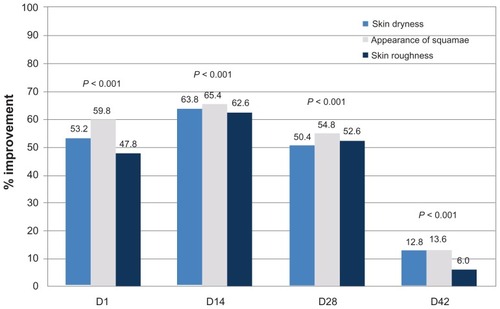
Clinical efficacy was assessed by a dermatologist. Assessments included visual examination of skin dryness and appearance of epithelial squamae, as well as tactile evaluation of skin roughness. A 10 cm visual analog scale was used, where 0 represented “none” and 10 was “severe”. Subject self-assessment involved a questionnaire with a five-point scale ranging from 1 (“agree”) to 5 (“disagree”). The number of responses from category 1 and 2 (ie, “agree” and “rather agree”) was combined for analysis. Measurements were made on the treated body areas (leg and inner forearm), as well as on an untreated area on the mid-thigh which served as a control site. Clinical assessments were performed only on the treated leg and on the control area.
Statistical analysis was performed using SPSS software (SPSS Inc, Chicago, IL). Data at each assessment time point are shown as the mean ± standard error of the mean. At each time point, data were compared with baseline and, where applicable, the difference between treated and untreated sites was compared, and the difference was expressed as a variation percentage. The Student’s paired t-test (for normal distribution) or the Wilcoxon test (for nonnormal distribution) was used at a significance level of P ≤ 0.05. All subjects involved in the study gave their written informed consent before enrolment. All studies described herein were conducted according to the ethical principles outlined in the Declaration of Helsinki and according to good clinical practices.
Results
Safety analysis
Of the 2565 men and women who enrolled in the 12 repeat insult patch testing studies, 274 discontinued for reasons other than a reaction to the test material. In the induction period, a total of 23 patients experienced a reaction. We observed 34 transient low-level grade ± reactions (ie, faint minimal erythema) in 20 subjects (including one patient with eight consecutive faint erythema readings), six transient low-level grade 1 reactions in six subjects, and mild erythema in one subject. In the challenge period, 17 patients had a reaction. This comprised 18 transient low-level grade ± reactions in 14 subjects, nine transient low-level grade 1 reactions in seven subjects, and five grade 1 reactions with edema in three subjects (). Edematous reactions were not confirmed in subsequent patch tests for two subjects. However, for the other subject, reactions were confirmed for the complete product.
Table 2 Summary of repeat insult patch testing studies
A total of 645 subjects were enrolled in the 12 safety-in-use studies, which were completed by 615 subjects. Seven studies tested skin reactions to different facial, body, and hair cleansers as well as creams in female subjects with normal to very dry skin and skin sensitivity ranging from not sensitive to very sensitive by means of subjective self-evaluation (UK studies, ). Among the 402 subjects who returned their questionnaires, 18 reported adverse reactions. The majority of these reactions (nine) were moderate in nature, followed by slight (seven) and severe (two) reactions. In another study, the reaction to a facial cleanser was evaluated in female subjects with normal to oily or dry skin, of whom 32% had a history of atopic dermatitis. No reaction was reported by the investigating dermatologist. Two studies evaluated the ocular tolerance of a facial cleanser in 43 female subjects with normally sensitive eyes. Eye reactions were documented in three of the subjects and confirmed by clinical ophthalmological evaluation, and in nine subjects according to self-evaluation (). Finally, two studies tested the reactions to two test materials in 80 adults and 30 children with normal to dry skin. A history of atopic dermatitis was reported for 27 of 80 adults and for 11 of 30 children who participated in these studies. For adult subjects, the application period was followed by a patch test using the diluted or undiluted product. No clinical reaction was observed during the application period by any of the 110 subjects nor was there a reaction after patch testing in the 80 adults.
Table 3 Summary of safety-in-use studies
Table 4 Summary of ocular tolerability testing under ophthalmological control (safety-in-use studies)
Efficacy analysis
A total of 47 subjects completed the study of the clinical efficacy of an oatmeal-containing body cream, with evaluation on day 14, and 46 subjects underwent evaluation on days 28 and 42.
Skin hydration
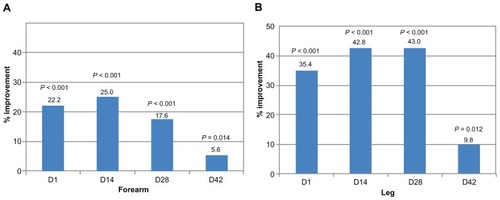
On the forearm, skin hydration was significantly higher at all time points, including at 2 weeks after cessation of application (day 42), compared with baseline. For the leg area, an increase in hydration was observed that was significantly higher than on the control site at all time points ().
Figure 1 Skin hydration increases during and after use of oatmeal-containing cream.
Desquamation index and dead cell surface area
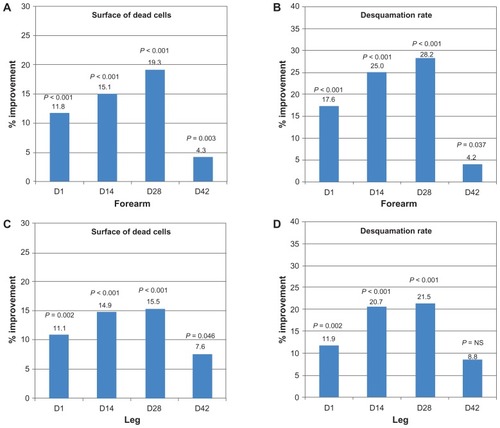
The surface area of dead epithelial cells and the epithelial desquamation index on the forearm were significantly reduced at all assessment time points compared with baseline. On the leg, a reduction in the dead cell surface area was observed that was significantly larger than that on the control area at all time points. In this area, the desquamation index also diminished to a significantly greater extent than in the control area during the application period ().
Figure 2 Surface area of dead cells and desquamation index diminish with use of oatmeal-containing cream. Surface of dead cells (A) and desquamation rate (B) were compared with baseline for the forearm area and their reduction is indicated as percentage improvement. Data derived from leg measurements was compared with the control area for surface of dead cells (C) and desquamation rate (D).
Clinical evaluation
Throughout the application period, all parameters (skin dryness, appearance of squamae, and skin roughness) assessed by the dermatologist on the leg were significantly more improved than on the control area (). This was still the case beyond cessation of treatment on day 42. No clinically significant adverse reactions were noted during the course of the study.
Self-evaluation
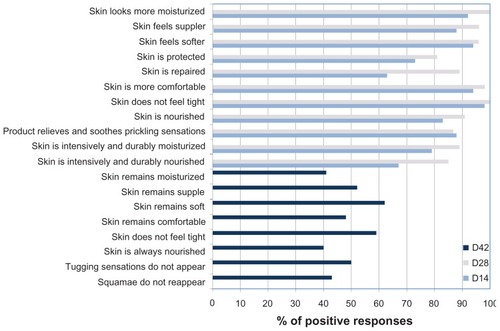
At days 14 and 28, 63%–100% of the subjects responded favorably (“agree” or “rather agree”) to a series of questions concerning subjective evaluation of signs of skin dryness after application of the product (). Two weeks after cessation of application, the proportion of positive responses was 40%–61%.
Discussion
We tested the irritation and sensitization potential of 12 oatmeal-containing personal care products in 2291 subjects as part of a series of repeat insult patch testing studies. We observed only low-level reactions corresponding to faint erythema, minimal erythema, or obvious erythema according to the scoring system used. All of the subjects concerned (23/2291, ie, 1.0%) experienced transient reactions, with the exception of one individual with persistent erythema. These data indicate a very low irritancy potential for the study materials. In the subsequent challenge period, we saw mostly transient low-level reactions (14/2291 subjects, ie, 0.6%). Three subjects had a low-level reaction accompanied by edema. One of these subjects had a persistent reaction on three consecutive readings. However, this reaction was doubtful because it was confirmed with the complete product and not with A. sativa. The test material in that case was a hand cream. The edematous reaction was transient in the two other subjects.
Furthermore, we performed safety-in-use testing of both “leave-on” (creams and lotions) and “wash/rinse-off” (shower oils and shower gels, shampoo, liquid hand wash, facial cleansers, intimate wash) oatmeal-containing products to assess their irritancy potential. The majority of these safety-in-use studies (8/12) included subjects with self-reported sensitive skin. Three studies also included subjects with a self-reported history of atopic dermatitis. The prevalence of sensitive skin among the subjects was 77%–100%, but was lower in two of the studies including atopic subjects (2%–20%). The proportion of subjects with sensitive skin in our studies was higher than that in the general population, as indicated by a frequency of self-reported sensitive skin in a random population sample in the UK of 51% in women and 38% in men and of 52% in women in the US.Citation5,Citation6 Of the tests involving self-assessed skin reactions, the highest percentage of reactions was observed for a leave-on night cream, which provoked reactions in 7/64 subjects (10.9%), followed by a face and eye cleanser (two of 22 subjects, 9.1%). Other test materials (mainly wash/rinse-off products) had a lower frequency of adverse reactions (0% for a face and eye cleanser and a body lotion, <4% for shower oils, gels, and a shampoo, 5% for a facial exfoliating cleanser, and 5.2% for a liquid hand wash). In one of the two studies including ophthalmological evaluation after use of a face and eye cleansing lotion, we observed 14% of clinical eye signs with possible implication of the product in one case and reactions only on one eye in two subjects.
For two studies, in which one third of subjects were atopic, we did not find any clinical signs of skin irritation in either adults or infants or when assessed, of allergic sensitization in adults. Atopic dermatitis is an inflammatory skin condition particularly affecting infants and children. It appears to be increasing in prevalence,Citation12,Citation13 and affects 10%–20% of individuals in the first decade of life.Citation14 In a recent study of 67 children with atopic dermatitis, it was suggested that use of moisturizers containing oat protein is a risk factor for oat sensitization.Citation8 In the same study, 15% of 302 children aged 4 months to 15 years with atopic dermatitis had a positive oat extract atopy patch test result and 19% had a positive skin prick test result. However, the frequency of oat sensitivity was much lower in another study performed in 202 atopic children, with sensitivity reported in 2.9% of children who were oat cream users and in 2.1% of those who had never used oat cream.Citation9 No sensitization to topical colloidal oatmeal was found in a randomized, double-blind study performed in 65 atopic and nonatopic children between 6 months and 2 years of age.Citation10 In our studies, we limited patch tests after in-use application of the test material to adults, and did not observe any allergic sensitization in 80 participants.
The skin hydrating properties of colloidal oatmeal have been ascribed to its propensity to form an occlusive film capable of binding water in the stratum corneum.Citation1 We observed a significant moisturizing effect of an oatmeal-containing cream on dry skin throughout the application period, which was sustained for 2 weeks afterwards. This was indicated by increased hydration, a reduced desquamation index, and a reduced surface area occupied by dead skin cells, as well as by clinical evaluation and subjective self-evaluation.
Conclusion
We demonstrated that the irritation and allergenic potential of a diverse range of oatmeal-containing personal care products is low. With the exception of one subject, in whom the reaction to A. sativa was doubtful, more than 2300 subjects did not show allergic sensitization. Moreover, in allergic patients we reported by consumers of 445,820 products sold during a 3-year period. In addition, we found a sustainable moisturizing effect of oatmeal-containing products on dry skin.
Acknowledgment
The authors thank Beate Gerstbrein for her help in preparation of the manuscript.
Disclosure
Vincent Walczak and Judith Nebus, both employees of Johnson & Johnson CPWW, provided information on the studies mentioned in this paper.
References
- KurtzESWalloWColloidal oatmeal: history, chemistry and clinical propertiesJ Drugs Dermatol20076216717017373175
- WuJAnti-inflammatory ingredientsJ Drugs Dermatol20087Suppl 7S13S1618681154
- GrimaltRMengeaudVCambazardFThe steroid-sparing effect of an emollient therapy in infants with atopic dermatitis: a randomized controlled studyDermatology20072141616717191050
- SurRNigamAGroteDLiebelFSouthallMDAvenanthramides, polyphenols from oats, exhibit anti-inflammatory and anti-itch activityArch Dermatol Res20083001056957418461339
- JourdainRde LacharriereOBastienPMaibachHIEthnic variations in self-perceived sensitive skin: epidemiological surveyContact Dermatitis200246316216912000326
- WillisCMShawSDe LacharriereOSensitive skin: an epidemiological studyBr J Dermatol2001145225826311531788
- AdamsRMMaibachHIA five-year study of cosmetic reactionsJ Am Acad Dermatol1985136106210694078100
- BoussaultPLeaute-LabrezeCSaubusseEOat sensitization in children with atopic dermatitis: prevalence, risks and associated factorsAllergy200762111251125617919139
- RancéFDargassiesJDupuyPSchmittAMGuerinLDutauGFaut-il contre-indiquer l’utilisation des émollients à base d’avoine chez l’enfant atopique? (Are there contra-indications in the use of oatmeal-based emollients in atopic children?)Rev Fr Allergol Immunol Clin200141477483 French
- PigattoPBigardiACaputoRAn evaluation of the allergic contact dermatitis potential of colloidal grain suspensionsAm J Contact Dermat1997842072099358111
- FisherAAContact DermatitisPhiladelphia, PALea and Febiger1986
- Schultz LarsenFDiepgenTSvenssonAThe occurrence of atopic dermatitis in north Europe: an international questionnaire studyJ Am Acad Dermatol1996345 Pt 17607648632070
- SpergelJMPallerASAtopic dermatitis and the atopic marchJ Allergy Clin Immunol20031126 SupplS118S12714657842
- KrakowskiACEichenfieldLFDohilMAManagement of atopic dermatitis in the pediatric populationPediatrics2008122481282418829806