Abstract
Fetuin-A is a plasma glycoprotein exhibiting multifaceted physiological and pathological functions. It has been determined to be involved in various essential biological functions, such as regulation of calcium metabolism, osteogenesis, and insulin signaling pathway. It also plays a crucial role in the pathogenesis of several disorders, including psoriasis. Psoriasis is a chronic systemic inflammatory disorder caused by a constellation of environmental, immunogenic, and genetic factors. It has been shown that dysregulation of cytokines mediated immune response is responsible for the development of psoriasis. Several recent publications suggest that dysregulation of fetuin-A correlates with psoriasis disease activities, revealing its putative role in the development of psoriasis. Furthermore, clinical application of fetuin-A as a diagnostic marker, prognostic predictor, and therapeutic target for different clinical conditions is in progress, and some are showing promising outcomes. This review primarily focuses on the current understanding of the role of fetuin-A in the pathogenesis of psoriasis and its potential clinical applications, with a brief highlight of psoriasis epidemiology and burden. The information was gathered systematically from various journals via electronic searches using various search engines: PubMed, Google Scholar, HINARI, and Cochrane Library from inception to 2022. The studies involved were restricted to English language. Conversely, articles written in other languages, studies done on fetuin B, or studies conducted on other dermatological diseases were excluded from the review article.
Introduction
Fetuin-A, also known as alpha2 Heremans-Schmid glycoprotein (AHSG), is a 60 kDa negatively charged plasma protein encoded by a gene located on the 3q27 chromosome and primarily expressed by hepatocytes. It is a dimeric protein possessing a 282 amino acid long A-chain and a 27 amino acid residue B-chain, linked by a single inter-disulfide bond.Citation1–Citation4 Fetuin-A is a pleiotropic protein that exhibits multifaceted physiological and pathological functions by binding with a plethora of receptors, such as insulin, TGF-β, and TLR receptors.Citation1,Citation5,Citation6 It has been discovered to be involved in myriads of biological activities, including regulation of calcium metabolism, osteogenesis and the insulin signaling pathway. Fetuin-A possesses cystatin-like domains that potentially inhibit the activities of cysteine proteinases, such as papain, calpain, cathepsin, and the caspase family.Citation7,Citation8 Fetuin-A has also been found to play a key role in the development of various clinical conditions, including psoriasis.Citation9
Psoriasis is a common inherited, chronic, and recurrent inflammatory disorder of the skin characterized by rounded, erythematous, dry, scaling patches.Citation10 It is a complex autoimmune disorder manifested by heightened epidermopoiesis (hyperproliferation) and aberrant differentiation (hyperkeratosis) of keratinocytes, infiltrative inflammatory cells in the dermis and epidermis, and vasodilation of dermal vessels in skin lesions, resulting in sharply demarcated erythematous squamous papules and plaques.Citation11 These lesions have a predilection for nails, scalp, genitalia, extensor surfaces, and the lumbosacral region. It ranges in severity from a few scattered red, scaly plaques to virtually the entire body surface being affected. It may get worse with age, or wax and wane in its severity depending on genetic and environmental factors.Citation12,Citation13 Psoriasis is found to be triggered by chronic inflammation induced by the continual release of immunological mediators and cytokines.Citation14 Although the dysregulation of the immune system induces the disease’s development, the clear immunopathogenesis of psoriasis has yet to be clearly elucidated. Currently, fetuin-A has been discovered to exhibit a novel putative role in the development of psoriasis. In light of its pathological role in the development of psoriasis, the clinical application of fetuin-A as a diagnostic marker and therapeutic target in psoriasis is nowadays in progress. Thus, this review primarily discusses the role of fetuin-A in the pathogenesis of psoriasis and its potential clinical applications.
Studies were systematically searched from various journals via electronic searches using different search engines: PubMed, Google Scholar, HINARI, and Cochrane Library using keywords: fetuin-A, alpha2 Heremans-Schmid Glycoprotein, structure of fetuin-A, functions of fetuin-A, psoriasis, epidemiology of psoriasis, comorbidities of psoriasis, role of fetuin-A in psoriasis, burden of psoriasis, pathogenesis of psoriasis, and clinical application of fetuin-A in psoriasis. All studies regarding fetuin-A in psoriasis were included in the study. Besides, articles providing data on psoriasis prevalence, comorbidities, and burden were included in the review. The studies involved were restricted to the English language. Conversely, articles written in languages other than English, studies done on fetuin B, or studies conducted on other dermatological diseases were excluded from the review article.
Overview on the Epidemiology and Burden of Psoriasis
Even though the worldwide epidemiological data on psoriatic disease is poorly understood, psoriasis affects nearly 2–3% of the global population.Citation15 Available data indicate that the occurrence of psoriasis varies according to age and geographic region (countries). Psoriasis is generally a common disease, with its higher estimates in adults than in other age groups, such as children.Citation13 In adults, the estimates of psoriasis prevalence range between 0.91% and 8.5%, while its incidence rate varies from 30.3/100,000 to 321/100,000 person-years.Citation13,Citation15 In children (age <18 years), the prevalence of psoriasis ranges from 0.0% to 2.1% and the incidence estimate reported was 40.8/100,000 person-years.Citation13 On the other hand, the prevalence of psoriasis ranges from 0.14% in East Asia to 1.10–1.50% in high-income southern Latin and North America, 1.83–1.92% in central and western Europe, and 1.99% in Australia. The proportion of psoriasis is higher in high-income countries, reaching 3.2% in the US and 8–11% in some European countries, with the onset most frequently occurring between 15 and 35 years of age.Citation16,Citation17
Psoriasis is found to have a significant impact on global healthcare systems and inflicts enormous burdens on economic, social, and private life, resulting in a low overall quality of life.Citation15,Citation18 The burden of psoriasis is generally greatest in the age group of 60–69 years, with a disproportionately greater burden in high-income countries.Citation15 Despite the fact that psoriasis is physically and emotionally debilitating and devastating on its own, the occurrence of comorbidities, such as metabolic diseases, cardiovascular diseases (CVD), inflammatory diseases, and psychiatric illness (depression/anxiety) in psoriatic patients increases the burden of the disease.Citation17,Citation19,Citation20 Psoriatic patients with comorbidities are more likely to seek urgent care, have higher hospitalization rates and more frequent outpatient visits, and incur greater costs than those without comorbidities.Citation20 The burden of these comorbidities is even worse as the severity of psoriasis increases.Citation19,Citation21
Pathophysiology of Psoriasis and the Role of Fetuin-A
Pathophysiology of Psoriasis
The pathogenesis and the precise mechanisms underlying psoriatic disease are complex and multifactorial. Even though further elucidating research is needed, the interplay between genetic, environmental, and immunologic factors is thought to be involved in the pathogenesis of the disease.Citation22,Citation23 Several risk factors have been identified to be involved in the development of psoriasis, including family history and environmental risk factors such as smoking, stress, obesity, and alcohol consumption.Citation24 More importantly, chronic inflammation mediated by immunological mediators and inflammatory cytokines is found to be a major player in the pathophysiology of psoriasis.Citation14,Citation25 The cytokines, chemokines, and inflammatory mediators that are involved in psoriasis development are chiefly produced by keratinocytes and immune cells such as T-cells, neutrophils, monocytes, macrophages, hepatocytes, epithelial, and endothelial cells.Citation26 Although there are several possible interactions between these cell types that could have a significant impact on psoriasis, it is likely that a cascade of cytokines secreted by various cells in the local environment of the psoriatic plaque plays a central role in the phenotypic responses in psoriasis.Citation27
More importantly, psoriasis is a T-cell mediated immunological condition primarily driven by cytokines and chemokines released by activated T-helper 1 (Th1) and Th17 lymphocytes.Citation14 The key pathomechanism of psoriatic lesion is the immune mediated keratinocyte changes by T-cells and secretions of other inflammatory cells. The T-cell secreted inflammatory cytokines such as TNF-α, interleukin-1 (IL-1), IL-2, IL-6, IL-8, IL-17, and IL-23 increase the release of chemokines, epidermal growth factor (EGF), and TGF-β1.Citation28 These factors regulate the migration of new inflammatory cells into the skin and increase the activity of these cells and keratinocytes, resulting in psoriasis.Citation14 Besides, inflammatory cytokines such as IL-1 are linked with proteolysis, signal transduction, adhesion, proliferation, and epidermal differentiation, implying their essential role in the initiation and formation of psoriatic lesions.Citation29 The pathogenesis of psoriasis also contains an autoimmune facet that presents as autoreactive T-cells, as evident from the association of psoriasis with several autoimmune diseases and the detection of autoreactive T-cells in patients.Citation30,Citation31
Psoriasis is not solely a disease involving the skin, but it is a systemic disease that also affects many other parts of the body, such as the joints, cardiovascular system, and central nervous system.Citation22 Due to the chronic nature of psoriatic disease, it has been observed to be associated with a wide range of comorbidities such as psoriatic arthritis, atherosclerosis, insulin resistance (IR), type 2 diabetes mellitus, obesity, hypertension, dyslipidemia, metabolic syndrome, CVD, stroke, osteoporosis, respiratory diseases, gastrointestinal diseases, autoimmune diseases, inflammatory bowel diseases, malignancies (lymphoma and skin cancers), infectious disease (hepatitis C virus), and bullous pemphigoid.Citation15,Citation17,Citation32–Citation39 All different manifestations (comorbidities) of psoriatic disease show common pathogenetic, immunological, and metabolic signatures.Citation40–Citation42 Although the exact mechanism underlying the comorbidities of psoriasis remains blurred, systemic chronic inflammation has been reported to be involved in their pathophysiology. Proinflammatory cytokines spill over from the active lesions of psoriasis into the bloodstream, and causing inflammation in distant tissues or comorbidities, which is known as the “psoriatic march”.Citation43,Citation44 These inflammatory cytokines that are involved in psoriasis pathology are thought to cause endothelial injury, immunological dysregulation, and metabolic changes, thereby deriving comorbidities.Citation14
Multiple epidemiological studies outline a possible correlation between psoriasis and respiratory diseases such as asthma, chronic obstructive pulmonary disease (COPD), and airway infections.Citation45–Citation47 Respiratory diseases share common inflammatory pathogenic mechanisms with other psoriasis comorbidities.Citation48 The systemic state of inflammation caused by psoriasis acts de novo on respiratory tissues and heightens the preexisting inflammation from asthma or COPD.Citation49–Citation51 Several immunogenetic studies have also suggested that the coexistence of psoriasis and CVD is caused by resistance-induced release of inflammatory markers such as TNF-α and IL-6 leading to skin inflammation.Citation52–Citation55
Moreover, psoriatic arthritis is a systemic inflammatory arthritis affecting about 30% of the psoriatic patients, with more prevalence in those with more extensive skin disease.Citation56 Dysregulation of the immune response, such as T-cell over-activation has also been proposed as a cause for the coexistence of psoriasis and psoriatic arthritis.Citation49 Furthermore, psoriasis also coexists with bullous pemphigoid and their association is found to be bidirectional. A large-scale population-based study by Kridin et al. confirms that patients with bullous pemphigoid are 2.6-times more at risk of psoriasis, while patients with a prior psoriasis history are 1.5-times more likely to have bullous pemphigoid compared to those without psoriasis.Citation39 The pathogenesis underlying this association is largely uncertain, but the generation of autoantibodies against the basement membrane, the release of neutrophil secreting metalloproteases that break down matrix proteins, cytokines, and T-cell polarization are factors involved in the coexistence of bullous pemphigoid and psoriasis.Citation39
The Role of Fetuin-A in the Pathogenesis of Psoriasis
Currently, a plethora of recent data report a strong correlation between fetuin-A and psoriasis disease activity, suggesting involvement in psoriasis pathogenesis though its role is still not entirely clear ().Citation25,Citation57 A substantial body of data found that people with psoriasis had significantly lower levels of fetuin-A than healthy controls.Citation58–Citation62 This result is in accordance with the findings of another study that reported significantly lower mean serum values of fetuin‑A in patients with psoriasis compared to the healthy control groups.Citation63 The systemic levels of fetuin‑A are down regulated in patients with psoriasis, most likely due to psoriasis-related inflammation (negative acute phase reactant). This is in agreement with Ataseven et al that reported an inverse relationship between serum levels of fetuin‑A and CRP in psoriatic patients.Citation64
Table 1 A Summary Table on the Relationship Between Fetuin-A and Psoriasis
According to different rat and human studies, the gene expression of fetuin-A is reduced by inflammation-induced inhibition of active HMGBP1 release and subsequent expression of inflammatory cytokines ().Citation63,Citation65 Inflammatory cytokines such as IL‑6, IL‑1β, interferon-γ (IFN-γ) and TNF-α repress the C/EBP regulatory sequences, resulting in downregulated fetuin-A gene expression and a robust inflammatory response.Citation66 Accumulated evidence indicated that the decreased level of fetuin-A may underlie the pathogenesis of psoriasis, though further clarification is still warranted. Thus, the decreased fetuin-A level is postulated to contribute to the development of psoriasis, which may be owing to the decreased anti-inflammatory function of fetuin-A that antagonizes inflammatory cytokines (such as TGF-β, TNF-α and IL‑6) and EGF.Citation9,Citation67,Citation68
Figure 1 Hypothetical role of fetuin-A in the pathogenesis of psoriasis. Early inflammatory mediators (IL-6, IL-1β, IFN-γ, TNF-α) are released from immune cells in response to primary triggers. These early mediators repress the C/EBP regulatory sequences and downregulates hepatic fetuin-A gene expression (release). Low fetuin-A decreases its anti-inflammatory function by counteracting inflammatory cytokines (TGF-β, TNF- α and IL-6, EGF) and promoting inflammation and vascular calcification, thereby psoriasis and comorbidities like CVD. On the other hand, the excess late pro-inflammatory mediators (HMGB1) stimulate hepatic release of fetuin-A. High level of fetuin-A stimulates keratinocyte proliferation by inhibiting TGF- β, activates keratinocyte and neutrophil migration directly or by inducing EGF and TGF-α, reduce the of adiponectin expression by adipocytes and thereby increases pro-inflammatory cytokines and decreased anti-inflammatory cytokines, ultimately cause inflammation and psoriasis. High level of fetuin-A, by impairing ISP and counteracting adiponectin action, results in IR, diabetes mellitus, obesity, NAFLD, and dyslipidemia, which may eventually ends with CVD.
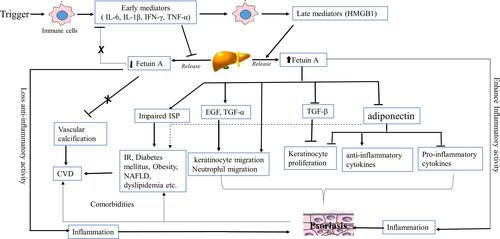
In contrast, a study by Uyar et al reported no significant relationship in fetuin-A values between the patients with psoriasis and the control group.Citation69 However, several other recent publications have shown positive correlations between fetuin-A with psoriasis and its concomitant disorders, which demonstrated elevated values of fetuin-A in patients with psoriasis than in healthy individuals.Citation25,Citation26,Citation57,Citation70 The elevated levels of fetuin-A demonstrated in patients with psoriasis could probably be due to the excess accumulation of late pro-inflammatory mediators (eg, HMGB1) in response to the strong inflammatory response underlying the disease. Subsequent HMGB1-stimulated hepatic expression of fetuin-A restores serum levels of fetuin-A in the disease process.Citation71 Furthermore, while a number of studies revealed no correlations between fetuin-A and the Psoriasis Area and Severity Index (PASI) score, results from several other studies claimed that fetuin-A levels are significantly and positively correlated with the disease severity of psoriasis (PASI score).Citation25,Citation26,Citation57,Citation59,Citation61,Citation62,Citation69 The elevated levels of fetuin-A in psoriasis indicate that the proinflammatory attributes of fetuin-A in mediating the development and progression of psoriasis.
Several mechanisms have been proposed for the role of fetuin-A in the development of psoriasis, albeit more research is needed. One proposed mechanism is that fetuin-A acts as an endogenous inhibitor of TGF-β, which inhibits keratinocyte proliferation. Hence, the increased level of fetuin-A is associated with reduced TGF-β and causes excessive proliferation of keratinocytes in patients with psoriasis.Citation9,Citation27 Furthermore, fetuin-A has recently been discovered to play a pivotal role in keratinocyte migration via signaling pathways resembling those pro-migratory pathways induced by EGF and TGF-α activation and to significantly induce actin-rich protrusions in human primary keratinocytes.Citation72 In addition, fetuin-A may cause psoriasis by reducing the expression of adiponectin by fat cells. Adiponectin found to have anti-inflammatory effect by acting on keratinocytes and T-cells to decrease the inflammation underlying psoriasis. Thus, fetuin-A induced lowering of adiponectin levels result in an increase in contents of pro-inflammatory cytokines and a decrease in levels of anti-inflammatory cytokines.Citation73 The decrease in adiponectin levels further leads to reduced E2F1 gene activation via the AMPK pathway in keratinocytes, thus leading to excessive keratinocyte proliferation and psoriasis.Citation73,Citation74 diagrammatically summarizes the hypothetical role of fetuin-A in the pathogenesis of psoriasis.
Serum fetuin-A level derangement is not only implicated in the pathogenesis of psoriasis but also responsible for the development of several comorbidities of psoriasis. Low level of fetuin-A is associated with suppressed anti-inflammatory (protective) effects and a high degree of calcium accumulation in soft tissues (eg, vascular calcification), leading to comorbidities like CVD.Citation59 On the other hand, conditions associated with elevated values of fetuin-A, such as IR, diabetes mellitus, dyslipidemia, and metabolic syndrome, may also cause CVD.Citation9,Citation63,Citation70
The Potential Clinical Applications of Fetuin-A in Psoriasis
Fetuin-A is a soluble protein that has nowadays gained researchers’ attention due to its potential clinical relevance in patients with a variety of clinical diseases, including psoriasis. In light of its key pathological role in psoriasis, fetuin-A found to have potential implications in the clinical practice as a diagnostic marker, prognostic predictor, and therapeutic target for psoriasis. Henceforth, this review highlights the potential clinical implications of fetuin-A in diagnosis and therapy of psoriasis and related comorbidities.
The Potential Diagnostic Implications of Fetuin-A
Collective body of evidence demonstrated that fetuin-A could be useful as a potential diagnostic marker for various disease conditions in clinical practice.Citation75 Many researchers have reported the correlation of fetuin-A with psoriasis and its role in the development of the disease.Citation25,Citation26,Citation57–Citation62,Citation70 Other studies have also shown a significant positive association between fetuin-A and the severity of psoriasis (PASI score).Citation25,Citation26 Altogether, these findings suggest the usefulness of fetuin-A as a possible diagnostic marker and severity indicator of psoriasis in clinical settings.
Fetuin-A levels in the blood and tissues could also serve as a useful marker in various clinical conditions that occur alongside or independently of psoriasis, such as atherosclerosis, CVDs, metabolic syndrome, chronic kidney disease (CKD), liver dysfunction, tumors, and other inflammatory diseases.Citation8 In recent years, there has been considerable interest with respect to fetuin-A and atherosclerosis. Fiore and coworkers reported a significantly positive correlation between serum levels of fetuin-A and intima-media thickness in the early stages, establishing it as a surrogate marker for early atheromatous alterations.Citation76 In contrast, more recent studies showed that fetuin-A level is inversely associated with the severity of atherosclerosis and plaque formation, indicating low fetuin A level that promotes vascular calcification, and hence it may serve as a novel biomarker of vascular calcification and atherosclerosis.Citation77,Citation78 Moreover, fetuin-A is a well-established pathological contributor to CVD, and it may serve as a biomarker.Citation79 A study by Borsky et al assessed the suitability of fetuin A for detection of the early stages of CVD in people with psoriasis and implied that this biomarker could be considered as an appropriate marker in detecting cardiac risk and early stages of CVDs.Citation58 However, the variable nature of the correlation of fetuin-A in the early and advanced stages of atherosclerosis as well as the biphasic association of fetuin-A with the occurrence of CVD depending on the stage of atherosclerosis may limit its clinical usefulness as a diagnostic biomarker.Citation79 In order to use fetuin-A in routine clinical practice, additional rigorous and extensive research is needed to provide supporting evidence harmonizing the contradictory results and prove the diagnostic importance of fetuin-A in CVD.
Fetuin-A is also found to be elevated in liver dysfunctions such as NAFLD, and it has been observed to have positive correlations with accumulated liver fat and liver fibrosis score index.Citation80 However, it is negatively associated with severe liver damage, which could be as a result of reduced hepatic fetuin-A synthesis.Citation8,Citation81 This shows that fetuin-A can serve as a marker for the progression of simple hepatic steatosis to advanced stages of NAFLD. Thus, fetuin-A could be employed as a liver function marker, severity predictor, and prognostic marker in patients with liver disease in the future.
Fetuin-A deficiency has also been linked with CKD, which is usually accompanied by vascular calcification, suggesting that fetuin-A may be clinically important in patients with severe CKD.Citation1,Citation82 Recent studies have also shown that fetuin A can be used as a promising biological marker for type 2 diabetes mellitus, obesity, tumors, neurodegenerative diseases, multiple sclerosis, cognitive decline, and COPD.Citation75,Citation83 Fetuin-A is currently reported to be a novel urinary biomarker of the progression of diabetic nephropathy in type 2 diabetes mellitus.Citation84 A substantial body of evidence shows anti-fetuin-A auto-antibodies are frequently seen in the blood of tumor patients and have potential as early diagnostic biomarkers.Citation83 Based on the finding that fetuin-A has a pathological role in the disease process of neurodegenerative disorders, its cerebrospinal fluid (CSF) level is has recently been identified as a potential biomarker in degenerative diseases such as multiple sclerosis.Citation85 On the other hand, Laughlin et al demonstrated that fetuin-A will be used as a new vascular biomarker of age-related cognitive decline in older adults.Citation75 In COPD, fetuin-A may serve as a biomarker of disease activity and progression, with lower fetuin-A indicating more severe disease.Citation86
Although human fetuin-A is one potential candidate as a diagnostic biomarker for psoriasis and various related diseases, there are several obstacles that limit its clinical utility. Many scholars have uncovered a number of flaws associated with fetuin-A that make it unsuitable for use as a metabolic biomarker at this time. The first is the absence of standardized reference values, leading to confusion and discordancy.Citation87 This perhaps associated with inconsistent reference ranges from different commercial enzyme-linked immunosorbent assays (ELISA) though the differences among the various ELISA not yet well elucidated. The second reason for the erratic results could be due to individual (genetic) variations in serum levels of fetuin-A.Citation75 The other reason is the unclear effect of post-translational processing on human fetuin-A measurements.Citation88 For instance, the species-specific glycosylation of fetuin-A is created by distinct expression systems, resulting in products with variable glycosylation patterns and thus serum values.Citation89,Citation90 Overall, to minimize misinterpretation and to use fetuin-A as a diagnostic marker consistently, standardization of fetuin-A assays and consistent normal reference ranges are urgently needed.
The Potential Therapeutic Role of Fetuin-A in Psoriasis
The Treatment Approaches of Psoriasis
Psoriasis is a lifelong disease with a chronic relapsing course requiring long-term treatment for most patients. The disease’s evolution is unpredictable, and the extent of skin involvement can range from mild to very severe forms. Remission of psoriasis is achieved through the long-term efficacious control of skin lesions.Citation91 The treatment approaches for psoriasis involve topical medication, systemic drugs, and phototherapy, which are considered as conventional treatments.Citation91 The therapeutic paradigms for psoriasis are changing due to poor tolerability, drug resistance, and adverse effects linked with the long-term use of conventional treatments. Especially, as molecular pathways of psoriasis immunopathogenesis are elucidated, a mechanistic approach to therapy has revolutionized medicine over the most recent decade. The therapeutic landscape for psoriasis has been revolutionized recently by biologic (targeted) therapies.Citation15,Citation91 Several commercially available biologic agents for treating psoriasis are constantly growing, including, but not limited to, inhibitors of TNF, IL-17 receptor, IL-17, IL-12/23, IL-23, phosphodiesterase, and Janus kinases (JAK).Citation15 Biologic drugs have been discovered to be game changers in psoriasis treatment due to their high efficacy and promising safety, and they are now the standard treatment for moderate-to-severe psoriasis.Citation92 These drugs are particularly effective in patients who do not respond or have contraindication to conventional therapy. Moreover, because of better tolerability and safety, biologics are more feasible for long-term use than conventional therapy.Citation91
Treatment goal for psoriasis is generally evaluated using the PASI between weeks 12 and 16 at the end of the induction therapy. The PASI is a useful tool to assess treatment efficacy, with a percentage reduction in the PASI score indicating the decrease in psoriasis severity and the increase in the effectiveness of treatments.Citation91 While PASI 75 is the current benchmark of primary endpoints for most clinical trials of psoriasis, PASI 90 response is the ultimate goal of therapy, which is currently considered as the most relevant treatment outcome, particularly in patients with severe disease.Citation93,Citation94 When there is no improvement by 50% in the PASI score, the patient is said to be a non-responder.Citation95
Despite significant therapeutic advances, psoriasis therapy has become challenging for clinicians in recent years, as more patients have developed resistance to the available biologic therapies such as TNF-α, IL-23 and IL12/23 inhibitors. This is due to loss of response, lack of response, discontinuation due to adverse effects, or the chronic nature of the disease.Citation15 Roughly 10–30% of the psoriatic patients treated with TNF or IL12/23 inhibitors are partial or non-responders, or experience adverse effects, leading to treatment discontinuation.Citation96,Citation97 This therapeutic challenge of psoriasis doubles when it coupled with psoriasis-related comorbidities that make systemic treatment difficult. If the treatment goal is not achieved, several therapeutic strategies can be adopted, such as raising the dose of the drug, reducing the time gap between administrations, combination therapy, drug switching, and targeting novel target site.Citation37,Citation91 These strategies play a pivotal role in limiting the detrimental progression of psoriasis and worsening of the patient’s quality of life as measured by the significant improvements in PASI and the Dermatology Life Quality Index (DLQI), respectively. Switching patients to other treatments is a relatively common practice that is indicated when the other mentioned strategies are ineffective or inappropriate.Citation98 The switch from IL-12/23 and TNF inhibitors to IL-17 inhibitors has been recently found to be a safe and effective therapeutic strategy in psoriatic patients.Citation98,Citation99 IL-17 inhibitors such as Ixekizumab display higher efficacy than both TNF and IL-12/23 inhibitors, which increased the treatment goal from PASI 75 to PASI 90 or even PASI 100.Citation100 In addition, therapeutically targeting fetuin-A currently proposed to be a potential option for dermatologists in the treatment of psoriasis.
The Potential Role of Fetuin-A in the Treatment of Psoriasis
Currently, fetuin-A has been discovered to serve as a therapeutic target for the treatment of various disease conditions, in addition to its possible use as a biomarker. The growing role of fetuin-A in the development of diseases such as psoriasis necessitates the development of appropriate therapies designed to manipulate fetuin-A levels. Thus, fetuin-A is expected to become a novel target for psoriasis treatment. Fetuin-A is thought to play a role in psoriasis development by modulating the chronic inflammation that underpin the disease. Thus, treating by targeting fetuin-A-mediated inflammatory pathways in psoriatic patients may provide a new therapeutic approach that improves clinical outcomes while more research is needed. Elevated level of fetuin-A in psoriasis is suggested to have anti-inflammatory role and calcification inhibitory activities. Thus, therapeutically increasing the level of fetuin-A in psoriatic illness may have a protective effect, opening up a new avenue for psoriasis therapy. It is supported by Ketter et al who reported that boosting fetuin-A levels may be a solution to undesirable extraosseous calcification, although its diverse activities need to be addressed during treatment.Citation1 Furthermore, since fetuin-A has been identified as an endogenous ligand for TLR4 promoting lipid-induced insulin resistance, it may serve as a new therapeutic target for treating IR and type 2 diabetes coexisting with psoriasis.Citation101
Extensive efforts are being made to develop effective therapeutic options for modulating fetuin-A levels. To date, adiponectin, salsalate, curcumin, and vitamin D have been identified as promising agents to modulate the serum levels of fetuin-A.Citation102–Citation104 A study by Jung et al shows adiponectin and salsalate has been proven to reduce palmitate-induced fetuin-A gene expression and ameliorate hepatic steatosis in hepG2 cells.Citation102 Moreover, curcumin administration reduces serum fetuin-A in rats on a high-fat diet, presumably by curcumin-mediated AMPK activation.Citation103 Treating rats with a megadose of vitamin D also lowers blood fetuin-A levels and enhances vascular calcification.Citation104
Discussion
The pathomechanisms underlying psoriatic disease are complex and multifactorial, and the interplay between genetic, environmental, and immunologic factors is thought to be involved in the pathogenesis of the disease. Notably, a systemic chronic inflammation mediated by immunological mediators and inflammatory cytokines was found to be a major player in the pathophysiology of psoriasis and its comorbidities. However, the exact pathophysiology of psoriasis is not entirely clear, which warrants further elucidating researches. Nowadays, a considerable body of data has reported the close association of fetuin-A derangement with psoriasis and its comorbidities, suggesting its novel putative pathological role in psoriasis. However, the study findings are still discordant and the role of fetuin-A in the pathogenesis of psoriasis remains vague. In other words, consensus has not been reached on whether fetuin-A has an exacerbating or protective effect on psoriasis. This inconsistent property can be due to the dual roles of fetuin-A in psoriasis, where it can act as an inflammatory and anti-inflammatory agent as well as due to the presence of confounding morbidities coexisting with psoriasis. Therefore, it still remains an area of active research that needs further extensive studies to resolve the opposing findings and to uncover the enigmatic issues and arrive at a conclusion.
In light of its role in the pathogenesis of psoriasis, fetuin-A nowadays attracts researchers’ interest towards its potential implications in clinical practice as a diagnostic marker, prognostic predictor, and therapeutic target for psoriasis. Thus, many attempts are currently being made to better understand the emerging clinical implications of fetuin-A for diagnostics as well as therapeutics. Several findings suggest the usefulness of fetuin-A as a possible diagnostic marker and severity indicator of psoriasis in clinical settings in the future. However, several obstacles such as absence of standardized reference values, individual variation, endogenous effect of post-translational processing on fetuin-A value may limit its clinical utility. Therefore, more research is needed to properly determine the concentration range for fetuin-A in psoriatic diseases and in specific group of patients.
Although several anti-psoriatic therapies are nowadays available in the pharmaceutical market, psoriatic patients may experience therapeutic failure even with the most effective biologics due to the chronic nature of the disease, leading to loss of response, lack of response or treatment discontinuation. Thus, several therapeutic strategies have been adopted, including increasing the drug dose, reducing the time gap between drug intake, combination therapy, drug switching, and targeting novel sites to limit the progression of psoriasis and worsening of patients’ quality of life. In this scenario, targeting fetuin-A is a great new street to approach patients with psoriasis. To date, adiponectin, salsalate, curcumin, and vitamin D have been identified as promising agents to modulate the serum levels of fetuin-A. Generally, fetuin-A could be a promising therapeutic target in future clinical practice, though therapeutic adaptation against different comorbid disorders such as vascular diseases needs to be considered in treatment planning. Hence, more in-depth studies that clearly show the role of fetuin-A in psoriasis and comorbidities are needed.
Despite our extensive effort to provide a comprehensive and up-to-date review on fetuin-A and psoriasis, this review primarily focuses on the role of fetuin-A in psoriasis, though it has an important pathological role in various other clinical conditions. Besides, this review did not incorporate the paralogue of fetuin-A, known as fetuin-B, that has critical role in different diseases.
Concluding Remarks
In summary, psoriasis is a multifactorial chronic inflammatory disease mediated by different inflammatory mediators that act as an important hallmark in its pathogenesis. Interestingly, fetuin-A has currently been observed to play an essential pathological role in psoriasis. It is recently found to be involved in the chronic inflammatory process underlying psoriasis, though its role is very complex and still not entirely clear. Various studies have shown the correlation between fetuin-A and psoriasis, but the results are contrasting and equivocal. More extensive studies are warranted to reconcile the opposing findings and to throw more light on the possible role of fetuin-A in psoriasis pathogenesis. Fetuin-A is currently attracting researchers’ attention due to its diagnostic and therapeutical clinical relevance. It is proposed to serve as a diagnostic tool for psoriasis and related comorbidities in future clinical practice. Despite the diagnostic potential of fetuin-A for psoriasis and the availability of multiple assays, fetuin-A readings are variable, limiting its clinical utility and necessitating standardization. Fetuin-A has also been found to be a promising therapeutic target for the treatment of psoriasis. Although therapeutic options are limited in modulating fetuin-A levels, salsalates, curcumin, and vitamin D are intriguing future agents. Nevertheless, the available information is still insufficient and the relevance of fetuin-A in psoriasis is still of concern.
Abbreviations
AHSG, α2-Heremans-Schmid glycoprotein; APP, acute phase protein; CKD, chronic kidney disease; CRP, C reactive protein; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; EGF, epidermal growth factor; ELISA, enzyme-linked immunosorbent assays; ESRD, end-stage renal disease; HMGBP1, high mobility group box protein 1; IBD, inflammatory bowel disease; IFN-γ, interferon-γ; IL-1, interleukin-1; IR, insulin resistance; NAFLD, non-alcoholic fatty liver disease; NF-1, nuclear factor-1; PASI, Psoriasis Area and Severity Index; RTK, TGF- β, transforming growth factor-β; TGF, transforming growth factor; TLR-4, Toll-like receptor 4; TNF-α, tumor necrosis factor-alpha.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no competing interests in this work.
References
- Kettler M, Bongartz P, Westenfeld R, et al. Association of low fetuin-A (AHSG) concentration in serum with cardiovascular mortality in patients on dialysis: a cross-sectional study. Lancet. 2003;361(9360):827–833. doi:10.1016/S0140-6736(03)12710-9
- Wojtysiak-Duma B, Malecha Jędraszek A, Burska A, et al. Serum fetuin-A levels in patients with type 2 diabetes mellitus. Ann UMCS Sect. 2010;1500:2.
- Mori K, Emoto M, Yokoyama H, et al. Association of serum fetuin-A with insulin resistance in type 2 diabetic and nondiabetic subjects. Diabetes Care. 2006;29(2):468. doi:10.2337/diacare.29.02.06.dc05-1484
- Osawa M, Umetsu K, Sato M, et al. Structure of the gene encoding human alpha2-HS glycoprotein (AHSG). Gene. 1997;196(1–2):121–125. doi:10.1016/S0378-1119(97)00216-3
- Dietzel E, Wessling J, Floehr J, et al. Fetuin-B, a liver-derived plasma protein is essential for fertilization. Dev Cell. 2013;25(1):106–112. doi:10.1016/j.devcel.2013.03.001
- Floehr J, Dietzel E, Schmitz C, et al. Down-regulation of the liver-derived plasma protein fetuin-B mediates reversible female infertility. MHR Basic Sci Reprod Med. 2016;23:1–11.
- Lin Y-H, Franc V, Heck AJ. Similar albeit not the same: in-depth analysis of proteoforms of human serum, bovine serum, and recombinant human fetuin. J Proteome Res. 2018;17(8):2861–2869. doi:10.1021/acs.jproteome.8b00318
- Dabrowska AM, Tarach JS, Wojtysiak-Duma B, et al. Fetuin-A (AHSG) and its usefulness in clinical practice. Review of the literature. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159(3):352–359. doi:10.5507/bp.2015.018
- Mori K, Emoto M, Inaba M. Fetuin-A and the cardiovascular system. Adv Clin Chem. 2012;56:176.
- Braun-Falco O, Burgdorf W, Plewig G, et al. Braun-Falco´ s dermatology. Braun-Falcos Dermatol. 2009;2009:1712–xix.
- Adya KA, Inamadar AC, Palit A. Dermatoses with “Collarette of Skin”. BLDE; 2018.
- Krueger G, Ellis CN. Psoriasis—recent advances in understanding its pathogenesis and treatment. J Am Acad Dermatol. 2005;53(1):S94–S100. doi:10.1016/j.jaad.2005.04.035
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Investig Dermatol. 2013;133(2):377–385. doi:10.1038/jid.2012.339
- Mehlis SL, Gordon KB. The immunology of psoriasis and biologic immunotherapy. J Am Acad Dermatol. 2003;49(2):44–50. doi:10.1016/S0190-9622(03)01134-4
- Damiani G, Bragazzi NL, Aksut CK, et al. The global, regional, and national burden of psoriasis: results and insights from the global burden of disease 2019 study. Front Med. 2021;8. doi:10.3389/fmed.2021.743180
- Egeberg A, Andersen YM, Thyssen JP. Prevalence and characteristics of psoriasis in Denmark: findings from the Danish skin cohort. BMJ open. 2019;9(3):e028116. doi:10.1136/bmjopen-2018-028116
- Feldman SR, Tian H, Gilloteau I, et al. Economic burden of comorbidities in psoriasis patients in the United States: results from a retrospective US database. BMC Health Serv Res. 2017;17(1):1–8. doi:10.1186/s12913-017-2278-0
- Strober B, Karki C, Mason M, et al. Characterization of disease burden, comorbidities, and treatment use in a large, US-based cohort: results from the Corrona Psoriasis Registry. J Am Acad Dermatol. 2018;78(2):323–332. doi:10.1016/j.jaad.2017.10.012
- Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149(10):1173–1179. doi:10.1001/jamadermatol.2013.5015
- Kimball A, Guerin A, Tsaneva M, et al. Economic burden of comorbidities in patients with psoriasis is substantial. J Eur Acad Dermatol Venereol. 2011;25(2):157–163. doi:10.1111/j.1468-3083.2010.03730.x
- Kim N, Thrash B, Menter A, editors. Comorbidities in psoriasis patients. In: Seminars in Cutaneous Medicine and Surgery. Vol. 29. WB Saunders; 2010:10–15. doi:10.1016/j.sder.2010.01.002
- Langley R, Krueger G, Griffiths C. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(suppl2):ii18–ii23. doi:10.1136/ard.2004.033217
- Duarte GV, Follador I, Cavalheiro CMA, et al. Psoriasis and obesity: literature review and recommendations for management. An Bras Dermatol. 2010;85:355–360. doi:10.1590/S0365-05962010000300009
- Huerta C, Rivero E, Rodríguez LAG. Incidence and risk factors for psoriasis in the general population. Arch Dermatol. 2007;143(12):1559–1565. doi:10.1001/archderm.143.12.1559
- Uysal S, Yilmaz F, Karatoprak K, et al. The levels of serum pentraxin3, CRP, fetuin-A, and insulin in patients with psoriasis. Eur Rev Med Pharmacol Sci. 2014;18(22):3453–3458.
- Okan G, Baki AM, Yorulmaz E, et al. Serum visfatin, fetuin a, and pentraxin 3 levels in patients with psoriasis and their relation to disease severity. J Clin Lab Anal. 2016;30(4):284–289. doi:10.1002/jcla.21850
- Mukhopadhyay S, Mondal SA, Kumar M, et al. Proinflammatory and antiinflammatory attributes of fetu Iν-A: a novel hepatokine modulating cardiovascular and glycemic outcomes in metabolic syndrome. Endocr Pract. 2014;20(12):1345–1351. doi:10.4158/EP14421.RA
- Wieduwilt M, Moasser M. The epidermal growth factor receptor family: biology driving targeted therapeutics. Cell Mol Life Sci. 2008;65(10):1566–1584. doi:10.1007/s00018-008-7440-8
- Yano S, Banno T, Walsh R, et al. Transcriptional responses of human epidermal keratinocytes to cytokine interleukin‐1. J Cell Physiol. 2008;214(1):1–13. doi:10.1002/jcp.21300
- Furue K, Ito T, Tsuji G, et al. Autoimmunity and autoimmune comorbidities in psoriasis. Immunology. 2018;154(1):21–27. doi:10.1111/imm.12891
- Schön MP. Adaptive and innate immunity in psoriasis and other inflammatory disorders. Front Immunol. 2019;10:1764. doi:10.3389/fimmu.2019.01764
- Ghazizadeh R, Shimizu H, Tosa M, et al. Pathogenic mechanisms shared between psoriasis and cardiovascular disease. Int J Med Sci. 2010;7(5):284. doi:10.7150/ijms.7.284
- Gisondi P, Ferrazzi A, Girolomoni G. Metabolic comorbidities and psoriasis. Acta dermatovenerologica Croatica. 2010;18(4):179.
- Pietrzak A, Michalak-Stoma A, Chodorowska G, et al. Lipid disturbances in psoriasis: an update. Mediators Inflamm. 2010;2010:1–13. doi:10.1155/2010/535612
- Khunger N, Gupta D, Ramesh V. Is psoriasis a new cutaneous marker for metabolic syndrome? A study in Indian patients. Indian J Dermatol. 2013;58(4):313. doi:10.4103/0019-5154.113958
- Boehncke S, Thaci D, Beschmann H, et al. Psoriasis patients show signs of insulin resistance. Br J Dermatol. 2007;157(6):1249–1251. doi:10.1111/j.1365-2133.2007.08190.x
- Damiani G, Odorici G, Pacifico A, et al. Secukinumab loss of efficacy is perfectly counteracted by the introduction of combination therapy (rescue therapy): data from a multicenter real-life study in a cohort of Italian psoriatic patients that avoided secukinumab switching. Pharmaceuticals. 2022;15(1):95. doi:10.3390/ph15010095
- Damiani G, Franchi C, Pigatto P, et al. Outcomes assessment of hepatitis C virus-positive psoriatic patients treated using pegylated interferon in combination with ribavirin compared to new Direct-Acting Antiviral agents. World J Hepatol. 2018;10(2):329. doi:10.4254/wjh.v10.i2.329
- Kridin K, Ludwig RJ, Schonmann Y, et al. The bidirectional association between bullous pemphigoid and psoriasis: a population-based cohort study. Front Med. 2020;7:511. doi:10.3389/fmed.2020.00511
- Yuan Y, Qiu J, Lin ZT, et al. Identification of novel autoantibodies associated with psoriatic arthritis. Arthritis Rheumatol. 2019;71(6):941–951. doi:10.1002/art.40830
- Lande R, Botti E, Jandus C, et al. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat Commun. 2014;5(1):1–16. doi:10.1038/ncomms6621
- Pietrzak A, Chabros P, Grywalska E, et al. Serum lipid metabolism in psoriasis and psoriatic arthritis–an update. AMS. 2019;15(2):369. doi:10.5114/aoms.2018.74021
- Damiani G, Radaeli A, Olivini A, et al. Increased airway inflammation in patients with psoriasis. 2016.
- Malerba M, Damiani G, Radaeli A, et al. Narrowband ultraviolet B phototherapy in psoriasis reduces proinflammatory cytokine levels and improves vitiligo and neutrophilic asthma. Br J Dermatol. 2015;173(6):1544–1545. doi:10.1111/bjd.13988
- Fang HY, Liao WC, Lin CL, et al. Association between psoriasis and asthma: a population based retrospective cohort analysis. Br J Dermatol. 2015;172(4):1066–1071. doi:10.1111/bjd.13518
- Santus P, Rizzi M, Radovanovic D, et al. Psoriasis and respiratory comorbidities: the added value of fraction of exhaled nitric oxide as a new method to detect, evaluate, and monitor psoriatic systemic involvement and therapeutic efficacy. Biomed Res Int. 2018;2018:1–10. doi:10.1155/2018/3140682
- Kao L-T, Lee C-Z, Liu S-P, et al. Psoriasis and the risk of pneumonia: a population-based study. PLoS One. 2014;9(12):e116077. doi:10.1371/journal.pone.0116077
- Naik HB, Natarajan B, Stansky E, et al. Severity of psoriasis associates with aortic vascular inflammation detected by FDG PET/CT and neutrophil activation in a prospective observational study. Arterioscler Thromb Vasc Biol. 2015;35(12):2667–2676. doi:10.1161/ATVBAHA.115.306460
- Damiani G, Pacifico A, Rizzi M, et al. Patients with psoriatic arthritis have higher levels of FeNO than those with only psoriasis, which may reflect a higher prevalence of a subclinical respiratory involvement. Clin Rheumatol. 2020;39(10):2981–2988. doi:10.1007/s10067-020-05050-2
- Östling J, van Geest M, Schofield JP, et al. IL-17–high asthma with features of a psoriasis immunophenotype. J Allergy Clin Immunol. 2019;144(5):1198–1213. doi:10.1016/j.jaci.2019.03.027
- Phillips BG, Wang Y, Ambati S, et al. Airways therapy of obstructive sleep apnea dramatically improves aberrant levels of soluble cytokines involved in autoimmune disease. Clin Immunol. 2020;221:108601. doi:10.1016/j.clim.2020.108601
- Johnston A, Arnadottir S, Gudjonsson JE, et al. Obesity in psoriasis: leptin and resistin as mediators of cutaneous inflammation. Br J Dermatol. 2008;159(2):342–350. doi:10.1111/j.1365-2133.2008.08655.x
- Muse ED, Feldman DI, Blaha MJ, et al. The association of resistin with cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2015;239(1):101–108. doi:10.1016/j.atherosclerosis.2014.12.044
- Pina T, Genre F, Lopez‐Mejias R, et al. Relationship of Leptin with adiposity and inflammation and Resistin with disease severity in Psoriatic patients undergoing anti‐TNF‐alpha therapy. J Eur Acad Dermatol Venereol. 2015;29(10):1995–2001. doi:10.1111/jdv.13131
- Huang H, Shen E, Tang S, et al. Increased serum resistin levels correlate with psoriasis: a meta-analysis. Lipids Health Dis. 2015;14(1):1–9. doi:10.1186/s12944-015-0039-9
- Husni ME, Merola JF, Davin S, editors. The psychosocial burden of psoriatic arthritis. In: Seminars in Arthritis and Rheumatism. Elsevier; 2017.
- Demirbaş A, Kurtipek GS, Tunçez A, et al. The role of cystatin C and fetuin A in the determination of early atherosclerotic risk in psoriasis patients. Dermatol Ther. 2020;33(6):e13898. doi:10.1111/dth.13898
- Borsky P, Fiala Z, Andrys C, et al. C-reactive protein, chemerin, fetuin-A and osteopontin as predictors of cardiovascular risks in persons with psoriasis vulgaris. Physiol Res. 2021;383–391. doi:10.33549/physiolres.934654
- Genc M, Can M, Guven B, et al. Evaluation of serum Fetuin-a and Osteoprotegerin levels in patients with psoriasis. Ind J Clin Biochem. 2017;32(1):90–94. doi:10.1007/s12291-016-0570-0
- Gulle S, Ozturk S, Kozaci LD, et al. Fetuin-A and its association with disease activity in psoriatic arteritis/Fetuin-A ve psoriyatik artrit hastalik aktivitesi ile iliskisi. J Turk Soc Rheumatol. 2021;13(2):47–54.
- Shehata W, Basha M, Gayed I, et al. Relationship between disease severity and fetuin-A levels in patients with psoriasis. Indian J Dermatol Venereol Leprol. 2020;86(5):586. doi:10.4103/ijdvl.IJDVL_355_19
- Agamia NF, Ae-a AF, El-Hadidy A, et al. YKL-40, fetuin-A plasma levels, and carotid intima-media thickness: do they have relations with subclinical atherosclerosis associated with psoriasis and the disease severity? J Egypt Womens Dermatol Soc. 2019;16(2):133. doi:10.4103/JEWD.JEWD_23_19
- Gerdes S, Osadtschy S, Buhles N, et al. Cardiovascular biomarkers in patients with psoriasis. Exp Dermatol. 2014;23(5):322–325. doi:10.1111/exd.12381
- Ataseven A, Kesli R, Akyurek F, et al. Decreased serum levels of alpha-2-Heremans Schmid glycoprotein (fetuin-a) in patients with psoriasis. Aperito J Dermatol. 2015;2:111.
- Tuttolomondo A, Di Raimondo D, Di Sciacca R, et al. Fetuin-A and CD40 L plasma levels in acute ischemic stroke: differences in relation to TOAST subtype and correlation with clinical and laboratory variables. Atherosclerosis. 2010;208(1):290–296. doi:10.1016/j.atherosclerosis.2009.07.032
- Falquerho L, Paquereau L, Vilarem MJ, et al. Functional characterization of the promoter of pp63, a gene encoding a natural inhibitor of the insulin receptor tyrosine kinase. Nucleic Acids Res. 1992;20(8):1983–1990. doi:10.1093/nar/20.8.1983
- Flisiak I, Zaniewski P, Rogalska M, et al. Effect of psoriasis activity on VEGF and its soluble receptors concentrations in serum and plaque scales. Cytokine. 2010;52(3):225–229. doi:10.1016/j.cyto.2010.09.012
- Flisiak I, Zaniewski P, Rogalska‐Taranta M, et al. Effect of psoriasis therapy on VEGF and its soluble receptors serum concentrations. J Eur Acad Dermatol Venereol. 2012;26(3):302–307. doi:10.1111/j.1468-3083.2011.04053.x
- Uyar B, Akyıldız M, Solak A, et al. Relationship between serum fetuin-A levels and carotid intima-media thickness in Turkish patients with mild to moderate psoriasis. A case-control study. Acta Dermatovenerologica Croatica. 2015;23(3):171.
- Grochowiec M, Narbutt J, Lesiak A. Serum concentration of endocan, fetuin-A, and endothelin in plaque psoriasis-coincidence or fate? A preliminary study. Dermatol Rev/Przeglad Dermatol. 2021;108(1):16–26.
- Li W, Zhu S, Li J, et al. A hepatic protein, fetuin-A, occupies a protective role in lethal systemic inflammation. PLoS One. 2011;6(2):e16945. doi:10.1371/journal.pone.0016945
- Wang XQ, Hung BS, Kempf M, et al. Fetuin‐A promotes primary keratinocyte migration: independent of epidermal growth factor receptor signalling. Exp Dermatol. 2010;19(8):e289–e92. doi:10.1111/j.1600-0625.2009.00978.x
- Yang SJ, Hong HC, Choi HY, et al. Effects of a three‐month combined exercise programme on fibroblast growth factor 21 and fetuin‐A levels and arterial stiffness in obese women. Clin Endocrinol (Oxf). 2011;75(4):464–469. doi:10.1111/j.1365-2265.2011.04078.x
- Jüllig M, Yip S, Xu A, et al. Lower fetuin-A, retinol binding protein 4 and several metabolites after gastric bypass compared to sleeve gastrectomy in patients with type 2 diabetes. PLoS One. 2014;9(5):e96489. doi:10.1371/journal.pone.0096489
- Laughlin GA, McEvoy LK, Barrett Connor E, et al. Fetuin‐A, a new vascular biomarker of cognitive decline in older adults. Clin Endocrinol (Oxf). 2014;81(1):134–140. doi:10.1111/cen.12382
- Fiore CE, Celotta G, Politi GG, et al. Association of high alpha2-Heremans–Schmid glycoprotein/fetuin concentration in serum and intima-media thickness in patients with atherosclerotic vascular disease and low bone mass. Atherosclerosis. 2007;195(1):110–115. doi:10.1016/j.atherosclerosis.2006.08.052
- Emoto M, Mori K, Lee E, et al. Fetuin-A and atherosclerotic calcified plaque in patients with type 2 diabetes mellitus. Metabolism. 2010;59(6):873–878. doi:10.1016/j.metabol.2009.10.005
- Szweras M, Liu D, Partridge EA, et al. α2-HS glycoprotein/fetuin, a transforming growth factor-β/bone morphogenetic protein antagonist, regulates postnatal bone growth and remodeling. J Biol Chem. 2002;277(22):19991–19997. doi:10.1074/jbc.M112234200
- Roos M, Oikonomou D, von Eynatten M, et al. Associations of fetuin-A levels with vascular disease in type 2 diabetes patients with early diabetic nephropathy. Cardiovasc Diabetol. 2010;9(1):1–7. doi:10.1186/1475-2840-9-48
- Yilmaz Y, Yonal O, Kurt R, et al. Serum fetuin A/α 2HS-glycoprotein levels in patients with non-alcoholic fatty liver disease: relation with liver fibrosis. Ann Clin Biochem. 2010;47(6):549–553. doi:10.1258/acb.2010.010169
- Kalabay L, Jakab L, Prohászka Z, et al. Human fetuin/α2HS-glycoprotein level as a novel indicator of liver cell function and short-term mortality in patients with liver cirrhosis and liver cancer. Eur J Gastroenterol Hepatol. 2002;14(4):389–394. doi:10.1097/00042737-200204000-00009
- Ix JH, Chertow GM, Shlipak MG, et al. Fetuin-A and kidney function in persons with coronary artery disease—data from the Heart and Soul Study. Nephrol Dial Transplant. 2006;21(8):2144–2151. doi:10.1093/ndt/gfl204
- Ochieng J, Nangami G, Sakwe A, et al. Impact of Fetuin-A (AHSG) on tumor progression and type 2 diabetes. Int J Mol Sci. 2018;19(8):2211. doi:10.3390/ijms19082211
- Inoue K, Wada J, Eguchi J, et al. Urinary fetuin-A is a novel marker for diabetic nephropathy in type 2 diabetes identified by lectin microarray. PLoS One. 2013;8(10):e77118. doi:10.1371/journal.pone.0077118
- Harris VK, Donelan N, Yan QJ, et al. Cerebrospinal fluid fetuin-A is a biomarker of active multiple sclerosis. Multiple Scleros J. 2013;19(11):1462–1472. doi:10.1177/1352458513477923
- Minas M, Mystridou P, Georgoulias P, et al. Fetuin-A is associated with disease severity and exacerbation frequency in patients with COPD. COPD. 2013;10(1):28–34. doi:10.3109/15412555.2012.727922
- Reinehr T, Roth CL. Fetuin-A and its relation to metabolic syndrome and fatty liver disease in obese children before and after weight loss. J Clin Endocrinol Metab. 2008;93(11):4479–4485. doi:10.1210/jc.2008-1505
- Robinson KN, Teran-Garcia M. From infancy to aging: biological and behavioral modifiers of fetuin-A. Biochimie. 2016;124:141–149. doi:10.1016/j.biochi.2015.12.016
- Rudd PM, Dwek RA. Glycosylation: heterogeneity and the 3D structure of proteins. Crit Rev Biochem Mol Biol. 1997;32(1):1–100. doi:10.3109/10409239709085144
- Arnold JN, Wormald MR, Sim RB, et al. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21–50. doi:10.1146/annurev.immunol.25.022106.141702
- Gisondi P, Del Giglio M, Girolomoni G. Treatment approaches to moderate to severe psoriasis. Int J Mol Sci. 2017;18(11):2427. doi:10.3390/ijms18112427
- Georgakopoulos JR, Ighani A, Phung M, et al. Drug survival of secukinumab in real-world plaque psoriasis patients: a 52-week, multicenter, retrospective study. J Am Acad Dermatol. 2018;78(5):1019–1020. doi:10.1016/j.jaad.2017.11.036
- Gisondi P, Altomare G, Ayala F, et al. Italian guidelines on the systemic treatments of moderate‐to‐severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2017;31(5):774–790. doi:10.1111/jdv.14114
- Daudén E, Puig L, Ferrándiz C, et al. Consensus document on the evaluation and treatment of moderate‐to‐severe psoriasis: Psoriasis Group of the Spanish Academy of Dermatology and Venereology. J Eur Acad Dermatol Venereol. 2016;30:1–18. doi:10.1111/jdv.13542
- Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303(1):1–10. doi:10.1007/s00403-010-1080-1
- Umezawa Y, Nobeyama Y, Hayashi M, et al. Drug survival rates in patients with psoriasis after treatment with biologics. J Dermatol. 2013;40(12):1008–1013. doi:10.1111/1346-8138.12353
- Warren RB, Smith CH, Yiu ZZ, et al. Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Investig Dermatol. 2015;135(11):2632–2640. doi:10.1038/jid.2015.208
- Damiani G, Conic RR, de Vita V, et al. When IL‐17 inhibitors fail: real‐life evidence to switch from secukinumab to Adalimumab or ustekinumab. Dermatol Ther. 2019;32(2):e12793. doi:10.1111/dth.12793
- Hu Y, Chen Z, Gong Y, et al. A review of switching biologic agents in the treatment of moderate-to-severe plaque psoriasis. Clin Drug Investig. 2018;38(3):191–199. doi:10.1007/s40261-017-0603-3
- Damiani G, Conic RR, Pigatto PD, et al. From randomized clinical trials to real life data. An Italian clinical experience with ixekizumab and its management. Dermatol Ther. 2019;32(3):e12886. doi:10.1111/dth.12886
- Pal D, Dasgupta S, Kundu R, et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012;18(8):1279–1285. doi:10.1038/nm.2851
- Jung TW, Youn B-S, Choi HY, et al. Salsalate and adiponectin ameliorate hepatic steatosis by inhibition of the hepatokine fetuin-A. Biochem Pharmacol. 2013;86(7):960–969. doi:10.1016/j.bcp.2013.07.034
- Öner-iyidoğan Y, Kocak H, Seyidhanoğlu M, et al. Curcumin prevents liver fat accumulation and serum fetuin-A increase in rats fed a high-fat diet. J Physiol Biochem. 2013;69(4):677–686. doi:10.1007/s13105-013-0244-9
- Price PA, Williamson MK, Nguyen TMT, et al. Serum levels of the fetuin-mineral complex correlate with artery calcification in the rat. J Biol Chem. 2004;279(3):1594–1600. doi:10.1074/jbc.M305199200
