Abstract
Introduction
Autophagy is an important process for maintaining intracellular homeostasis and is deregulated in ultraviolet B (UVB)-induced skin injury. Salidroside (SAL) is an active ingredient extracted from Rhodiola rosea, which is a herbal medicine that has shown protection against ultraviolet (UV) radiation. Here, we investigated the functions and mechanisms of SAL on UVB-induced skin cell oxidative damage and autophagy.
Methods
Human immortalized keratinocyte cell line HaCaT was used as a cell model of UV injury. HaCaT cells were exposed to UVB irradiation and then incubated with SAL to investigate cell viability, lactate dehydrogenase (LSD) in culture media, intracellular reactive oxygen species (ROS) level, oxidative stress, autophagy, and regulatory effects on SIRT1 protein.
Results
SAL pretreatment (25, 50 and 100 μM) increased cell viability and inhibited LDH release in UVB-challenged HaCaT cells. SAL (100 μM) significantly reduced intracellular ROS level and suppressed oxidative stress, with increased MDA content and increased SOD activity. In addition, SAL pretreatment enhanced autophagy in UVB-irradiated HaCaT cells, increased protein expressions of Beclin-1 and ATG7, and decreased protein expression of P62. We also found that pretreatment with SAL increased the SIRT1 protein in irradiated HaCaT cells. SAL protected UVB-induced damage in a dependent manner on autophagy and SIRT1, as SAL-induced increase in viability was significantly attenuated by specific autophagy inhibitor Wortmannin (1 μM) or SIRT1 inhibitor EX-527 (100 nM).
Discussion
The present study results speculate that SAL suppresses UVB-induced injury and autophagy by enhancing SIRT1 expression.
Introduction
Skin is a self-regulated protective barrier organ designed to maintain the homeostasis when exposed to environmental agents. Keratinocytes are an important component of the epidermal structure of human skin and play a key role in the construction and function of the epidermis, thus protecting the body from external invasion. Sunlight is the main source of ultraviolet (UV) radiation, among which UVB (320–280 nm) is the most destructive UV type and can reach the earth’s surface.Citation1 UVB primarily affects the epidermis of the skin and is considered a major risk factor that leads to the formation of free radicals, acute inflammation and increased risk of skin cancer.Citation2 Long-term UVB radiation can lead to oxidative stress damage, promote cell aging (photoaging),Citation3 produce signs of premature skin aging, such as pigmentation spots, deep wrinkles, and increased risk of skin cancer.Citation4 UVB directly induces DNA damage by promoting the formation of cyclobutane pyrimidine dimer. When the DNA damage exceeds the removal ability of nucleotide excision repair (NER), a large number of mutations are produced in normal skin exposed to sunlight, resulting in cell death, photoaging and cancer.Citation5,Citation6
Autophagy is an evolutionarily conserved lysosomal degradation process in which damaged organelles are engulfed by autophagosomes and delivered to lysosome degradation. Autophagy is a biological process that maintains homeostasis when cells undergo stress conditions such as starvation, oxidation damage, inflammatory response, immune response, etc, thus promoting cell survival. Activation of autophagy under various stresses can effectively clear intracellular aggregates and damaged organelles, thereby promoting survival.Citation7 DNA damage and genotoxic stress can also induce autophagy, leading to cytoprotective effects.Citation8 In keratinocytes irradiated with ultraviolet light, the autophagy response of the cells is inhibited.Citation9 In contrast, promoting cell autophagy can protect keratinocytes from UV-induced cell death.Citation10 Therefore, in UV-induced skin keratinocytes, cell autophagy plays a protective role, inhibits DNA damage and cell senescence, and thus prevents skin cancer.Citation11,Citation12
Salidroside (SAL) is the active component of Rhodiola rosea, which has shown protectection against premature senescence in human dermal fibroblasts and inhibit melanogenesis in guinea pig skin induced by UVB,Citation13,Citation14 and prevent chemical-induced skin cancer in mice by inhibiting inflammatory response.Citation15 In UV-irradiated keratinocytes, SAL can inhibit inflammatory response and oxidative stress.Citation16,Citation17
In this study, we investigated how SAL treatment regulates autophagy in UVB-induced human keratinocytes. Meanwhile, the expression of autophagy-related genes, such as Beclin 1, ATG7, P62 and SIRT1, was quantitatively analyzed. Furthermore, we investigated the roles of autophagy and SIRT1 in the protective effect of SAL in UVB-irradiated keratinocytes.
Materials and Methods
Cell Culture
The immortalized human epidermal keratinocyte cell line HaCaT cells (Chinese Academy of Sciences, Shanghai, China) were cultured in low glucose DMEM supplemented with 10% FBS (Gibco, NY, USA) at 37°C and 5% CO2. The cells (70–80% confluent) were pretreated with SAL (0–200 μM, 43,866, Sigma-Aldrich, Germany) for 2 h, then the media were removed and added with fresh phosphate buffer saline (PBS). These cells were then irradiated with UVB.
UVB Radiation
Mono layer of HaCaT cells reached 70–80% confluence and were washed with PBS. Then, the cells were rinsed with a thin layer of PBS and pretreated with SAL for 2 h and exposed to UVB by a UV fluorescent lamp (JCB35-24-01, Sigma, Shanghai, China) (range: 280–315 nm; peak intensity: 312 nm) at doses of 10, 25 and 50 mJ/cm2, respectively. During the irradiation, the plate of control cells was shielded and the medium was removed and then covered with a thin layer of PBS.Citation18 Then, the cells were cultured in fresh medium containing SAL for further 24 h.
Measurement of Cell Viability
Cell viability was assessed using an MTT assay (M2128, Sigma-Aldrich, Germany). HaCaT cells were seeded in 96-well plates at a density of 5×104 cell/well. After overnight growth, cells were pretreated with SAL for 2 h and exposed to UVB. In some experiments, cells were also co-incubated with Wortmannin (HY-10197, MedChemExpress) or EX-527 (HY-15452, MedChemExpress). Cells were washed with PBS and incubated with MTT solution (0.5 mg/mL, 20 µL/well) for 4 h at 37°C. Then, the supernatant was removed and DMSO (100 µL/well) was added to each well to dissolve the formazan crystals, and the absorbance was measured at 570 nm of optical density (OD) using a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Cell viability was calculated as the percentage of viable cells of the treated cells versus untreated cells.Citation19 Experiment was performed in triplicates and repeated at least three times.
LDH Release Assay
Cell injury was evaluated by measuring the release of LDH in the extracellular fluid using a commercial kit (A020-3, Nanjing Jiancheng, Nanjing, China). HaCaT cells were treated by SAL and UVB for indicated hours, then 0.2 mL culture medium was collected, and the OD of 490 nm was measured to determine the amount of LDH by spectrophotometry (U/dL).Citation18 Experiment was performed in triplicates and repeated at least three times.
Intracellular ROS Level Evaluation
Intracellular ROS levels were determined with the fluorescent indicator DCFH-DA (Sigma-Aldrich). DCFH-DA diffuses into cells and changes into DCFH, which reacts with ROS to form the DCF (green fluorescent). After HaCaT cells were seeded on top of cover slips in 24-well plates and treated by SAL and UVB, they were washed with PBS and then incubated with DCFH-DA (10 μM) in the dark for 30 min, and the intracellular DCFH fluorescence was determined using a fluorescence microscope (IX71, Olympus, Japan) (emission wavelength: 520 nm; excitation wavelength: 488 nm).Citation20 Experiment was performed in triplicates and repeated at least three times.
Oxidative Stress
MDA (A003-1, Nanjing Jiancheng) content and SOD (A001-1, Nanjing Jiancheng) activity in HaCaT cells were measured at 532 and 520 nm absorbance by a microplate reader and were expressed as nmol/mg protein and U/mg protein, respectively. Experiment was performed in triplicates and repeated at least three times.
Immunofluorescence
HaCaT cells were plated on cover slips and pretreated with SAL and exposed to UVB irradiation (25 mJ/cm2) for further 24 h. Cultures were fixed with 4% paraformaldehyde (pH 7.4) for 20 min, and blocked with 1% BSA and permeabilized with 0.1% Triton-X-100 for 10 min. Then, the cells were incubated with anti-LC3-II antibody (AF4650, Affinity Biosciences, China) at 4°C overnight, followed by FITC-conjugated secondary antibody for 1 h. After washing with PBS, cells were treated with DAPI (10 mg/mL) and photomicrographs were taken with a fluorescence microscope (IX71). The number of LC3-II-positive and DAPI-positive cells was counted, and of percentage LC3-II-positive cells (normalized to DAPI-positive cells) was calculated. At least 100 DAPI-positive cells were counted in each group.Citation21 Experiment was performed in triplicates and repeated at least three times.
Western Blotting
Total protein was extracted from HaCaT cells using RIPA extraction reagents (Solarbio, Beijing, China). Protein sample (50 μg) was separated with sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). After transferring to a PVDF membrane (Millipore, Bedford, MA), samples were incubated with specific primary antibodies (diluted 1:200): Beclin-1 (sc-48341, Santa Cruz Biotechnology, USA), ATG7 (sc-376212), P62 (sc-28359) and SIRT1 (sc-74504) at 4°C overnight. After PBS washing, the membrane was incubated with HRP-labeled secondary antibody (1:500). The density of the protein bands was visualized by ECL (Thermo, Waltham, MA, USA), analyzed using ImageJ software, and normalized to β-actin.
Statistical Analysis
All values are presented as mean ± standard deviation (SD) from at least three experiments, and analyzed by SPSS 20.0 statistical software (SPSS, Inc., Chicago, IL, USA). One-way ANOVA and Student–Newman–Keuls (SNK) test were applied to compare the differences between three or more groups. P<0.05 was considered as statistical significance.
Results
SAL Protected UVB Irradiation-Induced Injury of Keratinocytes
Human keratinocytes (HaCaT Cells) were exposed to UVB irradiation (0, 10, 25, 50 mJ/cm2) for 24 h. Cytotoxic character of UVB was examined by MTT assay. As expected, cell viability was decreased by UVB irradiation in a concentration-dependent manner (P<0.05) (). We investigated the effects of SAL (0–200 μM) against UVB-mediated decreases in cell viability. SAL showed no obvious toxic effect on HaCaT cells from 0 to 100 μM, and produced mild toxicity at 200 μM (P<0.05) (). We have taken 25, 50 and 100 μM of SAL for HaCaT cells with UVB (25 mJ/cm2). The UVB-induced reduction in cell viability was significantly attenuated by the pretreatment of cells with SAL in a dose-dependent manner (). We then detected LDH release into the culture medium from HaCaT cells. SAL pretreatment significantly decreased LDH content (P<0.05) (). Since SAL demonstrated greatest cell viability and least LDH release at 100 μM concentrations, we have taken 100 μM of SAL for optimum dose of further study.
Figure 1 Effect of SAL on cell viability of HaCaT cells with UVB. (A) UVB induces cytotoxicity in HaCaT cells. (B) Cell viability of HaCaT cells was not influenced by SAL. (C) SAL prevents UVB-induced cytotoxicity. (D) SAL inhibits LDH release from HaCaT cells. ANOVA was performed for analysis. Data are shown as the mean ± SD of three independent experiments. *P<0.05, ***P<0.001 vs control group; ##P<0.01, ###P<0.001 vs UVB group.
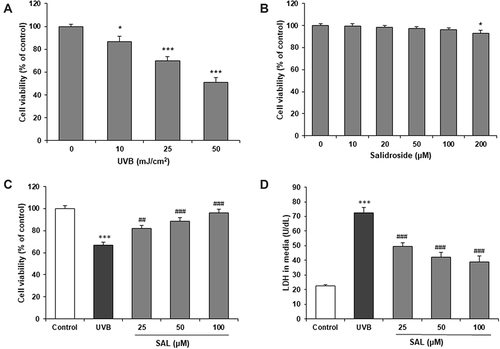
SAL Suppressed UVB-Induced Oxidative Stress
ROS levels were detected by DCFH-DA staining. Microscopical images showed widely augmented green fluorescence intensity in UVB-irradiated HaCaT cells, and SAL pretreatment attenuated the intracellular ROS production (). The DCFH-DA intensity of HaCaT cells was significantly increased by UVB, which was significantly attenuated by pretreatment with SAL (P<0.05) (). SAL also markedly reversed UVB irradiation-induced oxidative stress, with reduced MDA content and increased SOD activity comparted to cells with UVB alone (both P<0.05) ( and ).
Figure 2 Effect of SAL on oxidative injury in HaCaT cells. (A) Intracellular ROS production was detected by DCFH-DA staining, which shows green fluorescence (200×). (B) DCFH-DA fluorescence intensity is reduced by SAL in UVB-induced HaCaT cells. MDA content (C) and SOD activity (D) were measured to evaluate the oxidative stress. Data are shown as the mean ± SD of three independent experiments. ***P<0.001 vs control group; ###P<0.001 vs UVB group.
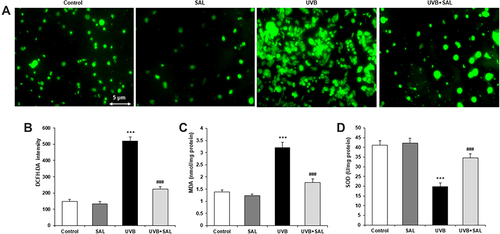
SAL Promoted Autophagy in UVB-Irradiated Human Keratinocytes
To evaluate the regulation of SAL on autophagy in UVB-irradiated keratinocytes, HaCaT cells were stained with LC3-II antibody. Autophagosomes were visualized by the yellow images, which were obtained from merging green puncta (LC3-II) with blue puncta (DAPI). Autophagy was suppressed by UVB irradiation in HaCaT cells. SAL enhanced autophagy and showed yellow fluorescence in HaCaT cells (). The percentage of LC3-II-positive cells was significantly higher in cells treated with SAL and UVB compared to cells with UVB alone (). Western blot was carried out to determine the protein expression of Beclin-1, ATG7 and P62 (). SAL prominently enhanced the Beclin-1 and ATG7 protein expressions ( and ). Also, SAL reduced the P62 protein expression ().
Figure 3 Effect of SAL on autophagy and related proteins in HaCaT cells. (A) HaCaT cells of control, UVB and/or SAL were stained with LC3-II antibody (green, ×200). (B) SAL significantly increases the number of cells with LC3-II dots. (C) Representative blots of autophagy-related proteins by Western blot analysis. SAL significantly increases the protein expression of Beclin-1 (D), ATG7 (E) and decreases the protein expression of P62 (F). Data are shown as the mean ± SD of three independent experiments. *P<0.05, **P<0.01, ***P<0.001 vs control group; ###P<0.001 vs UVB group.
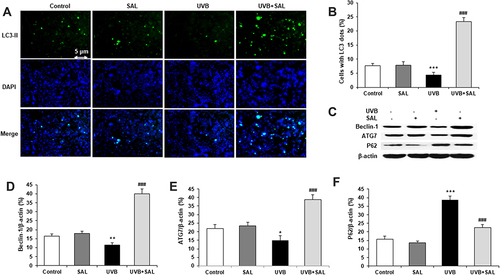
SAL Attenuated UVB-Induced Cell Injury by Up-Regulation of SIRT1 and Autophagy in in Human Keratinocytes
The protein expression of SIRT1 was determined by Western blot. UVB irradiation stimulated an decrease in expression of SIRT1 in HaCaT cells, but SAL pretreatment reversed the decrease in SIRT1 protein ( and ). To further investigate the functional role of autophagy and SIRT1 pathway in SAL-treated HaCaT cells, cells were pretreated with the autophagy inhibitor, Wortmannin (1 μM), or SIRT1 inhibitor EX-527 (100 nM), and then incubated with SAL and UVB irradiation. The results demonstrated that the protective effect of SAL (increased cell viability) was markedly reversed by the inhibition of autophagy or SIRT1 in UVB-irradiated HaCaT cells ( and ).
Figure 4 SIRT1 and autophagy involve{{?odel?}}s{{?cdel?}} the protective effects of SAL in UVB-irradiated HaCaT cells. (A) Representative blots of SIRT1 protein. (B) Quantification of Western blot for SIRT1 protein. Cells were pretreated with the autophagy inhibitor, Wortmannin (1 μM) (C), or SIRT1 inhibitor EX-527 (100 nM) (D). Data are shown as the mean{{?odel?}}s{{?cdel?}} ± SD of three independent experiments. ***P<0.001 vs control group; ##P<0.01, ###P<0.001 vs UVB group;{{?oins?}} $P<0.001 vs UVB + SAL group.{{?cins?}}{{?odel?}} {{?cdel?}}{{?odel?}}$P<0.001 vs UVB + SAL group.{{?cdel?}}
Discussion
In the present study, we explored the protective effect of SAL on UVB-exposed HaCaT. UVB irradiation reduced cell viability of HaCaT cells, which was attenuated by pretreatment with SAL. In HaCaT cells exposed to UVB irradiation, SAL suppressed intracellular ROS production and oxidative stress, enhanced autophagy and modulated autophagy-related protein expressions, including Beclin-1, ATG7 and P62. SAL reversed the decrease in protein expression of SIRT1 by UVB irradiation. Autophagy inhibitor Wortmannin or SIRT1 inhibitor EX-527 could abolish the increase in viability by SAL. Taken together, SAL protects HaCaT cells against UVB irradiation-induced injury by enhancing autophagy and SIRT1 expression.
In our results, UVB irradiation decreased viability () and increased ROS generation () in HaCaT cells. This suggests that UVB induces oxidative injury in HaCaT cells and causes cell death. Thus, ROS-derived oxidative injury could simulate photoaging of keratinocytes and risk of skin cancer. ROS is an important mediator for photoaging of keratinocytes.Citation22 Numerous antioxidants can protect keratinocytes against UV damage.Citation23 In addition, our previous study has shown that astragaloside IV has inhibitory effect on oxidative stress, and suppressed apoptosis in HaCaT cells with UVB-irradiation.Citation24 This study added SAL as another agent with suppressive effect on oxidative injury in HaCaT cells. These effects may be related to decreased apoptosis and enhanced Nrf2 expression by SAL.Citation17
Oxidative stress has been proved to play an important role in the pathogenesis of many diseases, in which free radicals such as reactive oxygen species (ROS) are the focus and target of disease prevention and treatment. At present, natural products with antioxidant activity are widely used to prevent and treat various systemic diseases caused by oxidative stress, such as Rhodiola rosea or its active ingredient SAL. In diabetes, glucose oxidation and lipid oxidation under hyperglycemia lead to excessive production of free radicals, imbalance of oxidation and antioxidant system, resulting in oxidative stress. Studies have shown that SAL shows antioxidant effects in different diabetes models. SAL enhanced the activity of SOD and reduced the content of MDA in blood samples of diabetic mice.Citation25 In addition, SAL improved hyperglycemia and alleviate oxidative stress in db/db mice induced by high-fat diet, accompanied by increased β-cell mass and β-cell replication.Citation26 SAL shows antioxidant effect on cardiomyocytes, which can significantly reduce the inhibitory effect of H2O2 on the growth of H9c2 cells, and reduce the cellular levels of ROS and MDA, and improve the activities of SOD and catalase (CAT).Citation27 Atherosclerosis is a chronic vascular inflammatory disease associated with oxidative stress. In an in vitro model of human umbilical vein endothelial cells (HUVEC) induced by oxidized low-density lipoprotein (ox-LDL), SAL treatment protected HUVEC from damage by inhibiting oxidative stress and improving mitochondrial dysfunction,Citation28 and protected against ox-LDL-induced endothelial injury and oxidative stress by enhancing autophagy mediated by SIRT1-FoxO1 pathway.Citation29 Taken together, SAL can improve diabetes and cardiovascular diseases through antioxidant effect, and the specific mechanisms are related to reduction of ROS production, inhibition of cell death, and enhanced autophagy. This study confirmed that SAL can reduce the keratinocyte damage induced by ultraviolet radiation by inhibiting oxidative stress. The specific mechanism of SAL regulating oxidative stress is still unclear and needs to be further investigated.
In the present study, SAL enhanced autophagy in UVB-irradiated HaCaT cells ( and ), which was validated by modulation of Beclin-1, ATG7 and P62 proteins ( and ). Beclin-1 is a key regulator of autophagy and endocytosis and involves the maintenance of epidermal barrier.Citation30 ATG7 expression was reduced in keratinocytes irradiated with ultraviolet.Citation9 The expression of p62 protein was downregulated in autophagy, and p62 protein was increased by an autophagy inhibitor 3-MA in HaCaT cells.Citation31 Our results show that UVB irradiation inhibited autophagy of HaCaT cells and SAL enhanced autophagy in UVB-irradiated HaCaT cells. SAL can modulate autophagy in various cell types. In human gastric cancer cells, SAL induced apoptosis and enhanced autophagy, and an autophagy inhibitor chloroquine enhanced SAL-induced apoptosis.Citation32 This indicates SAL-induced autophagy is protective, and this hypothesis is supported by the result that cell viability was decreased by specific autophagy inhibitor Wortmannin in HaCaT cells with SAL (). In addition, the effect of protective autophagy by SAL is also validated in other cell models. For instance, SAL enhanced autophagy and protected from cell death of neuronal and endothelial cells.Citation33,Citation34 Autophagy is capable of delaying the skin photoaging process caused by ultraviolet, thus acts as inhibitory process against skin cancer.Citation35 Autophagy activation mediated prevention of human keratinocytes from oxidative stress and cell death by an antioxidant.Citation36 On the contrary, keratinocytes with autophagy deficiency showed increased DNA damage and senescence in the condition of oxidative stress.Citation11
In this study, SAL increased SIRT1 protein expression ( and ), which was associated with the increase in cell viability by SAL ( and ). SIRT1 is a deacetylase protein and regulates various pathways in metabolism, aging and cancer. SIRT1 is expressed in keratinocytes, but its expression level was decreased by UV radiation and hydrogen peroxide exposure in an ROS-dependent manner.Citation37 SIRT1 expression or activation could enhance autophagy flux and protect human keratinocytes against hydrogen peroxide-induced damages.Citation38 This suggests that SAL enhances SIRT1 expression through inhibition of intracellular ROS and there might be an ROS-SIRT1-autophagy axis in keratinocytes. SAL enhanced autophagy through upregulation of SIRT1 expression and inhibition of oxidative stress in ox-LDL-induced endothelial injury.Citation29 SIRT1 activation-dependent autophagy was observed in UVB irradiation-induced skin aging human dermal fibroblasts.Citation39 Whether SAL also modulate this ROS-SIRT1-autophagy axis in keratinocytes is unclear.
SAL exhibits photoprotective effects in UVB-treated keratinocytes. In addition, SAL-regulated autophagy might involve the UVB effects on SIRT1. However, we could not clarify whether SAL regulates other signaling pathways in cultured and UVB-challenged keratinocytes. SIRT3 is a homologous protein of SIRT1, and increased protein of SIRT3 was associated with induction of autophagy and reduction of senescence and tissue damage in UV-irradiated mouse skin.Citation40 SAL can also increase protein expression of SIRT3 and attenuate endothelial cellular senescence and diabetic cardiomyopathy.Citation41,Citation42 Nrf2 is an antioxidant transcription factor, and its activation and nuclear translocation resulted in expression of antioxidant proteins in HaCaT cells, and is associated with reduced ROS production.Citation43 Nrf2 is also linked to autophagy regulation in keratinocytes by regulating Nrf2-p62 pathway.Citation44 SAL treatment upregulated Nrf2 nuclear translocation and transcription activity and reduced ROS production in HaCaT cells.Citation17 However, whether Nrf2 activation is associated with HaCaT autophagy is unclear. p38 MAPK pathway also regulated autophagy in HaCaT cells and UVB-induced photoaging mice.Citation45 SAL can also downregulate p38 protein expression and inhibit oxidative stress in lung cancer cells,Citation46 whether SAL regulates p38 in keratinocytes is unknown. The mechanism that mediates novel signal pathways by SAL in keratinocytes with UVB damage remains to be elucidated.
In conclusion, we report that SAL exhibits novel photoprotective effects in UVB-treated keratinocytes, such as inhibiting apoptosis, enhancing restoring autophagy, reducing oxidative stress, and upregulating SIRT1 protein. These findings suggest the potential application of SAL as an agent against skin photodamage. However, this was an in vitro study and its effects on photodamage need to be confirmed in further investigations in vivo. These findings suggest that SAL could induce protective autophagy, which might act as a photoprotective potential agent for preventing skin cancer.
Data Sharing Statement
The data in support of the results are available from the corresponding author on reasonable request.
Consent for Publication
All authors agreed to publish this manuscript.
Author Contributions
JK performed experiments and wrote the manuscript; JW performed experiments and collected data; XW analyzed the data and performed the statistical analysis; YHY designed and supervised the study, and revised the manuscript. All authors have read and approved the manuscript. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors declare that they have no conflicts of interest in this work.
Additional information
Funding
References
- Widel M, Krzywon A, Gajda K, Skonieczna M, Rzeszowska-Wolny J. Induction of bystander effects by UVA, UVB, and UVC radiation in human fibroblasts and the implication of reactive oxygen species. Free Radic Biol Med. 2014;68:278–287.
- Uco DP, Leite-Silva VR, Silva HDT, Duque MD, Grice J, Mathor MB. UVA and UVB formulation phototoxicity in a three-dimensional human skin model: photodegradation effect. Toxicol in Vitro. 2018;53:37–44.
- Kim HK. Protective Effect of Garlic on Cellular Senescence in UVB-exposed HaCaT human keratinocytes. Nutrients. 2016;8:pii: E464.
- Naylor EC, Watson RE, Sherratt MJ. Molecular aspects of skin ageing. Maturitas. 2011;69:249–256.
- Liu-Smith F, Jia J, Zheng Y. UV-induced molecular signaling differences in melanoma and non-melanoma skin cancer. Adv Exp Med Biol. 2017;996:27–40.
- Schuch AP, Moreno NC, Schuch NJ, Menck CFM, Garcia CCM. Sunlight damage to cellular DNA: focus on oxidatively generated lesions. Free Radic Biol Med. 2017;107:110–124.
- Tan Q, Wang M, Yu M, Zhang J, Bristow RG, Hill RP. Role of autophagy as a survival mechanism for hypoxic cells in tumors. Neoplasia. 2016;18:347–355.
- Chen LH, Chu PM, Lee YJ, Tu PH, Chi CW, Lee HC. Targeting protective autophagy exacerbates UV-triggered apoptotic cell death. Int J Mol Sci. 2012;13:1209–1224.
- Chen X, Li L, Xu S, Bu W, Chen K, Li M. Ultraviolet B radiation down-regulates ULK1 and ATG7 expression and impairs the autophagy response in human keratinocytes. J Photochem Photobiol B. 2018;178:152–164.
- Mou K, Liu W, Han D, Li P. HMGB1/RAGE axis promotes autophagy and protects keratinocytes from ultraviolet radiation-induced cell death. J Dermatol Sci. 2014;1:85.
- Song X, Narzt MS, Nagelreiter IM, Hohensinner P, Terlecki-Zaniewicz L, Tschachler E. Autophagy deficient keratinocytes display increased DNA damage, senescence and aberrant lipid composition after oxidative stress in vitro and in vivo. Redox Biol. 2017;11:219–230.
- Sun FD, Wang PC, Shang J, Zou SH, Du X. Ibrutinib presents antitumor activity in skin cancer and induces autophagy. Eur Rev Med Pharmacol Sci. 2018;22:561–566.
- Mao GX, Xing WM, Wen XL, et al. Salidroside protects against premature senescence induced by ultraviolet B irradiation in human dermal fibroblasts. Int J Cosmet Sci. 2015;37:321–328.
- Peng LH, Xu SY, Shan YH, et al. Sequential release of salidroside and paeonol from a nanosphere-hydrogel system inhibits ultraviolet B-induced melanogenesis in Guinea pig skin. Int J Nanomedicine. 2014;16:1897–1908.
- Kong YH, Xu SP. Salidroside prevents skin carcinogenesis induced by DMBA/TPA in a mouse model through suppression of inflammation and promotion of apoptosis. Oncol Rep. 2018;39:2513–2526.
- Wu D, Yuan P, Ke C, et al. Salidroside suppresses solar ultraviolet-induced skin inflammation by targeting cyclooxygenase-2. Oncotarget. 2016;7:25971–25982.
- Yuan XY, Pang XW, Zhang GQ, Guo JY. Salidroside’s protection against UVB-mediated oxidative damage and apoptosis is associated with the upregulation of Nrf2 expression. Photomed Laser Surg. 2017;35:49–56.
- Lin S, Li L, Li M, Gu H, Chen X. Raffinose increases autophagy and reduces cell death in UVB-irradiated keratinocytes. J Photochem Photobiol B. 2019;201:111653.
- Muzaffer U, Paul VI, Prasad NR, Karthikeyan R, Agilan B. Protective effect of Juglans regia L. against ultraviolet B radiation induced inflammatory responses in human epidermal keratinocytes. Phytomedicine. 2018;42:100–111.
- Chen F, Tang Y, Sun Y, Veeraraghavan VP, Mohan SK, Cui C. 6-shogaol, a active constituents of ginger prevents UVB radiation mediated inflammation and oxidative stress through modulating NrF2 signaling in human epidermal keratinocytes (HaCaT cells). J Photochem Photobiol B. 2019;197:111518.
- Tang ZL, Zhang K, Lv SC, Xu GW, Zhang JF, Jia HY. LncRNA MEG3 suppresses PI3K/AKT/mTOR signalling pathway to enhance autophagy and inhibit inflammation in TNF-α-treated keratinocytes and psoriatic mice. Cytokine. 2021;148:155657.
- Smirnov A, Panatta E, Lena A, Castiglia D, Di Daniele N, Melino G. FOXM1 regulates proliferation, senescence and oxidative stress in keratinocytes and cancer cells. Aging. 2016;8:1384–1397.
- Salucci S, Burattini S, Buontempo F, Martelli AM, Falcieri E, Battistelli M. Protective effect of different antioxidant agents in UVB-irradiated keratinocytes. Eur J Histochem. 2017;61:2784.
- Wang J, Ke J, Wu X, Yan Y. Astragaloside prevents UV-induced keratinocyte injury by regulating TLR4/NF-κB pathway. J Cosmet Dermatol. 2022;21:1163–1170.
- Li F, Tang H, Xiao F, Gong J, Peng Y, Meng X. Protective effect of salidroside from Rhodiolae Radix on diabetes-induced oxidative stress in mice. Molecules. 2011;16:9912–9924.
- Ju L, Wen X, Wang C, et al. Salidroside, a natural antioxidant, improves β-cell survival and function via activating AMPK pathway. Front Pharmacol. 2017;8:749.
- Gao H, Liu X, Tian K, Meng Y, Yu C, Peng Y. Insight into the protective effect of salidroside against H2O2-induced injury in H9C2 cells. Oxid Med Cell Longev. 2021;1060271:2021.
- Zhao D, Sun X, Lv S, et al. Salidroside attenuates oxidized low‑density lipoprotein‑induced endothelial cell injury via promotion of the AMPK/SIRT1 pathway. Int J Mol Med. 2019;43:2279–2290.
- Zhu Z, Li J, Zhang X. Salidroside protects against ox-LDL-induced endothelial injury by enhancing autophagy mediated by SIRT1-FoxO1 pathway. BMC Complement Altern Med. 2019;19:111.
- Noguchi S, Honda S, Saitoh T, et al. Beclin 1 regulates recycling endosome and is required for skin development in mice. Commun Biol. 2019;2:37.
- Yang Z, Zeng B, Pan Y, Huang P, Wang C. Autophagy participates in isoliquiritigenin-induced melanin degradation in human epidermal keratinocytes through PI3K/AKT/mTOR signaling. Biomed Pharmacother. 2018;97:248–254.
- Rong L, Li Z, Leng X. Salidroside induces apoptosis and protective autophagy in human gastric cancer AGS cells through the PI3K/Akt/mTOR pathway. Biomed Pharmacother. 2020;122:109726.
- Chen S, Cai F, Wang J, et al. Salidroside protects SH‑SY5Y from pathogenic α‑synuclein by promoting cell autophagy via mediation of mTOR/p70S6K signaling. Mol Med Rep. 2019;20:529–538.
- You L, Zhang D, Geng H, Sun F, Lei M. Salidroside protects endothelial cells against LPS-induced inflammatory injury by inhibiting NLRP3 and enhancing autophagy. BMC Complement Med Ther. 2012;21:146.
- Wang M, Charareh P, Lei X, Zhong JL. Autophagy: multiple mechanisms to protect skin from ultraviolet radiation-driven photoaging. Oxid Med Cell Longev. 2019;8135985:2019.
- Yoon JY, Jeon HO, Kim EJ, et al. Propofol protects human keratinocytes from oxidative stress via autophagy expression. J Dent Anesth Pain Med. 2017;17:21–28.
- Cao C, Lu S, Kivlin R, et al. SIRT1 confers protection against UVB- and H2O2-induced cell death via modulation of p53 and JNK in cultured skin keratinocytes. J Cell Mol Med. 2009;13:3632–3643.
- Lee JH, Moon JH, Nazim UM, et al. Melatonin protects skin keratinocyte from hydrogen peroxide-mediated cell death via the SIRT1 pathway. Oncotarget. 2016;7:12075–12088.
- Lim CJ, Lee YM, Kang SG, et al. Aquatide activation of SIRT1 Reduces cellular senescence through a SIRT1-FOXO1-autophagy axis. Biomol Ther (Seoul). 2017;25:511–518.
- Li YF, Ouyang SH, Tu LF, et al. Caffeine protects skin from oxidative stress-induced senescence through the activation of autophagy. Theranostics. 2018;8:5713–5730.
- Xing SS, Li J, Chen L, et al. Salidroside attenuates endothelial cellular senescence via decreasing the expression of inflammatory cytokines and increasing the expression of SIRT3. Mech Ageing Dev. 2018;175:1–6.
- Li Y, Wei X, Liu SL, Zhao Y, Jin S, Yang XY. Salidroside protects cardiac function in mice with diabetic cardiomyopathy via activation of mitochondrial biogenesis and SIRT3. Phytother Res. 2021;35:4579–4591.
- Yang HL, Lin CP, Vudhya Gowrisankar Y, et al. The anti-melanogenic effects of ellagic acid through induction of autophagy in melanocytes and suppression of UVA-activated α-MSH pathways via Nrf2 activation in keratinocytes. Biochem Pharmacol. 2021;185:114454.
- Yun CY, Choi N, Lee JU, et al. Marliolide derivative induces melanosome degradation via Nrf2/p62-mediated autophagy. Int J Mol Sci. 2021;22:3995.
- Gu Y, Xue F, Xiao H, Chen L, Zhang Y. Bamboo leaf flavonoids suppress oxidative stress-induced senescence of HaCaT cells and UVB-induced photoaging of mice through p38 MAPK and autophagy signaling. Nutrients. 2022;14:793.
- Wang J, Li JZ, Lu AX, Zhang KF, Li BJ. Anticancer effect of salidroside on A549 lung cancer cells through inhibition of oxidative stress and phospho-p38 expression. Oncol Lett. 2014;7:1159–1164.
