Abstract
Elevated levels of inflammatory mediators—including the interleukin IL-23—are implicated in the pathogenesis of pyoderma gangrenosum (PG), an autoinflammatory neutrophilic dermatosis characterized by rapidly enlarging, suppurative ulcers and cribriform scarring. Here, we present the first case report of significant response of isolated ulcerative PG with tildrakizumab, a biologic agent directed against the p19 subunit of IL-23, in an elderly woman with extensive treatment-refractory PG on her left leg. Tildrakizumab (100 mg subcutaneously at weeks 0 and 4, then every 8 weeks, and eventually increased in frequency to every 6 weeks), combined with acetic acid soaks each morning and chemical debridement every evening with 3% hydrogen peroxide, resulted in progressive decrease in ulcer size and depth, re-epithelialization, and recovery of sensory perception. This report describes the dramatic clinical response of ulcerative PG on the leg with tildrakizumab.
Introduction
Pyoderma gangrenosum (PG) is an autoinflammatory neutrophilic dermatosis with an overall prevalence of 5.8 cases per 100,000 adults.Citation1,Citation2 It is most common in women and individuals over 70 years of age and is characterized by rapidly enlarging, suppurative ulcers that heal with cribriform scarring.Citation2 Ulcerative PG, the most common subtype, classically manifests on the legs.Citation1
The management of PG is challenging, as there is no management guideline based on clinical evidence or expert consensus.Citation3,Citation4 Topical and/or systemic medical therapy, analgesia, compression, and diligent wound care are indicated.Citation1 Otherwise, treatment aims to minimize systemic inflammation, as the pathophysiology of PG—while multifactorial and poorly understoodCitation1,Citation5 is thought to involve inflammatory cytokines and signaling cascades including interleukin (IL)-23 and T-helper (Th)17 cell recruitment.Citation6,Citation7 However, there is no gold standard therapy, and more evidence is needed to establish a standardized treatment approach.
Tildrakizumab (ILUMYA®, Sun Pharmaceutical Industries, Inc., Princeton, NJ, USA), a biologic agent indicated for the treatment of moderate-to-severe plaque psoriasis, is a monoclonal antibody that neutralizes IL-23 overexpression by targeting the p19 subunit of IL-23 to inhibit binding to the IL-23 receptor (IL-23p19 inhibitor).Citation7 We present the first case report of treatment response to tildrakizumab of ulcerative PG of the leg previously unresponsive to standard therapies.
Case Report
An 85-year-old woman presented with extensive and severe sloughy, painful ulceration on most of her left leg. Her medical history included chronic skin ulcers since age 19, often secondary to trauma, and a family history of ulcerative cutaneous disease. In 2004, she developed an ulcer on the left shin that enlarged progressively and eventually extended to the calf. Unlike previous ulcers, it worsened despite attempts at debridement and daily wound care with normal saline and 10% povidone-iodine (Inadine™, Systagenix) dressings.
Initial wound swab cultures revealed moderate growth of ampicillin-, amoxicillin-, and penicillin-resistant Staphylococcus aureus, as well as tetracycline-resistant group B Streptococcus. After the patient was hospitalized for a urinary tract infection in August 2019 and the ulcer deteriorated, a subsequent wound swab culture confirmed Pseudomonas species. The patient recalled trialing a variety of oral antibiotics without success. Daily wound dressing changes were performed with gauze soaked with petrolatum or cadexomer iodine ointment (Iodosorb™, Smith & Nephew Healthcare, Watford, England, UK), povidone-iodine dressings, chlorhexidine acetate tulle (Bactigras™, Smith & Nephew Healthcare, Watford, England, UK), as well as silver and zinc oxide dressings. Surgical debridement was performed by scalpel.
Despite this treatment, the ulcer continued to worsen. A skin graft was considered but not performed due to potential complications involving the infected recipient site, and the patient also declined amputation. In January 2020, treatment was initiated with nonablative infrared laser (MLS Laser Therapy, Mphi 5 model, ASA Laser, Vicenza, Italy) 3 times a week and twice-daily wound cleansing with acetic acid soaks (household vinegar mixed with lukewarm boiled water approximating 0.3% acetic acid) for 30 minutes followed by exposure to natural sunlight for 10 minutes. The patient reported resolution of infection and significant improvement in the appearance of the wound with adjunctive non-ablative laser treatment from December 2019 to May 2020. This was ceased in June 2020 as no further benefit was apparent.
In March 2020, the patient was referred to a local dermatologist for ongoing ulcer management (). The clinical presentation fulfilled all major and minor PARACELSUS criteria except extreme pain. PG was diagnosed based on the patient’s total PARACELSUS score of 17, which indicates a high likelihood of PG, and differentiates PG from venous leg ulcers.Citation8 An excisional biopsy reported pseudoepitheliomatous hyperplasia, consistent with cellular proliferation from healing of the ulcer base. Lymphovascular involvement was absent. Histologic findings were confirmed by a senior dermatopathologist as consistent with PG.Citation9–11
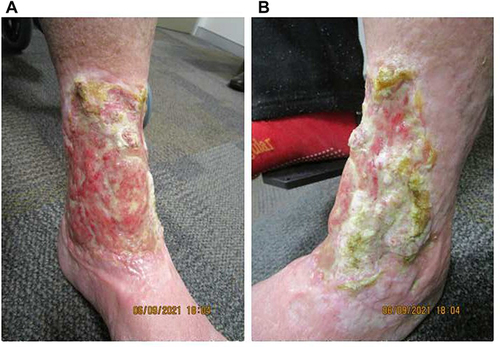
Figure 1 Ulcerative PG on the (A) anterolateral and (B) anteromedial aspects of the left leg on initial presentation, with extensive cribriform scarring and irregular overhanging borders.
The patient had severe derangement of renal function that precluded a trial of high-dose oral corticosteroid or cyclosporin. Treatment with 25 mg daily oral prednisolone and twice-daily topical betamethasone dipropionate ointment was initiated in March 2020. An adjunctive regimen of 400 mg oral pentoxifylline three times daily appeared to reduce venous stasis.
As systemic corticosteroid therapy did achieve significant improvement, in May 2020 oral dapsone 100 mg daily was commenced as the first steroid-sparing agent. The dose was reduced to 50 mg in June 2020 and subsequently discontinued due to treatment-emergent severe tremors. Oral cyclosporin 100 mg was commenced twice daily, with dose reduction to 50 mg twice daily due to severe known adverse effects in July 2020 followed by discontinuation in August due to limited efficacy. Blood investigations in late June 2020 revealed an erythrocyte sedimentation rate (ESR) of 81 mm/hour and a C-reactive protein (CRP) level of 69 mg/L.Citation12 Oral minocycline 100 mg daily was used in July 2020 as a supplementary anti-inflammatory agent following disruption to the supply of pentoxifylline in Australia.
In August 2020, subcutaneous tildrakizumab 100 mg was commenced, with a loading dose at week 4 followed by regular dosing every 8 weeks. Application of betamethasone dipropionate ointment continued daily following acetic acid soaks. Non-woven gauze and combine pads were applied overnight to absorb exudate. By October 20, both ulcer size and depth improved, with re-epithelialization (). Light touch and pain perception were regained over the ulcer, which was previously largely anaesthetic. Considering this encouraging response to treatment, the tildrakizumab dosage was increased by shortening administration intervals to 6 weeks. In November 2020, prednisolone was discontinued due to the patient’s history of bacterial infection, and pentoxifylline was resumed, replacing minocycline.
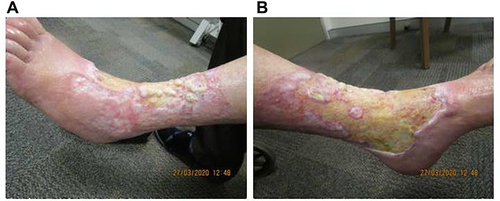
Figure 2 Re-epithelialization and reduction in ulcer size at week 8 of tildrakizumab treatment on the (A) medial and (B) anterolateral aspects of the left leg.
In December 2020, acetic acid soaks were ceased and the fibrinous floor of the ulcer became excessively thickened, hampering re-epithelization and resulting in heaped-up wound edges (). To address excess fibrin deposition, more rigorous daily wound care was initiated, including 3% hydrogen peroxide applied by non-woven gauze and deactivated by rinsing with water, complemented by fastidious daily debridement. The ulcer displayed noticeable re-epithelialization by January 2021. Simple debridement through daily wound care with betaine 0.1%/polyhexanide 0.1% solution (Prontosan® Wound Irrigation Solution, B. Braun) (BP) was initiated in late January 2021. The site was soaked in BP for 15–20 minutes each morning, followed by application of an antimicrobial silver dressing (Acticoat™ Flex 3, Smith & Nephew Healthcare). An outer dressing was added, comprising absorbent cellulose (Zetuvit® Plus, Hartmann AG) and combine pads. Over the following 6 weeks, the silver dressing was replaced every 3 days and reapplied during wound care on intervening days, along with BP. This regimen achieved dramatic clinical response by late February ().
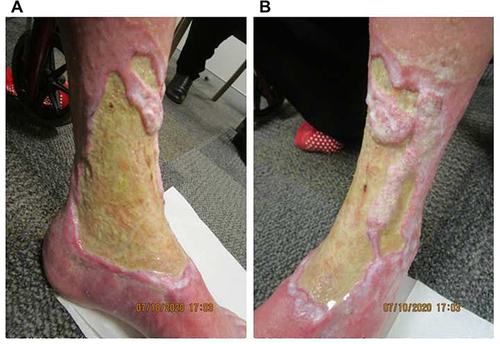
Figure 3 Excessive fibrin and thickening of the ulcer floor at week 18 of tildrakizumab treatment on the (A) medial and (B) anterolateral aspects of the left leg.
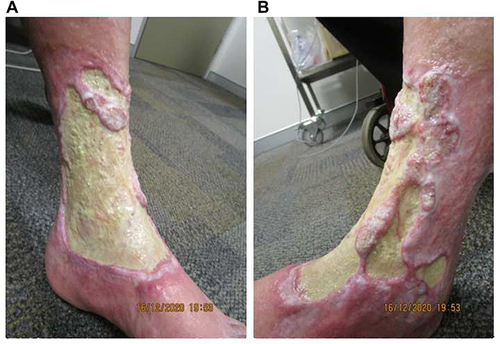
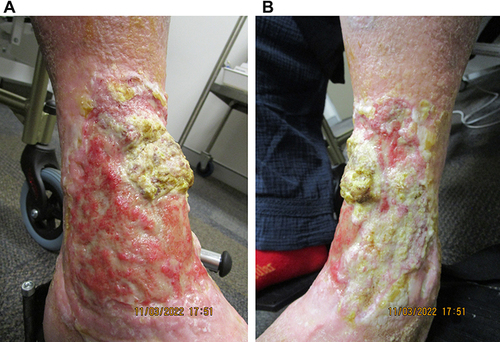
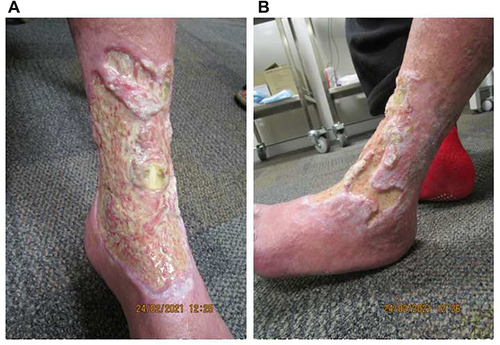
Figure 4 The underlying dermis at week 28 of tildrakizumab treatment, following judicious debridement on the (A) anteromedial and (B) anterolateral aspects of the left leg. The extensor digitorum longus tendon, previously obscured by thick fibrin, is exposed, flanked by prominent dermal proliferation.
In March 2021, a malodorous yellow-green slough suspicious for Pseudomonas aeruginosa infection was present. Oral ciprofloxacin 500 mg was administered empirically twice daily for a week, and the infection subsequently resolved. ESR and CRP levels in April 2021 were 99 mm/hour and 25 mg/L, respectively, comparing favorably with these parameters approximately a year earlier.
As of September 2021, the wound was healing progressively from its edges () upon continuation of this combination therapy of tildrakizumab injections, oral pentoxifylline, and wound care. Further re-epithelialization was observed as of the last follow-up in March 2022 (). No adverse effect has been reported to date.
Discussion
Several biologic agents targeting different cytokines have demonstrated at least some efficacy in PG.Citation6,Citation7,Citation13–18 Because PG involves cutaneous neutrophilic infiltration and IL-23 overexpression, IL-23p19 inhibition could reasonably be expected to result in clinical improvement.Citation6,Citation7 Significant improvement was observed in 2 patients, a 59-year-old woman with refractory leg PG and a 43-year-old woman with peristomal PG, following treatment with the IL-23p19 inhibitor risankizumab.Citation19,Citation20 Two other cases of ulcerative PG of the leg successfully resolved within 4 months of treatment with guselkumab, another IL-23p19 inhibitor.Citation21,Citation22 A single case of successful treatment of penile PG with tildrakizumab was previously described, while Murrell and Sheriff (2021) reported a case of recalcitrant PG responsive to tildrakizumab and ustekinumab combination therapy.Citation23 However, this is the first report of positive response to tildrakizumab monotherapy in recalcitrant ulcerative leg PG.
PG also presents as part of the neutrophilic autoinflammatory syndromes that feature constellations of disparate skin sites and manifestations, including pyogenic arthritis and acne (PAPA); acne and hidradenitis suppurativa (HS) (PASH); pyogenic arthritis, acne, and HS (PAPASH); and synovitis, acne, pustulosis, hyperostosis, and osteitis (SAPHO).Citation24,Citation25 In a case of PASH with an exacerbation of HS and leg PG following long-term stabilization on oral prednisolone, commencement of tildrakizumab enabled prednisolone dosage taper, although the PG component did not resolve.Citation26 PG and HS can also present concurrently. Tildrakizumab has been useful in HS,Citation27 where overexpression of IL-23 and bacterial biofilm formation may contribute significantly to pathogenesis.Citation28,Citation29 Importantly, our patient did not have PG as part of these syndromes.Citation26–30
The presence of slough and nonviable tissue (in PG), as well as microbiome dysregulation (in PG and HS), may promote biofilm formation. This is associated with impaired epithelialization and the formation of granulation tissue, triggering an inflammatory response that impedes wound healing.Citation31–34 We postulate that tildrakizumab may also have a secondary effect on biofilm colonies in PG. S. aureus may trigger Th1/Th17 responses,Citation35,Citation36 which are associated with biofilm formation and could exacerbate chronic infection.Citation36,Citation37 As IL-23 is required for the maintenance of Th17 cells,Citation36,Citation38 inhibition of IL-23 by tildrakizumab may additionally suppress these inflammatory host responses and hinder biofilm development in PG.
Interestingly, this patient experienced a marked improvement with acetic acid soaks, which may have helped prevent P. aeruginosa biofilm development.Citation39 Diluted acetic acid is also effective against S. aureus and group B Streptococcus.Citation40,Citation41 Hydrogen peroxide, potassium permanganate, povidone-iodine, and silver nitrate soaks have all been reported useful as part of the conservative management of PG.Citation29,Citation42–45 Debridement by laser ablation has a role, and there is anecdotal evidence that nonablative laser is also beneficial.Citation46–48 In our case, clinical improvement plateaued after several months of supportive treatment with this modality. Another option to consider would have been negative pressure wound therapy in combination with the above systemic anti-inflammatory and immunosuppressive therapies.Citation4
Over the course of treatment, excess fibrin deposition from inadequate debridement was the predominant factor hindering re-epithelialization.Citation49–51 In PG, avoidance of overzealous debridement is recommended due to the risk of pathergy.Citation52 However, judicious debridement removes necrotic tissue and fibrin, which in turn facilitates re-epithelialization.Citation53
Conclusions
This is the first case report of tildrakizumab treatment achieving a positive response in recalcitrant ulcerative leg PG, notably the most common PG subtype at its most common anatomical site.Citation1 Our findings are consistent with recent case reports of other IL-23p19 inhibitorsCitation19–22 and suggest that incorporating IL-23 inhibitors such as tildrakizumab as part of the systemic treatment ladder for PG could be beneficial. More studies are needed to confirm our observations.
Disclosure
Nicolas Zubrzycki has nothing to disclose. Liang Joo Leow has received education, writing, and travel support from Sun Pharmaceutical Industries, Inc.
Acknowledgments
We thank the patient for agreeing to publication. Medical writing and editorial assistance were provided by Elisabetta Lauretti, PhD, of AlphaBioCom, LLC, under the direction of the authors.
Additional information
Funding
References
- Fletcher J, Alhusayen R, Alavi A. Recent advances in managing and understanding pyoderma gangrenosum. F1000Res. 2019;8:F1000 Faculty Rev–2092. doi:10.12688/f1000research.19909.1
- Xu A, Balgobind A, Strunk A, Garg A, Alloo A. Prevalence estimates for pyoderma gangrenosum in the United States: an age- and sex-adjusted population analysis. J Am Acad Dermatol. 2020;83(2):425–429. doi:10.1016/j.jaad.2019.08.001
- John JM, Sinclair RD. Tildrakizumab for treatment of refractory pyoderma gangrenosum of the penis and polymyalgia rheumatica: killing two birds with one stone. Australas J Dermatol. 2020;61(2):170–171. doi:10.1111/ajd.13196
- Almeida IR, Coltro PS, Goncalves HOC, et al. The role of negative pressure wound therapy (NPWT) on the treatment of pyoderma gangrenosum: a systematic review and personal experience. Wound Repair Regen. 2021;29(3):486–494. doi:10.1111/wrr.12910
- Alavi A, French LE, Davis MD, Brassard A, Kirsner RS. Pyoderma gangrenosum: an update on pathophysiology, diagnosis and treatment. Am J Clin Dermatol. 2017;18(3):355–372. doi:10.1007/s40257-017-0251-7
- Guenova E, Teske A, Fehrenbacher B, et al. Interleukin 23 expression in pyoderma gangrenosum and targeted therapy with ustekinumab. Arch Dermatol. 2011;147(10):1203–1205. doi:10.1001/archdermatol.2011.168
- McKenzie F, Cash D, Gupta A, Cummings LW, Ortega-Loayza AG. Biologic and small-molecule medications in the management of pyoderma gangrenosum. J Dermatolog Treat. 2019;30(3):264–276. doi:10.1080/09546634.2018.1506083
- Jockenhofer F, Wollina U, Salva KA, Benson S, Dissemond J. The PARACELSUS score: a novel diagnostic tool for pyoderma gangrenosum. Br J Dermatol. 2019;180(3):615–620. doi:10.1111/bjd.16401
- Rostom M, Davidson D, Biswas A. Pyoderma gangrenosum and pseudoepitheliomatous hyperplasia: a poorly recognized association. Diag Histopath. 2017;23(5):229–233. doi:10.1016/j.mpdhp.2017.05.001
- George B, Tanveer N, Valaparla V, Boyars M. A case of pyoderma gangrenosum of head and neck mimicking purulent cellulitis. Am J Med. 2021;134(4):e281–e282. doi:10.1016/j.amjmed.2020.09.047
- Dorrell DN, Huang WW. Assessing the severity of pyoderma gangrenosum: a need for validated measurement tools. Br J Dermatol. 2019;180(1):217–218. doi:10.1111/bjd.17122
- Feldman S, Lacy F, Huang W. The safety of treatments used in pyoderma gangrenosum. Expert Opin Drug Saf. 2018;17(1):55–61. doi:10.1080/14740338.2018.1396316
- Fonder MA, Cummins DL, Ehst BD, Anhalt GJ, Meyerle JH. Adalimumab therapy for recalcitrant pyoderma gangrenosum. J Burns Wounds. 2006;5:e8.
- Cinotti E, Labeille B, Perrot JL, Pallot-Prades B, Cambazard F. Certolizumab for the treatment of refractory disseminated pyoderma gangrenosum associated with rheumatoid arthritis. Clin Exp Dermatol. 2014;39(6):750–751. doi:10.1111/ced.12393
- Brooklyn TN, Dunnill MG, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut. 2006;55(4):505–509. doi:10.1136/gut.2005.074815
- Santa Lucia G, DeMaio A, Karlin S, Elston D. A case of extracutaneous pyoderma gangrenosum in a patient with persistent cutaneous and systemic symptoms: implications for differential diagnosis and treatment. JAAD Case Rep. 2021;15:85–87. doi:10.1016/j.jdcr.2021.07.019
- Patel F, Fitzmaurice S, Duong C, et al. Effective strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta Derm Venereol. 2015;95(5):525–531. doi:10.2340/00015555-2008
- Herberger K, Dissemond J, Bruggestrat S, Sorbe C, Augustin M. Biologics and immunoglobulins in the treatment of pyoderma gangrenosum - analysis of 52 patients. J Dtsch Dermatol Ges. 2019;17(1):32–41.
- Burgdorf B, Schlott S, Ivanov IH, Dissemond J. Successful treatment of a refractory pyoderma gangrenosum with risankizumab. Int Wound J. 2020;17(4):1086–1088. doi:10.1111/iwj.13359
- Weigelt MA, Kirsner RS. Peristomal pyoderma gangrenosum responding to risankizumab. Adv Skin Wound Care. 2021;34(6):327–329. doi:10.1097/01.ASW.0000744324.59877.df
- Reese AM, Erickson K, Reed KB, Ortega-Loayza AG. Modified dose of guselkumab for treatment of pyoderma gangrenosum. JAAD Case Rep. 2022;21:38–42. doi:10.1016/j.jdcr.2021.11.030
- Baier C, Barak O. Guselkumab as a treatment option for recalcitrant pyoderma gangrenosum. JAAD Case Rep. 2021;8:43–46. doi:10.1016/j.jdcr.2020.12.005
- Sheriff TM, Murrell DF. Ustekinumab and tildrakizumab in pyoderma gangrenosum. Australas J Dermatol. 2021;62:114–115.
- Nguyen MT, Borchers A, Selmi C, Naguwa SM, Cheema G, Gershwin ME. The SAPHO syndrome. Semin Arthritis Rheum. 2012;42(3):254–265. doi:10.1016/j.semarthrit.2012.05.006
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18(4):555–562. doi:10.1007/s40257-017-0265-1
- Kok Y, Nicolopoulos J, Varigos G, Howard A, Dolianitis C. Tildrakizumab in the treatment of PASH syndrome: a potential novel therapeutic target. Australas J Dermatol. 2020;61(3):e373–e374. doi:10.1111/ajd.13285
- Kok Y, Nicolopoulos J, Howard A, Varigos G, Kern J, Dolianitis C. Tildrakizumab in the treatment of moderate-to-severe hidradenitis suppurativa. Australas J Dermatol. 2020;61(4):e488–e490. doi:10.1111/ajd.13377
- Schlapbach C, Hanni T, Yawalkar N, Hunger RE. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65(4):790–798. doi:10.1016/j.jaad.2010.07.010
- Kim TH, Oh SY, Myung SC. Pyoderma gangrenosum of the penis. J Korean Med Sci. 2009;24(6):1200–1202. doi:10.3346/jkms.2009.24.6.1200
- Hsiao JL, Antaya RJ, Berger T, Maurer T, Shinkai K, Leslie KS. Hidradenitis suppurativa and concomitant pyoderma gangrenosum: a case series and literature review. Arch Dermatol. 2010;146(11):1265–1270. doi:10.1001/archdermatol.2010.328
- Ead JK, Snyder RJ, Wise J, Cuffy C, Jafary H, Fischborn K. Is PASH syndrome a biofilm disease?: a case series and review of the literature. Wounds. 2018;30(8):216–223.
- Gurjala AN, Geringer MR, Seth AK, et al. Development of a novel, highly quantitative in vivo model for the study of biofilm-impaired cutaneous wound healing. Wound Repair Regen. 2011;19(3):400–410. doi:10.1111/j.1524-475X.2011.00690.x
- Metcalf DG, Bowler PG. Biofilm delays wound healing: a review of the evidence. Burns Trauma. 2013;1(1):5–12. doi:10.4103/2321-3868.113329
- Percival SL, Suleman L. Slough and biofilm: removal of barriers to wound healing by desloughing. J Wound Care. 2015;24(11):498–510. doi:10.12968/jowc.2015.24.11.498
- Prabhakara R, Harro JM, Leid JG, Harris M, Shirtliff ME. Murine immune response to a chronic Staphylococcus aureus biofilm infection. Infect Immun. 2011;79(4):1789–1796. doi:10.1128/IAI.01386-10
- Prabhakara R, Harro JM, Leid JG, Keegan AD, Prior ML, Shirtliff ME. Suppression of the inflammatory immune response prevents the development of chronic biofilm infection due to methicillin-resistant Staphylococcus aureus. Infect Immun. 2011;79(12):5010–5018. doi:10.1128/IAI.05571-11
- Aujla SJ, Dubin PJ, Kolls JK. Th17 cells and mucosal host defense. Semin Immunol. 2007;19(6):377–382. doi:10.1016/j.smim.2007.10.009
- McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27(1):17–23. doi:10.1016/j.it.2005.10.003
- Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol. 2012;13(3):191–211. doi:10.2165/11595240-000000000-00000
- Bjarnsholt T, Alhede M, Jensen PO, et al. Antibiofilm properties of acetic acid. Adv Wound Care. 2015;4(7):363–372. doi:10.1089/wound.2014.0554
- Ryssel H, Kloeters O, Germann G, Schafer T, Wiedemann G, Oehlbauer M. The antimicrobial effect of acetic acid – an alternative to common local antiseptics? Burns. 2009;35(5):695–700.
- Alam M, Grossman ME, Schneiderman PI, Blume RS, Benvenisty AI. Surgical management of pyoderma gangrenosum: case report and review. Dermatol Surg. 2000;26(11):1063–1066. doi:10.1046/j.1524-4725.2000.0260111063.x
- Adışen E, Erduran F, Gürer MA. Pyoderma gangrenosum: a report of 27 patients. Int J Low Extrem Wounds. 2016;15(2):148–154. doi:10.1177/1534734616639172
- McGarity WC, Robertson DB, McKeown PP, Amerson JR, Darden WA. Pyoderma gangrenosum at the parastomal site in patients with Crohn’s disease. Arch Surg. 1984;119(10):1186–1188. doi:10.1001/archsurg.1984.01390220064014
- Widgerow A, Leak K. Hypergranulation tissue: evolution, control and potential elimination. Wound Healing Southern Africa. 2010;3(2):7–9.
- Caliskan E. Er: YAG laser ablation: an adjuvant treatment for medically resistant pyoderma gangrenosum. Dermatol Surg. 2017;43(11):1405–1407. doi:10.1097/DSS.0000000000001100
- Park J, Choi M, Goo B, Cho S. Refractory pyoderma gangrenosum effectively treated using a 10,600-nm carbon dioxide fractional laser. Dermatol Surg. 2013;39(3):pt1. doi:10.1111/dsu.12020
- Tippett A. Minimizing the risk of pathergy in treating pyoderma gangrenosum. Availale from: https://www.woundsource.com/blog/minimizing-risk-pathergy-treating-pyoderma-gangrenosum. Accessed August 10, 2021.
- Pastar I, Stojadinovic O, Yin NC, et al. Epithelialization in wound healing: a comprehensive review. Adv Wound Care. 2014;3(7):445–464. doi:10.1089/wound.2013.0473
- Rousselle P, Braye F, Dayan G. Re-epithelialization of adult skin wounds: cellular mechanisms and therapeutic strategies. Adv Drug Deliv Rev. 2019;146:344–365. doi:10.1016/j.addr.2018.06.019
- Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020;10(9):200223. doi:10.1098/rsob.200223
- Leiphart PA, Lam CC, Foulke GT. Suppression of pathergy in pyoderma gangrenosum with infliximab allowing for successful tendon debridement. JAAD Case Rep. 2018;4(1):98–100. doi:10.1016/j.jdcr.2017.08.009
- Falabella AF. Debridement and wound bed preparation. Dermatol Ther. 2006;19(6):317–325. doi:10.1111/j.1529-8019.2006.00090.x