Abstract
Background
Bullous pemphigoid (BP) and atopic dermatitis (AD) are both type 2 inflammatory skin diseases with similar clinical features. Thymic stromal lymphopoietin (TSLP) is an epithelial-derived cytokine which is upregulated in AD. However, the expression of TSLP in BP and the correlation between TSLP and inflammatory infiltrations have not been fully studied.
Objective
To characterize the serum Th2 cytokines level and Th2 inflammatory cell infiltrations in BP and AD. To study TSLP levels in serum, blister fluids and expression in lesional skin in patients with BP and AD.
Methods
TSLP level in serum and blister fluids was measured by enzyme-linked immunosorbent assay (ELISA). Inflammatory cells (CD4+ T cells, CD8+ T cells, CD1a+ cells, eosinophils and mast cells) were stained immunohistochemically and quantified by image analysis.
Results
TSLP level was significantly increased in blister fluids of BP and was highly expressed in lesional skin of BP and AD. Serum levels of IL-6, IL-4, IL-22, IFN-γ and thymic activation regulates chemokines (TARC) were significantly higher in patients with BP and AD than in healthy controls. CD4+ T cells, CD8+ T cells and CD1a+ cells were significantly more in upper dermis of BP and AD lesions. Eosinophils were found more in BP lesions while mast cells were found more in AD lesions than in healthy controls. A distinct correlation was found between TSLP levels and the intensities of CD4+ T cells, CD1a+ cells infiltrations.
Conclusion
TSLP was significantly higher in blister fluids and skin lesions of BP, suggesting that it might contribute to the pathogenesis of BP. BP exhibited a similar type 2 immune response and a slight difference in cells infiltrations with AD.
Introduction
Type 2 inflammation is characterized by Th2 cells, innate lymphoid cells (ILCs) and type 2 cytokines. Allergens, infection and pollutants can derive type 2 responses and promote secretion of Th2 cytokines.Citation1 A specific type 2 set of cytokines such as IL-4, IL-5, IL-9, IL-13 and TSLP are produced during the induction and maintenance of allergic immune response with the contribution of T cells, innate lymphoid cells (ILC), eosinophils and mast cells (MC).Citation2 Many allergic diseases such as atopic dermatitis (AD) and asthma are characterized by type 2 immune responses.
Bullous pemphigoid (BP) is a common autoimmune blistering disease mostly affecting the elderly. The pathogenesis of BP has not been fully understood. Although BP is classified as a blistering disease and autoantibodies to BP180 and BP230 can be found in patients with BP, the cutaneous manifestations of BP are extremely polymorphic, including urticaria-like and eczema-like lesions.Citation3 Moreover, patients with BP often have severe itching and increased peripheral blood eosinophils and serum IgE level.Citation4 These clinical features can also be found in patients with AD. Atopic dermatitis (AD) is an inflammatory skin disease characterized by chronic recurrent dermatitis with pruritus. Elevated serum IgE levels, eosinophilia and increased expression of thymic stromal lymphopoietin (TSLP) were always found in AD.Citation5
Thymic stromal lymphopoietin (TSLP) is an epithelial derived cytokine. TSLP is expressed primarily by epithelial cells at barrier surfaces such as the skin, gut and lung.Citation6 TSLP is known to have wide-ranging impacts on both hematopoietic and nonhematopoietic cell lineages, including eosinophils, mast cells, T cells, B cells and epithelial cells.Citation7 Recent studies have implicated important role of TSLP in atopic dermatitis.Citation8 However, the role of TSLP in BP has not been studied.
Although BP and AD are independent entities, some patients with BP have urticaria-like, eczematous and papular lesions with itching, which was similar to AD.Citation9 Moreover, increased levels of type 2 cytokines (such as IL-4, IL-5, IL-10) and Th22 cytokines have been founded in skin lesions and serum of patients with AD.Citation10 Increased CCL17/TARC (thymic activation regulates chemokines), CCL22/MDC (produced by Langerhans cells), serum total IgE levels and peripheral eosinophils were often found in patients with AD and BP,Citation11 suggesting a close relationship between BP and AD.
In this study, the expression of TSLP in serum, blister fluids and lesional skin were studied in patients with BP and AD. Th2 cytokines and type 2 inflammatory cell infiltrations in BP and AD were also detected.
Methods
Subjects
10 adult patients with BP (7 male and 3 female), 15 adult patients with AD (10 male and 5 female) and 15 age- and sex-matched healthy controls were included in this study. All the AD patients satisfied the criteria of Hanifin and RajkaCitation12 and Chinese criteria.Citation13 Diagnosis of BP was established based on clinical histopathological and direct immunofluorescence findings of BP180. All patients were in active stage of disease. Circulating BP-180 antibody was also measured by indirect immunofluorescence assay. The informed consents were obtained from each patient and healthy control. This study was approved by the Ethics Committee of the Institutional Review Board of Peking University People’s Hospital.
TSLP and Cytokines Measurement
Blood samples were obtained from each patient and healthy control. TSLP, IL-4, IL-6, IL-22, TARC and IFN-γ levels were measured by ELISA (eBioscience, Germany) according to the manufacturer’s instructions. Blister fluids from patients with herpes zoster were used as controls.
Immunohistochemistry
Skin punch biopsies were taken from patients with BP and AD. Control specimens were obtained from the normal skin area of pigmented nevus surgeries excision. The biopsies were formalin fixed and tissue slides were subjected to hematoxylin and eosin (H&E) staining. CD4, CD8 and CD1a were stained by immunohistochemistry. The sliced sections were air dried for 30 min. Then the sections were fixed in cold acetone for 10 min. After blocking 5 min for endogenous peroxidase, using 0.2% sodium azide, they were washed in phosphate buffered saline (PBS) for 10 min. Then samples were incubated by primary antibodies overnight. The following primary antibodies (mouse antihuman) were used: anti-CD4 (no. 11056-2-AP, Proteintech, Beijing, China, 1:200), anti-CD8 (no. 66868-1-Ig, Proteintech, Beijing, China, 1:200), anti-CD1a (no.ab108309, Abcam, Cambridge, UK, 1:500), anti-TSLP (no.ab47943, Abcam, Cambridge, UK, 1:500). Samples were washed in PBS for 15 minutes and repeated for 3 times and incubated by secondary antibody for an hour (biotinylated rabbit anti-mouse IgG, Servicebio, China). A colour deconvolution technique was applied to separate the colour mixture of haematoxylin. The sliced sections were also stained with 1% toluidine blue for detection of mast cells. In toluidine blue staining, mast cell granules are seen purplish red and the nuclei sky blue in color. An image analysis technique was employed for quantitative scoring of the stained slides.
Image Analysis
All slides were read by a pathologist that. Quantification of inflammatory cell was performed at ×200 magnification. At high magnification, CD4, CD8, CD1a and toluidine blue positive cells in upper epidermis and deep epidermis were counted in five randomly selected fields. The eosinophils were counted in H-E staining slices. Quantitative cell counts were expressed in “Positive cells per high power field (HPF)”.Citation2
The images of immunohistochemical staining of TSLP were captured using the image capture system consisting of a light microscope connected to a desktop computer. Epidermal regions were analyzed interactively using Image pro plus (IPP), which allowed for image analysis of densitometry expressed as optical density (OD) over the background.
Statistical Analysis
All the data were analyzed by SPSS 22.0. Qualitative data were presented as the mean ± SEM. Differences between groups were tested using one-way analysis of variance (ANOVA) and unpaired Student’s t-test. Pearson correlation index were applied to describe the correlation between measurement data. P < 0.05 was considered significant.
Results
TSLP Levels in Serum and Blister Fluids
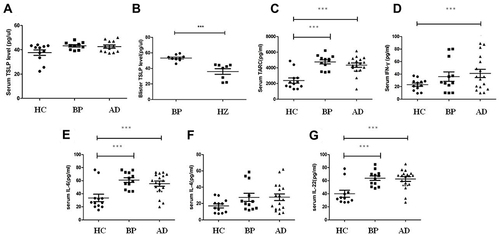
The serum TSLP levels are shown in . Serum TSLP levels in BP patients (43.22 pg/μL), AD patients (42.58pgl/μL) were slightly but not significantly higher than in healthy controls (37.67pg/μL, ). The TSLP level of BP blister fluids was higher than that from herpes zoster fluids (53.48pg/μL vs 36.11pg/μL, P<0.001, ).
Figure 1 The expression of TSLP in serum and blister fluids of BP and Th2 cytokines in BP/AD. (A) Serum TSLP levels in BP and AD. (B) TSLP levels in blister fluids of BP and herpes zoster. (C-G) Serum levels of TARC, IFN-γ, IL-6, IL-4 and IL-22 in BP and AD. ***P<0.001 versus HC.
Serum Cytokine Levels in Patients with BP and AD
The expression of TARC (), IFN-γ (), IL-6 (), IL-4 () and IL-22 () are shown in . Serum levels of IL-6, IL-22 and TARC were significantly increased in patients with BP and AD compared with healthy control (P<0.001). Serum IFN-γ in AD patients was significantly higher than healthy control. No difference was found between BP patients and AD patients.
TSLP Expression in Skin Lesions of BP and AD
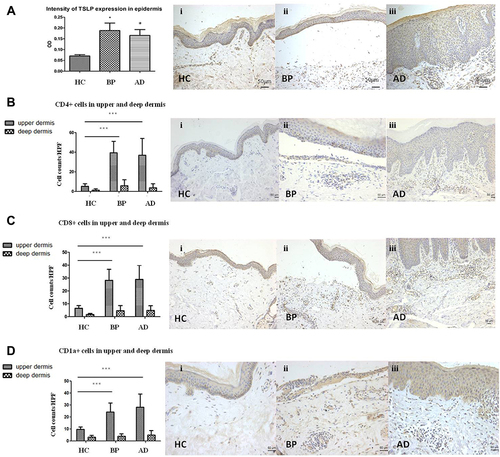
The expression of TSLP in lesional skin of BP and AD are shown in . In normal skin, the TSLP staining was faint in all layers of epidermis (). The TSLP expression in lesions of BP (P=0.016) and AD (P=0.045) were significantly higher than healthy controls. In BP lesions, TSLP staining was faint in the granular and upper spinous layers, and became darker towards lower spinous and basal layers (). In AD lesions, TSLP staining was uniformly positive in all epidermal layers ().
Figure 2 Immunohistochemistry of TSLP, CD4, CD8 and CD1a in normal skin and skin lesions of BP and AD. (A) Expression of TSLP in normal skin and lesions of BP and AD. The mean optical densities (OD) of BP, AD and healthy controls were 0.188±0.10, 0.162±0.08 and 0.07±0.01, respectively (Ai, Aii, Aiii×200). (B–D) The number of CD4+ T cells (B), CD8+ T cells (C) and CD1a cells (D) in upper and deep dermis of normal skin and lesions of BP and AD. (Bi, Biii, Ci, Cii Ciii×100; Bii, Ci, Cii, Ciii×200). *P<0.05 versus HC; ***P<0.001 versus HC.
Inflammatory Infiltrations of BP and AD in Upper Dermis
The inflammatory cell counts in the upper dermis per HPF are shown in . CD4+ T cells (), CD8+ T cells () and CD1a+ cells () were abundantly present in BP (CD4+ T cells: 39.4±13.7/HPF, CD8+ T cells: 28.2±8.5/HPF, CD1a+ cells: 24.4±13.7/HPF) and AD (CD4+ T cells: 36.9±16.8/HPF, CD8+ T cells: 28.9±11.1/HPF, CD1a+ cells: 28.2±11.0/HPF) than normal skin. These inflammatory cells infiltration were similar between BP and AD (P>0.05).
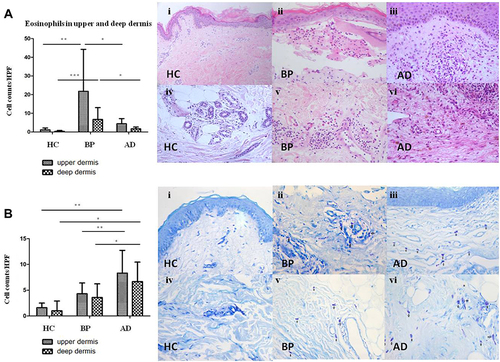
Eosinophils were significantly more observed in BP (21.7±22.5/HPF) than in AD (4.4±2.7/HPF, , P<0.05) and in healthy control (P<0.01). Mast cells were significantly more in AD (8.3±4.4/HPF) than in BP (4.3±2.1/HPF, , P<0.01) and in healthy control (P<0.01).
Figure 3 The number of eosinophils and mast cells in upper and deep dermis of BP, AD and normal skin. (A) Eosinophils in upper/deep dermis of HC (Ai/Aiv), BP (Aii/Av) and AD (Aiii/Avi) by histopathology (Aii, Aiii, Aiv, Av, Avi×200, Ai×100). (B) Mast cells in upper/deep dermis of HC (Bi/Biv), BP (Bii/Bv) and AD (Biii/Bvi) by toluidine blue staining. Mast cells were highlighted by black arrows. *P<0.05; **P<0.01; ***P<0.001. (Bii, Biii, Biv, Bv, Bvi×200, Bi×100).
Inflammatory Infiltrations of BP and AD in Deep Dermis
The inflammatory cell counts in the deep dermis per HPF are shown in . CD4+ T cells (), CD8+ T cells () and CD1a+ cells () were sporadic in deep dermis of BP lesions (CD4+ T cells: 5.8±6.1/HPF, CD8+ T cells: 4.6±4.0/HPF, CD1a+ cells: 4.0±2.1/HPF) and AD (CD4+ T cells: 3.7±4.1/HPF, CD8+ T cells: 4.8±4.7/HPF, CD1a+ cells: 5.0±3.9/HPF). Eosinophils were more in BP (6.7±6.4/HPF) than in AD (1.7±20.9/HPF, , P<0.05). Mast cells were significantly more in AD (6.6±3.3/HPF) than in BP (3.6±2.6/HPF, , P<0.05).
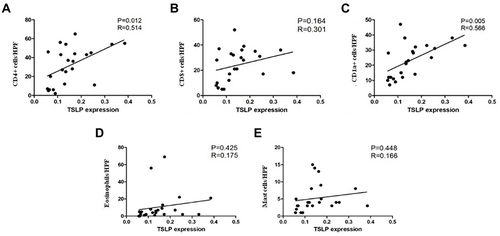
The Correlation Between TSLP, CD4+ T Cells and CD1a+ Cells
A distinct correlation was found between TSLP expression in epidermis and CD4+ T cells (P=0.012, R=0.514, ) and CD1a+ cells (P=0.005, R=0.566, ) in BP and AD patients. No correlation was found between TSLP expression and CD8+ T cells (), eosinophils () or mast cells ().
Figure 4 Scatter plot and linear regression between OD value of TSLP expression and the number of infiltrating CD4+ T cells (A), CD8+ T cells (B), CD1a+ cells (C), eosinophils (D) and mast cells (E). Significant correlation were found between TSLP level and CD4+ T cells (P=0.012, R=0.514) and CD1a+ cells (P=0.005, R=0.566).
Discussion
Bullous pemphigoid (BP) is the most common autoimmune bullous disorder which is characterized by intensely pruritic erythematous papules, vesicles or bulla, often accompanied with increased peripheral blood eosinophils and serum IgE.Citation4 These clinical features are quite similar to AD.Citation14 Zhang et alCitation15 reported that dysfunction of BP180 in mouse models can trigger spontaneous AD-like skin inflammation. In this study, increased serum levels of IL-6, IL-4, IFN-γ, IL-22 and TARC were found in both AD and BP patients. It is reported that BP is prominently induced and sustained by a Th2-driven autoimmune response. BP180-reactive T cells produce Th2 cytokines (IL-4, IL-5, IL-6, IL-10 and IL-13), which induce the autoantibody production of the Th2-dependent IgG4 subtype in the active stages.Citation16 TARC/CCL17 is also designated as Th2 type chemokine. TARC has been proven to be increased in AD, especially in moderate to severe patients and in active stage.Citation17 About 50% of BP patients have eosinophilia and about 70% have elevated serum IgE, which was commonly seen in AD.Citation4 In addition, more than 70% patients have serum IgE antibodies against BP180. Degranulation of eosinophils at the BMZ occurs early in BP and is most pronounced in lesions of urticaria and AD. All these indicated a Th2-polarized autoimmune response in BP and AD.Citation18
Recent studies have highlighted TSLP in various inflammatory diseases.Citation6 TSLP-induced Th2 responses are associated with the pathogenesis of allergic inflammatory diseases including AD, asthma and rhinitis.Citation19 Soumelis et alCitation20 reported that TSLP was highly expressed in AD. Cytokines that are found at high levels in lesional skin in AD patients (such as TNF-α, IL-4, and IL-13) can also synergize to induce TSLP expression by keratinocytes.Citation21 Wilson et alCitation22 reported that signaling between epithelial cells and innate immune cells via the TSLP is thought to drive AD and the atopic march. Epithelial cells can directly communicate to cutaneous sensory neurons via TSLP to promote itch, suggesting that TSLP may play an important role in AD. In this study, we found that TSLP levels were significantly increased in lesional skin of BP and AD and in blister fluids of BP, suggesting that it might also play important roles in BP.
In this study, we found that the distributions of CD4+ T cells, CD8+ T cells and CD1a+ cells in upper dermis of BP were significantly increased. AD and BP shared some common histopathological features especially in non-bullous BP, such as spongiosis, papillary dermal edema, and superficial lymphohistiocytic infiltrates without infiltrations in deep perivasculatures. This characteristic is supported by the similarity of the density of CD4+ T cells, CD8+ T cells and CD1a+ cells in deep dermis. Studies have showed a predominance of T cells and Langerhans cells infiltration in skin lesions of BP. It has been postulated that an imbalance of autoreactive Th cells and CD4+ T regulatory cells (Tregs) is critical to BP formation.Citation16 Although CD4+ T lymphocytes are well known to play roles in the development of skin inflammation, CD8+ T lymphocytes were also found in the dermis in both BP and AD lesions. The presence of CD8+ T cells was scarcely reported. In this study, we found that BP and AD also shared a similarity of the densities of CD1a+ dendritic cells. Emtestam et alCitation23 found a redistribution of the dendritic cells towards the basal membrane with increased total number of dendritic cells in BP. CD1a+ cells are predominant high-affinity IgE receptor bearing cells of lesional AD and BP.Citation24 We also identified elevated dendritic cells in dermis of BP and AD.
The impressive predominance of eosinophils in upper and deep dermis in BP lesions was also observed. In AD patients, Th2 cytokines such as IL-4 and IL-5 has been shown to increase the survival of lesional eosinophils, which were often seen in superficial dermis. Eosinophilia and increased eosinophilic cationic protein (ECP) are two characteristic markers for the activity of AD.Citation11 In BP, eosinophils, rather than other inflammatory cells, are the major cells seen in the lesional skin and they are also the most predominant biomarkers in peripheral blood. Early prebullous, urticarial-like lesions may demonstrate eosinophilic spongiosis histologically.Citation25 Th2 cells and cytokines may account for eosinophil recruitment from blood vessels to skin. Eosinophil production of proteolytic enzymes, such as collagenase, plasminogen activators, plasmin and matrix metalloproteinase, lead to breakdown of dermal-epidermal cohesion and further contributes to tissue damage such as blister formation.Citation26
We also found that mast cells were significantly increased in lesional skin of AD and BP. And, mast cell predominance was more obvious in lesional skin of AD compared with BP. Mast cells can be sensitized by IgE via the high-affinity IgE receptor (FcεRI) and release of pruritogenic substances which induces scratching and disruption of the skin barrier.Citation9 Mast cells can produce IL-4, IL-5 and IL-13, thereby playing important roles in skin inflammation. Mast cells were also significantly increased in chronic lesions of AD, especially in areas of lymphocytic infiltration in the papillary dermis.Citation27 Degranulation of mast cells had been proven to be related with inflammatory response of AD. Mast cell degranulation is followed by eosinophil migration and release of mediators, which might contribute to the inflammatory cells recruitment and activation.Citation28 Besides that, although mast cells also play a crucial role in neutrophil recruitment of BP through release of IL-8, the production of matrix metallopeptidase-9 (MMP) by eosinophils rather than mast cells can further contribute to tissue damage and blister formation. That’s the reason why a prominent eosinophil involvement was found in BP contrary to AD.Citation4
In this study, we found a distinct correlation between TSLP expression and the number of infiltrating CD4+ T cells (R=0.514, P=0.012) and CD1a+ cells (R=0.566, P=0.005). TSLP can be produced by a variety of cells including T cells, B cells and CD1a+ dendritic cells (DC).Citation29 TSLP can promote T-cell proliferation and differentiation both in vivo and in vitro. TSLP responsiveness of CD4+ T cells plays a triggering role in the activation, recruitment of IgE-producing B cells, mast cells, and eosinophils. It is a critical feature of allergic inflammation.Citation9 TSLP can also promote naive CD4+ and CD8+ T cells to develop into Th2 cells, because TSLPR (TSLP receptor) activation induces IL-4 gene transcription, which further upregulates TSLPR on CD4+ T cells, resulting in a positive feedback loop.Citation6
We also found correlation between dermal CD1a+ cells and TSLP expression levels (P=0.005). CD1a is a lipid-presenting molecule that is abundantly expressed on epidermal Langerhans cells and dermal dendritic cells. It has been reported that a major TSLP-responsive cellular subset in both human and mice are myeloid-derived dendritic cells.Citation30 Watanabe et alCitation31 reported that co-culture of TSLP-activated DCs with naive syngenic CD4+ T cells led to T cell proliferation but not differentiation, suggesting an important role for DCs and CD4+ T cell homeostasis. These results indicated that TSLP-activated DCs can induce the proliferation of inflammatory Th2 cells. Our results suggested that TSLP might play a crucial role in CD1a+ cell infiltrations of BP and AD.
We acknowledge a few inherent limitations of this study. It is often difficult to separate cells, especially when the immunohistochemical staining is suboptimal. Furthermore, our study is also restricted by its small sample size. More large-scale studies for verification are needed. In conclusion, our study defines the similarities and differences in type 2 inflammatory response between BP and AD. TSLP is of great significance in diagnosis of Th2 inflammatory skin diseases such as BP, which might point to a future role for biological therapy.
Ethics Approval Statement
The study design was approved by the Institutional Ethics Committee. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s human research committee.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no conflicts of interest to declare.
Additional information
Funding
References
- Akdis CA, Arkwright PD, Bruggen MC, et al. Type 2 immunity in the skin and lungs. Allergy. 2020;75(7):1582–1605. doi:10.1111/all.14318
- Garcovich S, Maurelli M, Gisondi P, Peris K, Yosipovitch G, Girolomoni G. Pruritus as a distinctive feature of type 2 inflammation. Vaccines-Basel. 2021;9(3). doi:10.3390/vaccines9030303
- Borradori L, Van Beek N, Feliciani C, et al. Updated S2 K guidelines for the management of bullous pemphigoid initiated by the European Academy of Dermatology and Venereology (EADV). J Eur Acad Dermatol Venereol. 2022;36(10):1689–1704. doi:10.1111/jdv.18220
- Miyamoto D, Santi CG, Aoki V, Maruta CW. Bullous pemphigoid. An Bras Dermatol. 2019;94(2):133–146. doi:10.1590/abd1806-4841.20199007
- Wollenberg A, Oranje A, Deleuran M, et al. ETFAD/EADV Eczema task force 2015 position paper on diagnosis and treatment of atopic dermatitis in adult and paediatric patients. J Eur Acad Dermatol Venereol. 2016;30(5):729–747. doi:10.1111/jdv.13599
- Cianferoni A, Spergel J. The importance of TSLP in allergic disease and its role as a potential therapeutic target. Expert Rev Clin Immunol. 2014;10(11):1463–1474. doi:10.1586/1744666X.2014.967684
- Ziegler SF. Thymic stromal lymphopoietin and allergic disease. J Allergy Clin Immunol. 2012;130(4):845–852. doi:10.1016/j.jaci.2012.07.010
- Heo WI, Park KY, Lee MK, Moon NJ, Seo SJ. TSLP polymorphisms in atopic dermatitis and atopic march in Koreans. Ann Dermatol. 2018;30(5):529–535. doi:10.5021/ad.2018.30.5.529
- Li SZ, Jin XX, Ge XL, Zuo YG, Jin HZ. Thymic stromal lymphopoietin is implicated in the pathogenesis of bullous pemphigoid by dendritic cells. J Immunol Res. 2020;4594630. doi:10.1155/2020/4594630
- Lo Schiavo A, Ruocco E, Brancaccio G, Caccavale S, Ruocco V, Wolf R. Bullous pemphigoid: etiology, pathogenesis, and inducing factors: facts and controversies. Clin Dermatol. 2013;31(4):391–399. doi:10.1016/j.clindermatol.2013.01.006
- Furue M, Chiba T, Tsuji G, et al. Atopic dermatitis: immune deviation, barrier dysfunction, IgE autoreactivity and new therapies. Allergol Int. 2017;66(3):398–403. doi:10.1016/j.alit.2016.12.002
- Hanifin JM. Diagnostic criteria for atopic dermatitis: consider the context. Arch Dermatol. 1999;135(12):1551. doi:10.1001/archderm.135.12.1551
- Liu P, Zhao Y, Mu ZL, et al. Clinical features of adult/adolescent atopic dermatitis and Chinese criteria for atopic dermatitis. Chin Med J. 2016;129(7):757–762. doi:10.4103/0366-6999.178960
- Kamiya K, Aoyama Y, Nishio E, Horio A, Tokura Y. Management of erythematous skin lesions in bullous pemphigoid associated with atopic dermatitis. J Dermatol. 2016;43(9):1102–1103. doi:10.1111/1346-8138.13330
- Zhang Y, Hwang B-J, Liu Z, et al. BP180 dysfunction triggers spontaneous skin inflammation in mice. Proc Natl Acad Sci U S A. 2018;115(25):6434–6439. doi:10.1073/pnas.1721805115
- Giusti D, Le Jan S, Gatouillat G, Bernard P, Pham BN, Antonicelli F. Biomarkers related to bullous pemphigoid activity and outcome. Exp Dermatol. 2017;26(12):1240–1247. doi:10.1111/exd.13459
- Uysal P, Birtekocak F, Karul AB. The relationship between serum TARC, TSLP and POSTN Levels and childhood atopic dermatitis. Clin Lab. 2017;63(7):1071–1077. doi:10.7754/Clin.Lab.2017.161107
- Hammers CM, Stanley JR. Mechanisms of disease: pemphigus and bullous pemphigoid. Annu Rev Pathol. 2016;11:175–197. doi:10.1146/annurev-pathol-012615-044313
- Zhang Y, Zhou B. Functions of thymic stromal lymphopoietin in immunity and disease. Immunol Res. 2012;52(3):211–223. doi:10.1007/s12026-012-8264-z
- Soumelis V, Reche PA, Kanzler H, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3(7):673–680. doi:10.1038/ni805
- Debinska A. New treatments for atopic dermatitis targeting skin barrier repair via the regulation of FLG expression. J Clin Med. 2021;10(11):2506. doi:10.3390/jcm10112506
- Wilson SR, The L, Batia LM, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155(2):285–295. doi:10.1016/j.cell.2013.08.057
- Emtestam L, Hovmark A, Lindberg M, Asbrink E. Human epidermal Langerhans’ cells in bullous pemphigoid. Acta Derm Venereol. 1987;67(6):529–532.
- Kwiek B, Lesniewska A, Kowalewski C, Wozniak K. Langerhans cells are predominant high affinity immunoglobulin E receptor bearing cells in the epidermis of bullous pemphigoid skin. J Dermatol Sci. 2017;85(1):60–63. doi:10.1016/j.jdermsci.2016.09.012
- Kasperkiewicz M, Zillikens D. The pathophysiology of bullous pemphigoid. Clin Rev Allergy Immunol. 2007;33(1–2):67–77. doi:10.1007/s12016-007-0030-y
- Grando SA. Pemphigus autoimmunity: hypotheses and realities. Autoimmunity. 2012;45(1):7–35. doi:10.3109/08916934.2011.606444
- Liu FT, Goodarzi H, Chen HY. IgE, mast cells, and eosinophils in atopic dermatitis. Clin Rev Allergy Immunol. 2011;41(3):298–310. doi:10.1007/s12016-011-8252-4
- van Beek N, Schulze FS, Zillikens D, Schmidt E. IgE-mediated mechanisms in bullous pemphigoid and other autoimmune bullous diseases. Expert Rev Clin Immunol. 2016;12(3):267–277. doi:10.1586/1744666X.2016.1123092
- Soumelis V, Liu YJ. The discovery of human TSLP as a critical epithelial cytokine in type 2 immunity and allergic disease. Nat Immunol. 2020;21(12):1471–1473. doi:10.1038/s41590-020-0720-7
- Kitajima M, Lee HC, Nakayama T, Ziegler SF. TSLP enhances the function of helper type 2 cells. Eur J Immunol. 2011;41(7):1862–1871. doi:10.1002/eji.201041195
- Watanabe N, Hanabuchi S, Soumelis V, et al. Human thymic stromal lymphopoietin promotes dendritic cell-mediated CD4+ T cell homeostatic expansion. Nat Immunol. 2004;5(4):426–434. doi:10.1038/ni1048
