Abstract
The outbreak of coronavirus disease 2019 (COVID-19) represented a new worldwide challenge, strongly impacting on the global economy, overall health and lifestyle. Since then, several strategies have been adopted to contain the widespread of infection. Among these, vaccination is currently the most important measure to fight against the pandemic. However, several concerns such as slower-than-hoped-for rollout, the hurried approval with limited data, the mechanism of action (in particular mRNA-based), and the uncertain duration of protection they afforded were initially raised. Moreover, even if cutaneous reactions have been rarely reported in clinical trials, global mass vaccination showed several dermatologic reactions not initially recognized, leaving dermatologists to decide how to diagnose and treat them. In this scenario, dermatologists should be ready to promptly recognize these clinical manifestations. Thus, the aim of this manuscript is to review current literature on cutaneous reactions following COVID-19 vaccination, particularly inflammatory dermatological diseases, in order to help clinicians to better understand these dermatological conditions and to provide an extensive overview of all the vaccine-related skin manifestations.
Introduction
The outbreak of coronavirus disease 2019 (COVID-19) represented a new worldwide challenge, strongly impacting on the global economy, overall health and lifestyle.Citation1 Since then, several strategies have been adopted to contain the widespread of infection.Citation2,Citation3 Dermatologists played a key role during the pandemic, fighting against several challenges such as cutaneous reactions caused by COVID-19 disease, the hesitancy on the efficacy and safety of conventional treatment and biologic drugs in this period, the worsening of several dermatosis due to the wearing of personal protection equipment and the introduction of a new lifestyle.Citation4–6 Indeed, the “stay-at-home” policy and the restrictive measures adopted by the Italian Government during the COVID-19 pandemic period strongly affected the quality of life.Citation7 In addition, COVID-19 restriction measures affect the epidemiology of infectious diseases and skin cancers.Citation5,Citation8–10
Among the developed public health strategies to control the spread of COVID-19, vaccination is currently the most important measure to fight against the pandemic. However, several concerns such as slower-than-hoped-for rollout, the hurried approval with limited data, the mechanism of action (in particular mRNA-based), and the uncertain duration of protection they afforded were initially raised.Citation11,Citation12 Fortunately, worldwide vaccination campaign was a success, showing to be the most effective weapon to prevent and control COVID-19 epidemic, disease progression, hospitalization and mortality.Citation13
According to the WHO COVID-19 dashboard accessed on 11 September 2021, more than 608 million confirmed cased of COVID-19 have been reported, with almost 6.51 million deaths.Citation14 Nowadays, licensed vaccines for COVID-19, use nucleic acid-based vaccination platforms, such as viral vector platforms, messenger ribonucleic acid and inactivated virus.Citation13
Four vaccines have been approved by the European Medicines Agency (EMA): 2 mRNA-based vaccines (Pfizer/BioNTech; BNT162b2 and Moderna; mRNA-1273) and 2 viral-vector-based vaccines (AstraZeneca; AZD1222 and Johnson & Johnson; Ad26.COV2.S).Citation15 However, other vaccines have been approved in other countries such as “CoronaVac” (Sinovac), “Sputnik V” (Gamaleya Research Institute), and “Convidecia” (CanSino Biologics).Citation13 Currently, more than 5.3 billion people have received at least one dose of COVID-19 vaccine.Citation16
Similar to other drugs, some people reported mild-to-moderate adverse events following vaccination, including fatigue, headache, diarrhea, redness or pain at the injection site, fever, muscle aches, chills.Citation17–19 Fortunately, most of the side effects are limited, with a duration of few days.Citation17–19
Even if cutaneous reactions have been rarely reported in clinical trials, global mass vaccination showed several dermatologic reactions not initially recognized, leaving dermatologists to decide how to recognize and treat them. In particular, a wide spectrum of cutaneous reactions has been reported.Citation20 However, the significance of these reactions is still unknown. In this scenario, dermatologists should be ready to promptly recognize these clinical manifestations, which should be considered in personalized medicine.Citation21,Citation22
Thus, the aim of this manuscript is to review current literature on cutaneous reactions following COVID-19 vaccination, particularly inflammatory dermatological diseases, in order to help clinicians to better understand these dermatological conditions and to provide an extensive overview of all the vaccine-related skin manifestations.
Materials and Methods
For the current review, literature research was carried out on the PubMed, Embase, Cochrane Skin, Google Scholar, EBSCO and MEDLINE databases (until September 11, 2022). Research was performed by using the following keywords: “COVID-19”, “vaccination”, “vaccine”, “cutaneous”, “side effects”, “adverse events”, “skin manifestations”, “mRNA”, “viral-vector”, “Pfizer/BioNTech”, “BNT162b2”, “Moderna”, “mRNA-1273”, “AstraZeneca”, “AZD1222”, “Johnson & Johnson”, “Ad26.COV2.S”, “atopic dermatitis”, “psoriasis”, “lichen planus”, “bullous disease”, “pemphigus”, “pemphigoids”, “hidradenitis suppurativa”, “urticaria”, “rash”, “herpes”, “pityriasis rosea”, “chilblains”, “vitiligo”, “erythematous eruption”, “alopecia”, “local-injection”, “angioedema”, “eczema”. Analyzed articles included meta-analyses, reviews, letter to editor, real-life studies, case series and reports. The most relevant manuscripts were considered. Studies were selected if they provided information on cutaneous reactions following COVID-19 vaccination with BNT162b2, mRNA-1273, AZD1222 and Ad26.COV2.S, both first and second doses, if applicable. Cutaneous reactions following other vaccines, or the booster dose were excluded. Articles regarding skin reactions reported in clinical trials or with a limited number of cases were excluded. Manuscripts reporting local injection site reactions, both immediate and delayed, rash or unspecified cutaneous eruption and delayed inflammatory reactions to dermal hyaluronic acid filler were not considered. Moreover, articles where the vaccine leading to cutaneous reaction was not specified were excluded. Thus, the research was refined by reviewing the texts and the abstracts of collected articles. The bibliography was also reviewed to include articles that could have been missed. Only English language manuscripts were considered. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors. Details of the included studies are reported in .
Table 1 Main Cutaneous Reaction Following COVID-19 Vaccination
Results
A total of 1922 reports were initially found searching literature. Subsequently, 523 articles and 71 manuscripts were excluded since they were duplicates and in non-English languages, respectively. Then, literature review was refined following inclusion and exclusion criteria. Finally, a total of 183 articles involving 456 patients were selected in the current review. Main findings are summarized in .
Several cases of new onset or exacerbation of inflammatory skin diseases have been reported () as well as the type of vaccine causing these reactions has been investigated (). As regards psoriasis, a total of 98 reports on psoriasis following COVID-19 vaccination were reported.Citation23–54 In particular, flare of pre-existing disease and new-onset disease were reported in 81 and 17 cases, respectively. Moreover, several phenotypes of psoriasis were reported, with plaque subtype as the most frequent. Of note, even if biological treatments showed excellent results in terms of effectiveness and safety in psoriasis management,Citation55–58 they seem to reduce the possibility of disease worsening following vaccination, without nullifying the risk. Moreover, the effectiveness of COVID-19 vaccines in patients undergoing treatment with biologics is debated.Citation59,Citation60
Figure 1 Cutaneous reactions investigated and number of cases.
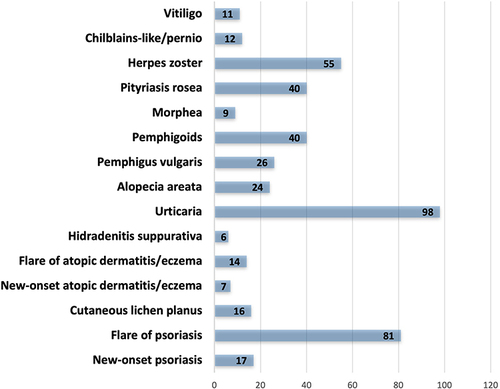
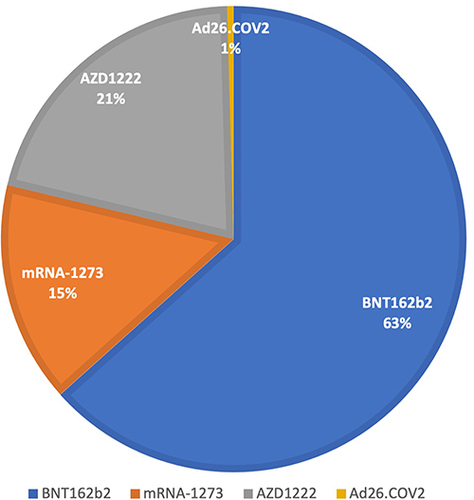
Figure 2 Percentage of vaccine types investigated that cause cutaneous reactions.
Lichen planus is a chronic, inflammatory, autoimmune disease with an unknown pathogenesis.Citation61 To date, 13 cases of new-onset cutaneous lichen planus and 3 cases of cutaneous lichen planus exacerbation have been reported.Citation62–76 Like psoriasis, also cases of new onset and flare of atopic dermatitis or eczema have been reported (7 and 14, respectively).Citation77–83 However, there is not a clear correlation with clinical phenotypes.Citation84 Moreover, undergoing treatment with dupilumab does not seem to prevent the possibility of a flare of the disease, even if its efficacy and safety have been largely demonstrated.Citation85,Citation86 No data of atopic dermatitis worsening in patients undergoing treatment with janus kinase inhibitors are available.Citation87,Citation88 Concerning hidradenitis suppurativa, there are currently few cases of new-onset disease (n = 1)Citation89 or disease exacerbation (n = 5).Citation90 However, patients with hidradenitis suppurativa are not at higher risk for any COVID-19 vaccine‐related adverse outcomes.Citation91,Citation92
Urticarial rashes are the second most common cutaneous reaction following COVID-19 vaccination reported, following local injection site reactions, such as “Covid-arm”.Citation93 Globally, 98 cases of urticarious eruptions following COVID-19 vaccination have been collected in our review,Citation77,Citation78,Citation83,Citation94–107 also during treatment with omalizumab.Citation108
Alopecia areata has been reported following COVID-19 vaccination.Citation109–119 The largest study on 77 patients developing alopecia areata (39) or a worsening of the disease (38) has been reported by Nguyen et al. Unfortunately, it is not possible to correlate alopecia areata development and the type of vaccine.Citation120
Regarding bullous disorders, a total of 26 cased of pemphigus vulgaris have been reported following COVID-19 vaccination,Citation121–135 with several implications in treatment and management.Citation136 Moreover, 40 cases of pemphigoids have been described.Citation137–151
Regarding other cutaneous diseases developed following COVID-19 vaccination, 9, 40, 55, 12 and 11 cases of morphea,Citation152–157 pityriasis rosea,Citation158–177 herpes zoster,Citation178–187 chilblains,Citation188–197 and vitligoCitation198–208 have been reported.
Finally, several other dermatoses have been described, even if data are limited. Among these, we want to highlight pityriasis rubra pilaris, leukocytoclastic vasculitis, morbilliform rash, livedo racemosa, fixed drug eruption, erythema annulare centrifugum, granuloma annulare, fascial neutrophilic eruption, annular rash, Henoch-Schonlein purpura, dermatomyositis, regression of viral wart, raynaud phenomenon, eruptive angiomatosis, lichen striatus, pityriasis lichenoides et varioliformis acuta, Rowell’s syndrome, acrocyanosis, … suggesting that a wide type of dermatoses may be triggered by COVID-19 vaccination.Citation209–221 However, most of these are limited to 1 or 2 case reports.
Discussion
COVID-19 pandemic revolutionized daily clinical practice. Indeed, several strategies were adopted to contain the spreading of the infection.Citation222 Dermatologists had to change their clinical routine in order to avoid the reduction in detection and treatment of several conditions, particularly skin cancer.Citation223–227 Among these, teledermatology allowed physicians to continuously assist patients’ dermatologic conditions with excellent results in terms of treatment adherence and clinical outcomes.Citation228–230 Vaccination campaign is the most important strategy showing excellent results in terms of safety and effectiveness.Citation231,Citation232 Indeed, it allowed to reduce the severity and the impact of COVID-19 pandemic. However, several skin diseases induced or exacerbated by COVID-19 vaccination have been reported. Fortunately, most of them were mild and self-limited, not requiring medical attention. In our review, we highlighted several cutaneous reactions following COVID-19 vaccination such as psoriasis, atopic dermatitis, bullous disease, etc. Even if not specifically investigated, local injection-site reaction was the commonest cutaneous vaccine-related adverse event reported. Of note, cutaneous reactions were reported following vaccination with both mRNA and viral vector-based vaccines, suggesting that the pathogenetic mechanism underlying the cutaneous reaction is not directly related to the vaccine mechanism of action itself.Citation99 Certainly, further studies are needed to understand pathogenetic mechanisms linking cutaneous reaction and COVID-19 vaccination in order to identify “at-risk” subjects and to adopt preventive measures.
Of note, among the articles reviewed in our work, the diagnosis of cutaneous reactions was confirmed by histopathological examination in most of the cases. However, a shared immune process was not found assessing the histological reports.
Overall, mRNA vaccines, particularly BNT162b2, seem to be most commonly associated with cutaneous reactions. However, mRNA vaccines were previously authorized, produced and administered worldwide. Thus, the different number of administered vaccines may explain the difference between the number of skin reactions following mRNA or viral vector-based vaccines. Further epidemiological studies will clarify if the percentage of cutaneous reactions following vaccination is significantly higher in one of the two types of vaccines, with clinical implications.
To sum up, our review analyzed several dermatoses exacerbated or developed following COVID-19 vaccination. However, the temporal association between the administration of the vaccine and the development of skin reaction may be casual.
As regards the dose of vaccination, cutaneous reactions were reported following both the first and the second dose of vaccine. Furthermore, skin reactions following both the doses in the same patient have been reported as well. In our opinion, clinicians should be prepared also to cutaneous reaction following the booster dose.Citation233
Strengths and Limitations
Main strengths of our review are the systematic method during the literature research and the high number of analyzed article and cutaneous reactions analyzed. Main limitations should be discussed. First, only the four vaccines approved by EMA have been considered. Moreover, several articles reporting registry-based studies did not allow the direct correlation between type of vaccine and cutaneous reaction. Finally, dermatological conditions developed following COVID-19 vaccination are usually mild and patients do not seek for medical attention.
Conclusion
With the worldwide advance of vaccination programs, several cutaneous reactions have been reported. Fortunately, the percentage of these adverse events is extremely low if compared with the number of vaccines administered. In our opinion, other cutaneous reactions following COVID-19 vaccination will be reported. Moreover, the pathogenetic mechanisms linking vaccination and skin reactions should be clarified. Clinicians should keep in mind the possibility of the exacerbation of the new onset of several dermatoses following vaccination in order to promptly recognize and differentiate vaccine-induced cutaneous manifestations from other clinical entities. Certainly, vaccination should not be discouraged.
Disclosure
The authors report no conflicts of interest in this work.
References
- Shivalkar S, Pingali MS, Verma A, et al. Outbreak of COVID-19: a detailed overview and its consequences. Adv Exp Med Biol. 2021;1353:23–45.
- Marasca C, Annunziata MC, Camela E, et al. Teledermatology and inflammatory skin conditions during COVID-19 era: new perspectives and applications. J Clin Med. 2022;11(6):1511. doi:10.3390/jcm11061511
- Ibrahim AE, Magdy M, Khalaf EM, et al. Teledermatology in the time of COVID-19. Int J Clin Pract. 2021;75(12):e15000. doi:10.1111/ijcp.15000
- Villani A, Fabbrocini G, Annunziata MC, et al. Maskne prevalence and risk factors during the COVID −19 pandemic. J Eur Acad Dermatol Venereol. 2022;36(9):e678–e680. doi:10.1111/jdv.18248
- Ruggiero A, Martora F, Fornaro L, et al. The impact of COVID-19 pandemic on nonmelanoma skin cancers: report of a Southern Italy referral centre [published online ahead of print, 2022 Jun 21]. Clin Exp Dermatol. 2022. doi:10.1111/ced.15307
- Martora F, Marasca C, Fabbrocini G, et al. Strategies adopted in a southern Italian referral centre to reduce Adalimumab discontinuation: comment on ‘Can we increase the drug survival time of biologic therapies in hidradenitis suppurativa?’. Clin Exp Dermatol. 2022;47(10):1864–1865. doi:10.1111/ced.15291
- Tull MT, Edmonds KA, Scamaldo KM, et al. Psychological outcomes associated with stay-at-home orders and the perceived impact of COVID-19 on daily life. Psychiatry Res. 2020;289:113098. doi:10.1016/j.psychres.2020.113098
- Villani A, Scalvenzi M, Fabbrocini G, et al. Effects of COVID-19 pandemic on malignant melanoma diagnosis. J Eur Acad Dermatol Venereol. 2022. doi:10.1111/jdv.18545
- Picone V, Gallo L, Fabbrocini G, et al. Impact of COVID-19 pandemic and the use of telemedicine on the diagnosis and treatment of tinea corporis: an experience of Southern Italy center. Dermatol Ther;2022. e15789. doi:10.1111/dth.15789
- De Lucia M, Potestio L, Costanzo L, et al. Scabies outbreak during COVID-19: an Italian experience. Int J Dermatol. 2021;60(10):1307–1308. doi:10.1111/ijd.15809
- Hudson A, Montelpare WJ. Predictors of vaccine hesitancy: implications for COVID-19 public health messaging. Int J Environ Res Public Health. 2021;18(15):8054. doi:10.3390/ijerph18158054
- Olusanya OA, Bednarczyk RA, Davis RL, et al. Addressing parental vaccine hesitancy and other barriers to childhood/adolescent vaccination uptake during the coronavirus (COVID-19) pandemic. Front Immunol. 2021;12:663074. doi:10.3389/fimmu.2021.663074
- Sharif N, Alzahrani KJ, Ahmed SN, et al. Efficacy, immunogenicity and safety of COVID-19 vaccines: a systematic review and meta-analysis. Front Immunol. 2021;12:714170. doi:10.3389/fimmu.2021.714170
- COVID-19 dashboard by the Center For Systems Science And Engineering (CSSE) at Johns Hopkins University (JHU). Available from: https://coronavirus.jhu.edu/map.html. Accessed September 22, 2022.
- European Medicines Agency. COVID-19 vaccines: authorised. Available from: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-authorised. Accessed November 2, 2022.
- COVID-19 vaccine tracker and landscape. Available from: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines. Accessed September 11, 2022.
- Klein NP, Lewis N, Goddard K, et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021;326(14):1390–1399. doi:10.1001/jama.2021.15072
- Rosenblum HG, Hadler SC, Moulia D, et al. Use of COVID-19 vaccines after reports of adverse events among adult recipients of Janssen (Johnson & Johnson) and mRNA COVID-19 vaccines (pfizer-biontech and moderna): update from the advisory committee on immunization practices - United States, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70(32):1094–1099. doi:10.15585/mmwr.mm7032e4
- Walsh EE, Frenck RWJ, Falsey AR, et al. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi:10.1056/NEJMoa2027906
- Sun Q, Fathy R, McMahon DE, et al. COVID-19 vaccines and the skin: the landscape of cutaneous vaccine reactions worldwide. Dermatol Clin. 2021;39(4):653–673. doi:10.1016/j.det.2021.05.016
- Camela E, Potestio L, Fabbrocini G, et al. New frontiers in personalized medicine in psoriasis. Expert Opin Biol Ther;2022. 1–3. doi:10.1080/14712598.2022.2113872
- Camela E, Potestio L, Ruggiero A, et al. Towards personalized medicine in psoriasis: current progress. Psoriasis. 2022;12:231–250. doi:10.2147/PTT.S328460
- Tran TNA, Nguyen TTP, Pham NN, et al. New onset of psoriasis following COVID −19 vaccination. Dermatol Ther. 2022;35(8):e15590. doi:10.1111/dth.15590
- Ouni N, Korbi M, Chahed F, et al. New-onset guttate psoriasis following coronavirus disease 2019 vaccination: about two cases. Dermatol Ther. 2022;35:e15617. doi:10.1111/dth.15617
- Romagnuolo M, Pontini P, Muratori S, et al. De novo annular pustular psoriasis following mRNA COVID-19 vaccine. J Eur Acad Dermatol Venereol. 2022;36:e603–e605. doi:10.1111/jdv.18114
- Ruggiero A, Potestio L, Battista T, et al. Reply to ‘Nail psoriasis: a rare mRNA COVID-19 vaccine reaction’ by Lamberti A et al. J Eur Acad Dermatol Venereol. 2022. doi:10.1111/jdv.18537
- Ricardo JW, Lipner SR. Case of de novo nail psoriasis triggered by the second dose of Pfizer-BioNTech BNT162b2 COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;17:18–20. doi:10.1016/j.jdcr.2021.09.009
- Pesqué D, Lopez-Trujillo E, Marcantonio O, et al. New-onset and exacerbations of psoriasis after mRNA COVID-19 vaccines: two sides of the same coin? J Eur Acad Dermatol Venereol. 2022;36:e80–e81. doi:10.1111/jdv.17690
- Huang Y-W, Tsai T-F. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med. 2021;8:812010. doi:10.3389/fmed.2021.812010
- Sotiriou E, Tsentemeidou A, Bakirtzi K, et al. Psoriasis exacerbation after COVID-19 vaccination: a report of 14 cases from a single centre. J Eur Acad Dermatol Venereol. 2021;35(12):e857–e859. doi:10.1111/jdv.17582
- Koumaki D, Krueger-Krasagakis S-E, Papadakis M, et al. Psoriasis flare-up after AZD1222 and BNT162b2 COVID-19 mRNA vaccines: report of twelve cases from a single centre. J Eur Acad Dermatol Venereol. 2022;36:e411–e415. doi:10.1111/jdv.17965
- Megna M, Potestio L, Gallo L, et al. Reply to “Psoriasis exacerbation after COVID-19 vaccination: report of 14 cases from a single centre” by Sotiriou E et al. J Eur Acad Dermatol Venereol. 2022;36(1):e11–e13. doi:10.1111/jdv.17665
- Durmaz I, Turkmen D, Altunisik N, et al. Exacerbations of generalized pustular psoriasis, palmoplantar psoriasis, and psoriasis vulgaris after mRNA COVID −19 vaccine: a report of three cases. Dermatol Ther. 2022;35:e15331. doi:10.1111/dth.15331
- Tran TB, Pham NTU, Phan HN, et al. Generalized erythrodermic psoriasis triggered by vaccination against severe acute respiratory syndrome coronavirus 2. Dermatol Ther. 2022;35(6):e15464. doi:10.1111/dth.15464
- Nagrani P, Jindal R, Goyal D. Onset / flare of psoriasis following the ChAdOx1 nCoV-19 Corona virus vaccine (Oxford-AstraZeneca / Covishield): report of two cases. Dermatol Ther. 2021;34(5):1–2. doi:10.1111/dth.15085
- Piccolo V, Russo T, Mazzatenta C, et al. COVID vaccine-induced pustular psoriasis in patients with previous plaque type psoriasis. J Eur Acad Dermatol Venereol. 2022;36:e330–e332. doi:10.1111/jdv.17918
- Bostan E, Elmas L, Yel B, Yalici‐Armagan B. Exacerbation of plaque psoriasis after inactivated and BNT162b2 mRNA COVID-19 vaccines: a report of two cases. Dermatol Ther. 2021;34(6):1–3. doi:10.1111/dth.15110
- Pavia G, Gargiulo L, Spinelli F, et al. Generalized pustular psoriasis flare in a patient affected by plaque psoriasis after BNT162b2 mRNA COVID-19 vaccine, successfully treated with risankizumab. J Eur Acad Dermatol Venereol. 2022;36:e502–e505. doi:10.1111/jdv.18032
- Durmus O, Akdogan N, Karadag O, et al. Erythroderma related with the first dose of Pfizer-BioNTech BNT16B2b2 COVID-19 mRNA vaccine in a patient with psoriasis. Dermatol Ther. 2022;35(5):e15363. doi:10.1111/dth.15363
- Fang W-C, Chiu L-W, Hu -SC-S. Psoriasis exacerbation after first dose of AstraZeneca coronavirus disease 2019 vaccine. J Dermatol. 2021;48:e566–e567. doi:10.1111/1346-8138.16137
- Krajewski PK, Matusiak Ł, Szepietowski JC. Psoriasis flare-up associated with second dose of Pfizer-BioNTech BNT16B2b2 COVID-19 mRNA vaccine. J Eur Acad Dermatol Venereol. 2021;35:e632–e634. doi:10.1111/jdv.17449
- Trepanowski N, Coleman EL, Melson G, et al. Erythrodermic psoriasis after COVID-19 vaccination. JAAD Case Rep. 2022;28:123–126. doi:10.1016/j.jdcr.2022.07.041
- Mieczkowska K, Kaubisch A, McLellan BN. Exacerbation of psoriasis following COVID-19 vaccination in a patient previously treated with PD-1 inhibitor. Dermatol Ther. 2021;34(5):10–11. doi:10.1111/dth.15055
- Lopez ED, Javed N, Upadhyay S, et al. Acute exacerbation of psoriasis after COVID-19 Pfizer vaccination. Proceedings (Baylor University. Medical Center); 2022: 199–201.
- Perna D, Jones J, Schadt CR. Acute generalized pustular psoriasis exacerbated by the COVID-19 vaccine. JAAD Case Rep. 2021;17:1–3. doi:10.1016/j.jdcr.2021.08.035
- Song WJ, Lim Y, Jo SJ. De novo guttate psoriasis following coronavirus disease 2019 vaccination. J Dermatol. 2022;49:e30–e31. doi:10.1111/1346-8138.16203
- Tsunoda K, Watabe D, Amano H. Exacerbation of psoriasis following vaccination with the Pfizer-BioNTech BTN162b2 mRNA COVID-19 vaccine during risankizumab treatment. J Dermatol. 2022. doi:10.1111/1346-8138.16505
- Nia AM, Silva MM, Spaude J, et al. Erythrodermic psoriasis eruption associated with SARS-CoV −2 vaccination. Dermatol Ther. 2022;35:e15380. doi:10.1111/dth.15380
- Frioui R, Chamli A, Zaouak A, et al. A case of new-onset acute generalized pustular psoriasis following Pfizer-BioNTech COVID-19 vaccine. Dermatol Ther. 2022;35:e15444. doi:10.1111/dth.15444
- Cortonesi G, Orsini C, Rubegni P, et al. New-onset psoriasis after Comirnaty (BNT162b2, BioNTech /Pfizer) vaccine successfully treated with ixekizumab. Dermatol Ther. 2022;35:e15606. doi:10.1111/dth.15606
- Lehmann M, Schorno P, Hunger RE, et al. New onset of mainly guttate psoriasis after COVID-19 vaccination: a case report. J Eur Acad Dermatol Venereol. 2021;35(11):e752–e755. doi:10.1111/jdv.17561
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol. 2022;47:153–155. doi:10.1111/ced.14895
- Wei N, Kresch M, Elbogen E, et al. New onset and exacerbation of psoriasis after COVID-19 vaccination. JAAD Case Rep. 2022;19:74–77. doi:10.1016/j.jdcr.2021.11.016
- Lamberti A, Lora V, Graceffa D, et al. Nail psoriasis: a rare mRNA COVID −19 vaccine reaction. J Eur Acad Dermatol Venereol. 2022;36(10). doi:10.1111/jdv.18255
- Ruggiero A, Picone V, Martora F, et al. Guselkumab, risankizumab, and tildrakizumab in the management of psoriasis: a review of the real-world evidence. Clin Cosmet Investig Dermatol. 2022;15:1649–1658. doi:10.2147/CCID.S364640
- Megna M, Potestio L, Fabbrocini G, et al. Tildrakizumab: a new therapeutic option for erythrodermic psoriasis? Dermatol Ther. 2021;34(5):e15030. doi:10.1111/dth.15030
- Megna M, Potestio L, Fabbrocini G, et al. Treating psoriasis in the elderly: biologics and small molecules. Expert Opin Biol Ther;2022. 1–18. doi:10.1080/14712598.2022.2089020
- Megna M, Potestio L, Ruggiero A, et al. Risankizumab treatment in psoriasis patients who failed anti-IL17: a 52-week real-life study. Dermatol Ther. 2022;35(7):e15524. doi:10.1111/dth.15524
- Megna M, Potestio L, Battista T, et al. Immune response to Covid-19 mRNA vaccination in psoriasis patients undergoing treatment with biologics. Clin Exp Dermatol. 2022. doi:10.1111/ced.15395
- Ruggiero A, Martora F, Picone V, et al. The impact of COVID-19 infection on patients with psoriasis treated with biologics: an Italian experience. Clin Exp Dermatol. 2022. doi:10.1111/ced.15336
- Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84(1):53–60.
- Merhy R, Sarkis A-S, Kaikati J, et al. New-onset cutaneous lichen planus triggered by COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35(11):e729–e730. doi:10.1111/jdv.17504
- Camela E, Guerrasio G, Patruno C, et al. Reply to “New-onset cutaneous lichen planus triggered by COVID-19 vaccination” by Merhy et al. J Eur Acad Dermatol Venereol. 2022;36:e249–e251. doi:10.1111/jdv.17866
- Gamonal SBL, Gamonal ACC, Marques NCV, et al. Lichen planus and vitiligo occurring after ChAdOx1 nCoV-19 vaccination against SARS-CoV-2. Dermatol Ther. 2022;35(5):e15422.
- Alrawashdeh HM, Al-Habahbeh O, Naser AY, et al. Lichen planus eruption following oxford-AstraZeneca COVID-19 vaccine administration: a case report and review of literature. Cureus. 2022;14:e22669. doi:10.7759/cureus.22669
- Shakoei S, Kalantari Y, Nasimi M, et al. Cutaneous manifestations following COVID-19 vaccination: a report of 25 cases. Dermatol Ther. 2022;35(8):e15651. doi:10.1111/dth.15651
- Hiltun I, Sarriugarte J, Martínez-de-Espronceda I, et al. Lichen planus arising after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:e414–e415. doi:10.1111/jdv.17221
- Herzum A, Burlando M, Molle MF, et al. Lichen planus flare following COVID-19 vaccination: a case report. Clin Case Rep. 2021;9(12):e05092. doi:10.1002/ccr3.5092
- Kato J, Kamiya T, Handa T, et al. Linear lichen planus after COVID-19 vaccination. Australas J Dermatol. 2022. doi:10.1111/ajd.13902
- Diab R, Araghi F, Gheisari M, et al. Lichen planus and lichen planopilaris flare after COVID −19 vaccination. Dermatol Ther. 2022;35(3):e15283. doi:10.1111/dth.15283
- Zagaria O, Villani A, Ruggiero A, et al. New-onset lichen planus arising after COVID-19 vaccination. Dermatol Ther. 2022;35(5):e15374. doi:10.1111/dth.15374
- Awada B, Abdullah L, Kurban M, et al. Inverse lichen planus post Oxford-AstraZeneca COVID-19 vaccine. J Cosmet Dermatol. 2022;21(3):883–885. doi:10.1111/jocd.14738
- Picone V, Fabbrocini G, Martora L, et al. A case of new-onset lichen planus after COVID-19 vaccination. Dermatol Ther. 2022;12(3):801–805. doi:10.1007/s13555-022-00689-y
- Hlaca N, Zagar T, Kastelan M, et al. New-onset lichen planus and lichen planus flare in elderly women after COVID −19 vaccination. J Cosmet Dermatol. 2022;21(9):3679–3681. doi:10.1111/jocd.15185
- Zengarini C, Piraccini BM, La Placa M. Lichen Ruber Planus occurring after SARS-CoV-2 vaccination. Dermatol Ther. 2022;35:e15389. doi:10.1111/dth.15389
- Masseran C, Calugareanu A, Caux F, et al. Extensive cutaneous lichen planus triggered by viral vector COVID-19 vaccination (ChAdOx1 nCoV-19). J Eur Acad Dermatol Venereol. 2022;36(4):e263–5. doi:10.1111/jdv.17899
- Rerknimitr P, Puaratanaarunkon T, Wongtada C, et al. Cutaneous adverse reactions from 35,229 doses of Sinovac and AstraZeneca COVID-19 vaccination: a prospective cohort study in healthcare workers. J Eur Acad Dermatol Venereol. 2022;36:e158–61. doi:10.1111/jdv.17761
- Holmes GA, Desai M, Limone B, et al. A case series of cutaneous COVID-19 vaccine reactions at Loma Linda university department of dermatology. JAAD Case Rep. 2021;16:53–57. doi:10.1016/j.jdcr.2021.07.038
- Leasure AC, Cowper SE, McNiff J, et al. Generalized eczematous reactions to the Pfizer-BioNTech COVID-19 vaccine. J Eur Acad Dermatol Venereol. 2021;35(11):e716–e717. doi:10.1111/jdv.17494
- Bekkali N, Allard T, Lengellé C, et al. [Eczematiform eruption after Pfizer-BioNTech COVID-19 vaccine]. Therapie. 2021;76:364–365. French. doi:10.1016/j.therap.2021.04.012
- Larson V, Seidenberg R, Caplan A, et al. Clinical and histopathological spectrum of delayed adverse cutaneous reactions following COVID −19 vaccination. J Cutan Pathol. 2022;49(1):34–41. doi:10.1111/cup.14104
- Potestio L, Napolitano M, Bennardo L, et al. Atopic dermatitis exacerbation after Covid-19 vaccination in Dupilumab-treated patients. J Eur Acad Dermatol Venereol. 2022;36(6):e409–e411. doi:10.1111/jdv.17964
- Niebel D, Wenzel J, Wilsmann-Theis D, et al. Single-center clinico-pathological case study of 19 patients with cutaneous adverse reactions following COVID-19 vaccines. Dermatopathol. 2021;8(4):463–476. doi:10.3390/dermatopathology8040049
- Patruno C, Potestio L, Napolitano M. Clinical phenotypes of adult atopic dermatitis and related therapies. Curr Opin Allergy Clin Immunol. 2022;22(4):242–249. doi:10.1097/ACI.0000000000000837
- Patruno C, Potestio L, Scalvenzi M, et al. Dupilumab for the treatment of adult atopic dermatitis in special populations. J Dermatolog Treat;2022. 1–6. doi:10.1080/09546634.2022.2102121
- Napolitano M, Maffei M, Patruno C, et al. Dupilumab effectiveness for the treatment of patients with concomitant atopic dermatitis and chronic rhinosinusitis with nasal polyposis. Dermatol Ther. 2021;34(6):e15120. doi:10.1111/dth.15120
- Napolitano M, Fabbrocini G, Genco L, et al. Rapid improvement in pruritus in atopic dermatitis patients treated with upadacitinib: a real-life experience. J Eur Acad Dermatol Venereol. 2022;36(9):1497–1498. doi:10.1111/jdv.18137
- Cantelli M, Martora F, Patruno C, et al. Upadacitinib improved alopecia areata in a patient with atopic dermatitis: a case report. Dermatol Ther. 2022;35(4):e15346. doi:10.1111/dth.15346
- Alexander H, Patel NP. Response to Martora et al’s “Hidradenitis suppurativa flares following COVID-19 vaccination: a case series”. JAAD Case Rep. 2022;25:13–14. doi:10.1016/j.jdcr.2022.05.005
- Martora F, Picone V, Fabbrocini G, et al. Hidradenitis suppurativa flares following COVID-19 vaccination: a case series. JAAD Case Rep. 2022;23:42–45. doi:10.1016/j.jdcr.2022.03.008
- Pakhchanian H, Raiker R, DeYoung C, et al. Evaluating the safety and efficacy of COVID −19 vaccination in patients with hidradenitis suppurativa. Clin Exp Dermatol. 2022;47:1186–1188. doi:10.1111/ced.15090
- Martora F, Marasca C, Battista T, et al. Management of patients with hidradenitis suppurativa during COVID −19 vaccination: an experience from southern Italy. Comment on: ‘Evaluating the safety and efficacy of COVID −19 vaccination in patients with hidradenitis suppurativa’. Clin Exp Dermatol. 2022;47(11):2026–2028. doi:10.1111/ced.15306
- Picone V, Martora F, Fabbrocini G, et al. “Covid arm”: abnormal side effect after Moderna COVID −19 vaccine. Dermatol Ther. 2022;35(1):e15197. doi:10.1111/dth.15197
- Magen E, Yakov A, Green I, et al. Chronic spontaneous urticaria after BNT162b2 mRNA (Pfizer-BioNTech) vaccination against SARS-CoV-2. Allergy Asthma Proc. 2022;43(1):30–36. doi:10.2500/aap.2022.43.210111
- Potestio L, Genco L, Villani A, et al. Reply to ‘Cutaneous adverse effects of the available COVID-19 vaccines in India: a questionnaire-based study’. by Bawane J et al. J Eur Acad Dermatol Venereol. 2022. doi:10.1111/jdv.18341
- Riad A, Pokorná A, Attia S, et al. Prevalence of COVID-19 vaccine side effects among healthcare workers in the Czech Republic. J Clin Med. 2021;10(7):1428. doi:10.3390/jcm10071428
- Sidlow JS, Reichel M, Lowenstein EJ. Localized and generalized urticarial allergic dermatitis secondary to SARS-CoV-2 vaccination in a series of 6 patients. JAAD Case Rep. 2021;14:13–16. doi:10.1016/j.jdcr.2021.05.018
- Peigottu MF, Ferreli C, Atzori MG, et al. Skin adverse reactions to novel messenger RNA coronavirus vaccination: a case series. Disease. 2021;9(3):58. doi:10.3390/diseases9030058
- McMahon DE, Kovarik CL, Damsky W, et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: a registry-based study. J Am Acad Dermatol. 2022;86(1):113–121. doi:10.1016/j.jaad.2021.09.002
- Fernandez-Nieto D, Hammerle J, Fernandez-Escribano M, et al. Skin manifestations of the BNT162b2 mRNA COVID-19 vaccine in healthcare workers. “COVID-arm”: a clinical and histological characterization. J Eur Acad Dermatol Venereol. 2021;35:e425–e427. doi:10.1111/jdv.17250
- Bianchi L, Biondi F, Hansel K, et al. Skin tests in urticaria/angioedema and flushing to Pfizer-BioNTech SARS-CoV-2 vaccine: limits of intradermal testing. Allergy. 2021;76(8):2605–2607. doi:10.1111/all.14839
- Corbeddu M, Diociaiuti A, Vinci MR, et al. Transient cutaneous manifestations after administration of Pfizer-BioNTech COVID-19 Vaccine: an Italian single-centre case series. J Eur Acad Dermatol Venereol. 2021;35:e483–e485. doi:10.1111/jdv.17268
- Baraldi C, Boling LB, Patrizi A, et al. Unique case of urticarial skin eruptions after COVID-19 vaccination. Am J Dermatopathol. 2022;44:198–200. doi:10.1097/DAD.0000000000002036
- Choi E, Liew CF, Oon HH. Cutaneous adverse effects and contraindications to COVID-19 vaccination; four cases and an illustrative review from an Asian country. Dermatol Ther. 2021;34:e15123. doi:10.1111/dth.15123
- Patruno C, Napolitano M, Stingeni L, et al. Skin rashes after SARS-CoV-2 vaccine: which relationship if any? Immunity Inflamm Dis. 2021;(3):622–623. doi:10.1002/iid3.428
- Burlando M, Herzum A, Cozzani E, et al. Acute urticarial rash after COVID-19 vaccination containing polysorbate 80. Clin Exp Vaccine Res. 2021;10(3):298–300. doi:10.7774/cevr.2021.10.3.298
- Thomas J, Thomas G, Chatim A, et al. Chronic spontaneous urticaria after COVID-19 vaccine. Cureus. 2021;13(9):e18102.
- Picone V, Napolitano M, Martora F, et al. Urticaria Relapse after mRNA COVID-19 vaccines in patients affected by chronic spontaneous urticaria and treated with antihistamines plus omalizumab: a single-center experience. Dermatol Ther;2022. e15838. doi:10.1111/dth.15838
- Scollan ME, Breneman A, Kinariwalla N, et al. Alopecia areata after SARS-CoV-2 vaccination. JAAD Case Rep. 2022;20:1–5. doi:10.1016/j.jdcr.2021.11.023
- Babadjouni A, Phong CH, Nguyen C, et al. COVID-19 vaccination related exacerbations of hair loss in patients with moderate to severe alopecia areata on systemic therapy. JAAD Case Rep. 2022;29:181–185. doi:10.1016/j.jdcr.2022.08.016
- Rossi A, Magri F, Michelini S, et al. Recurrence of alopecia areata after covid-19 vaccination: a report of three cases in Italy. J Cosmet Dermatol. 2021;20(12):3753–3757. doi:10.1111/jocd.14581
- Chen C-H, Chen Y-Y, Lan C-CE. Intractable alopecia areata following the second dose of COVID-19 vaccination: report of two cases. Dermatol Ther. 2022;35:e15689. doi:10.1111/dth.15689
- Abdalla H, Ebrahim E. Alopecia areata universalis precipitated by SARS-CoV-2 vaccine: a case report and narrative review. Cureus. 2022;14:e27953. doi:10.7759/cureus.27953
- Gamonal SBL, Marques NCV, Pereira HMB, et al. New-onset systemic lupus erythematosus after ChAdOX1 nCoV-19 and alopecia areata after BNT162b2 vaccination against SARS-CoV-2. Dermatol Ther. 2022;35:e15677. doi:10.1111/dth.15677
- Ho JD, McNish A, McDonald L, et al. Alopecia universalis with unusual histopathologic features after vaccination with ChAdOx1 nCoV-19 (AZD1222). JAAD Case Rep. 2022;25:4–8. doi:10.1016/j.jdcr.2022.05.002
- Su H-A, Juan C-K, Chen Y-C. Alopecia areata following ChAdOx1 nCoV-19 vaccination (Oxford/AstraZeneca). J Formosan Med Assoc. 2022;121(10):2138–2140. doi:10.1016/j.jfma.2022.03.006
- Gallo G, Mastorino L, Tonella L, et al. Alopecia areata after COVID-19 vaccination. Clin Exp Vaccine Res. 2022;11(1):129–132. doi:10.7774/cevr.2022.11.1.129
- May Lee M, Bertolani M, Pierobon E, et al. Alopecia areata following COVID −19 vaccination: vaccine-induced autoimmunity? Int J Dermatol. 2022;61:634–635. doi:10.1111/ijd.16113
- Essam R, Ehab R, Al-Razzaz R, et al. Alopecia areata after ChAdOx1 nCoV-19 vaccine (Oxford/AstraZeneca): a potential triggering factor? J Cosmet Dermatol. 2021;20:3727–3729. doi:10.1111/jocd.14459
- Nguyen B, Tosti A. Alopecia areata after COVID-19 infection and vaccination: a cross-sectional analysis. J Eur Acad Dermatol Venereol. 2022. doi:10.1111/jdv.18491
- Martora F, Fabbrocini G, Nappa P, et al. Reply to ‘Development of severe pemphigus vulgaris following SARS-CoV −2 vaccination with BNT162b2’ by Solimani et al. J Eur Acad Dermatol Venereol. 2022;36(10):e750–e751. doi:10.1111/jdv.18302
- Zou H, Daveluy S. Pemphigus vulgaris after COVID-19 infection and vaccination. J Am Acad Dermatol. 2022;87(3):709–710. doi:10.1016/j.jaad.2022.05.013
- Gui H, Young PA, So JY, et al. New-onset pemphigus vegetans and pemphigus foliaceus after SARS-CoV-2 vaccination: a report of 2 cases. JAAD Case Rep. 2022;27:94–98. doi:10.1016/j.jdcr.2022.07.002
- Rouatbi J, Aounallah A, Lahouel M, et al. Two cases with new onset of Pemphigus Foliaceus after SARS-CoV-2 vaccination. Dermatol ther. 2022;e15827. doi:10.1111/dth.15827
- Aryanian Z, Balighi K, Azizpour A, et al. Coexistence of pemphigus vulgaris and lichen planus following COVID-19 vaccination. Case Rep Dermatol Med. 2022;2022:2324212. doi:10.1155/2022/2324212
- Koutlas IG, Camara R, Argyris PP, et al. Development of pemphigus vulgaris after the second dose of the mRNA-1273 SARS-Cov-2 vaccine. Oral Dis. 2021. doi:10.1111/odi.14089
- Knechtl GV, Seyed Jafari SM, Berger T, et al. Development of pemphigus vulgaris following mRNA SARS-CoV-19 BNT162b2 vaccination in an 89-year-old patient. J Eur Acad Dermatol Venereol. 2022;36(4):e251–e253. doi:10.1111/jdv.17868
- Ong SK, Darji K, Chaudhry SB. Severe flare of pemphigus vulgaris after first dose of COVID-19 vaccine. JAAD Case Rep. 2022;22:50–52. doi:10.1016/j.jdcr.2022.01.027
- Yıldırıcı Ş, Yaylı S, Demirkesen C, et al. New onset of pemphigus foliaceus following BNT162b2 vaccine. Dermatol Ther. 2022;35:e15381. doi:10.1111/dth.15381
- Singh A, Bharadwaj SJ, Chirayath AG, et al. Development of severe pemphigus vulgaris following ChAdOx1 nCoV-19 vaccination and review of literature. J Cosmet Dermatol. 2022;21(6):2311–2314. doi:10.1111/jocd.14945
- Norimatsu Y, Yoshizaki A, Yamada T, et al. Pemphigus vulgaris with advanced hypopharyngeal and gastric cancer following SARS-CoV −2 vaccination. J Dermatol. 2022. doi:10.1111/1346-8138.16539
- Agharbi F-Z, Basri G, Chiheb S. Pemphigus vulgaris following second dose of mRNA-(Pfizer-BioNTech) COVID-19 vaccine. Dermatol Ther. 2022;35(10):e15769. doi:10.1111/dth.15769
- Almasi-Nasrabadi M, Ayyalaraju RS, Sharma A, et al. New onset pemphigus foliaceus following AstraZeneca COVID −19 vaccination. J Eur Acad Dermatol Venereol. 2022. doi:10.1111/jdv.18484
- Corrá A, Barei F, Genovese G, et al. Five cases of new-onset pemphigus following vaccinations against coronavirus disease 2019. J Dermatol. 2022. doi:10.1111/1346-8138.16554
- Solimani F, Mansour Y, Didona D, et al. Development of severe pemphigus vulgaris following SARS-CoV-2 vaccination with BNT162b2. J Eur Acad Dermatol Venereol. 2021;35(10):e649–51. doi:10.1111/jdv.17480
- Martora F, Battista T, Nappa P, et al. Pemphigus vulgaris and COVID-19 vaccination: management and treatment. J Cosmet Dermatol. 2022. doi:10.1111/jocd.15374
- Maronese CA, Caproni M, Moltrasio C, et al. Bullous pemphigoid associated with COVID-19 vaccines: an Italian multicentre study. Front Med. 2022;9:841506. doi:10.3389/fmed.2022.841506
- Maronese CA, Di Zenzo G, Genovese G, et al. Reply to “New-onset bullous pemphigoid after inactivated Covid-19 vaccine: synergistic effect of the Covid-19 vaccine and vildagliptin”. Dermatol Ther. 2022;35:e15496. doi:10.1111/dth.15496
- Hali FS, Araqi LJ, Marnissi F, et al. Autoimmune bullous dermatosis following COVID-19 vaccination: a series of five cases. Cureus. 2022;14:e23127. doi:10.7759/cureus.23127
- Gambichler T, Hamdani N, Budde H, et al. Bullous pemphigoid after SARS-CoV-2 vaccination: spike-protein-directed immunofluorescence confocal microscopy and T-cell-receptor studies. Br J Dermatol. 2022;186(4):728–731. doi:10.1111/bjd.20890
- Shanshal M. Dyshidrosiform bullous pemphigoid triggered by COVID-19 vaccination. Cureus. 2022;14:e26383. doi:10.7759/cureus.26383
- Desai AD, Shah R, Haroon A, et al. Bullous pemphigoid following the moderna mRNA-1273 vaccine. Cureus. 2022;14:e24126. doi:10.7759/cureus.24126
- Fu P-A, Chen C-W, Hsu Y-T, et al. A case of acquired hemophilia A and bullous pemphigoid following SARS-CoV-2 mRNA vaccination. J Formosan Med Assoc. 2022;121(9):1872–1876. doi:10.1016/j.jfma.2022.02.017
- Alshammari F, Abuzied Y, Korairi A, et al. Bullous pemphigoid after second dose of mRNA- (Pfizer-BioNTech) Covid-19 vaccine: a case report. Ann Med Surg. 2022;75:103420. doi:10.1016/j.amsu.2022.103420
- Hung W-K, Chi C-C. Incident bullous pemphigoid in a psoriatic patient following mRNA-1273 SARS-CoV-2 vaccination. J Eur Acad Dermatol Venereol. 2022;36:e407–e409. doi:10.1111/jdv.17955
- Pauluzzi M, Stinco G, Errichetti E. Bullous pemphigoid in a young male after COVID-19 mRNA vaccine: a report and brief literature review. J Eur Acad Dermatol Venereol. 2022;36:e257–e259. doi:10.1111/jdv.17891
- Dell’Antonia M, Anedda S, Usai F, et al. Bullous pemphigoid triggered by COVID-19 vaccine: rapid resolution with corticosteroid therapy. Dermatol Ther. 2022;35:e15208. doi:10.1111/dth.15208
- Pérez-López I, Moyano-Bueno D, Ruiz-Villaverde R. Bullous pemphigoid and COVID-19 vaccine. Med Clin. 2021;157(10):e333–e334. doi:10.1016/j.medcli.2021.05.005
- Agharbi F-Z, Eljazouly M, Basri G, et al. Bullous pemphigoid induced by the AstraZeneca COVID-19 vaccine. Ann Dermatol Venereol. 2022;149:56–57. doi:10.1016/j.annder.2021.07.008
- Young J, Mercieca L, Ceci M, et al. A case of bullous pemphigoid after the SARS-CoV-2 mRNA vaccine. J Eur Acad Dermatol Venereol. 2022;36(1):e13–e16. doi:10.1111/jdv.17676
- Nakamura K, Kosano M, Sakai Y, et al. Case of bullous pemphigoid following coronavirus disease 2019 vaccination. J Dermatol. 2021;48(12):e606–e607. doi:10.1111/1346-8138.16170
- Paolino G, Campochiaro C, Di Nicola MR, et al. Generalized morphea after COVID-19 vaccines: a case series. J Eur Acad Dermatol Venereol. 2022;36:e680–e682. doi:10.1111/jdv.18249
- Antoñanzas J, Rodríguez-Garijo N, Estenaga Á, et al. Generalized morphea following the COVID vaccine: a series of two patients and a bibliographic review. Dermatol Ther. 2022;35(9):e15709. doi:10.1111/dth.15709
- Oh DAQ, Heng YK, Tee S-I. Morphoea following COVID-19 Vaccination. Clin Exp Dermatol. 2022. doi:10.1111/ced.15349
- Metin Z, Celepli P. A case of morphea following the COVID-19 mRNA vaccine: on the basis of viral spike proteins. Int J Dermatol. 2022;61:639–641. doi:10.1111/ijd.16062
- Aryanian Z, Balighi K, Hatami P, et al. Morphea in two patients after being infected to and being vaccinated against SARS-CoV-2 infection. Clin Case Rep. 2022;10(4):e05667. doi:10.1002/ccr3.5667
- Martora F, Battista T, Ruggiero A, et al. Reply to “Morphoea following COVID-19 Vaccination”. Clin Exp Dermatol. 2022. doi:10.1111/ced.15396
- Temiz SA, Abdelmaksoud A, Dursun R, et al. Pityriasis rosea following SARS-CoV-2 vaccination: a case series. J Cosmet Dermatol. 2021;20(10):3080–3084. doi:10.1111/jocd.14372
- Ramot Y, Nanova K, Faitatziadou S-M, et al. Six cases of pityriasis rosea following SARS-CoV-2 vaccination with BNT162b2. J der Deutschen Dermatologischen Gesellschaft. 2022;20:1123–1124.
- Martora F, Fabbrocini G, Marasca C. Pityriasis rosea after Moderna mRNA-1273 vaccine: a case series. Dermatol Ther. 2022;35(2):e15225. doi:10.1111/dth.15225
- Khattab E, Christaki E, Pitsios C. Pityriasis rosea induced by COVID-19 vaccination. Eur J Case Rep Intern Med. 2022;9(2):3164.
- Cyrenne BM, Al-Mohammedi F, DeKoven JG, et al. Pityriasis rosea-like eruptions following vaccination with BNT162b2 mRNA COVID-19 Vaccine. J Eur Acad Dermatol Venereol. 2021;35:e546–e548. doi:10.1111/jdv.17342
- Valk B, Bender B. Pityriasis rosea associated with COVID-19 vaccination: a common rash following administration of a novel vaccine. Cutis. 2021;108(6):317–318. doi:10.12788/cutis.0411
- Buckley JE, Landis LN, Rapini RP. Pityriasis rosea-like rash after messenger RNA COVID-19 vaccination: a case report and review of the literature. JAAD Int. 2022;7:164–168. doi:10.1016/j.jdin.2022.01.009
- Wang C-S, Chen -H-H, Liu S-H. Pityriasis Rosea-like eruptions following COVID-19 mRNA-1273 vaccination: a case report and literature review. J Formosan Med Assoc. 2022;121:1003–1007. doi:10.1016/j.jfma.2021.12.028
- Shin SH, Hong JK, Hong SA, et al. Pityriasis rosea shortly after mRNA-1273 COVID-19 vaccination. Int J Infect Dis. 2022;114:88–89.
- Bostan E, Jarbou A. Atypical pityriasis rosea associated with mRNA COVID-19 vaccine. J Med Virol. 2022;94:814–816. doi:10.1002/jmv.27364
- Leerunyakul K, Pakornphadungsit K, Suchonwanit P. Case report: pityriasis rosea-like Eruption following COVID-19 vaccination. Front med. 2021;8:752443. doi:10.3389/fmed.2021.752443
- Cohen OG, Clark AK, Milbar H, et al. Pityriasis rosea after administration of Pfizer-BioNTech COVID-19 vaccine. Hum Vaccin Immunother. 2021;17(11):4097–4098. doi:10.1080/21645515.2021.1963173
- Dormann H, Grummt S, Karg M. Pityriasis rosea as a possible complication of vaccination against COVID-19. Dtsch Arztebl Int. 2021;118(25):431. doi:10.3238/arztebl.m2021.0257
- Bin Rubaian NF, Almuhaidib SR, Aljarri SA, et al. Pityriasis rosea following pfizer-BioNTech vaccination in an adolescent girl. Cureus. 2022;14:e27108. doi:10.7759/cureus.27108
- Fenner B, Marquez JL, Pham M, et al. Inverse pityriasis rosea secondary to COVID-19 vaccination. Proceedings (Baylor University. Medical Center); 2022: 342–343.
- Marcantonio-Santa Cruz OY, Vidal-Navarro A, Pesque D, et al. Pityriasis rosea developing after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:e721–e722. doi:10.1111/jdv.17498
- Abdullah L, Hasbani D, Kurban M, et al. Pityriasis rosea after mRNA COVID-19 vaccination. Int J Dermatol. 2021;60(9):1150–1151. doi:10.1111/ijd.15700
- Carballido Vázquez AM, Morgado B. Pityriasis rosea-like eruption after Pfizer-BioNTech COVID-19 vaccination. Br J Dermatol. 2021;185(2):e34. doi:10.1111/bjd.20143
- Martora F, Picone V, Fornaro L, et al. Can COVID-19 cause atypical forms of pityriasis rosea refractory to conventional therapies? J Med Virol. 2022;94(4):1292–1293. doi:10.1002/jmv.27535
- Drago F, Ciccarese G, Parodi A. Pityriasis rosea and pityriasis rosea-like eruptions after COVID-19 vaccines. JAAD int. 2022;9:127. doi:10.1016/j.jdin.2022.03.018
- Naoum C, Hartmann M. Herpes zoster reactivation after COVID-19 vaccination - A retrospective case series of 22 patients. Int J Dermatol. 2022;61:628–629. doi:10.1111/ijd.16116
- Agrawal S, Verma K, Verma I, et al. Reactivation of herpes zoster virus after COVID-19 vaccination: is there any association? Cureus. 2022;14(5):e25195. doi:10.7759/cureus.25195
- Monastirli A, Pasmatzi E, Badavanis G, et al. Herpes Zoster after mRNA COVID-19 vaccination: a case series. Skinmed. 2022;20(4):284–288.
- Furer V, Zisman D, Kibari A, et al. Herpes zoster following BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology. 2021;60(SI):SI90–5. doi:10.1093/rheumatology/keab345
- Vastarella M, Picone V, Martora F, et al. Herpes zoster after ChAdOx1 nCoV-19 vaccine: a case series. J Eur Acad Dermatol Venereol. 2021;35(12):e845–e846. doi:10.1111/jdv.17576
- Palanivel JA. Herpes zoster after COVID-19 vaccination-Can the vaccine reactivate latent zoster virus? J Cosmet Dermatol. 2021;20:3376–3377. doi:10.1111/jocd.14470
- Vallianou NG, Tsilingiris D, Karampela I, et al. Herpes zoster following COVID-19 vaccination in an immunocompetent and vaccinated for herpes zoster adult: a two-vaccine related event? Metab Open. 2022;13:100171. doi:10.1016/j.metop.2022.100171
- Jiang Z-H, Wong L-S, Lee C-H, et al. Disseminated and localised herpes zoster following Oxford-AstraZeneca COVID-19 vaccination. Indian J Dermatol Venereol Leprol. 2022;88:445. doi:10.25259/IJDVL_819_2021
- You I-C, Ahn M, Cho N-C, Case A. Report of herpes zoster ophthalmicus and meningitis after COVID-19 vaccination. J Korean Med Sci. 2022;37:e165. doi:10.3346/jkms.2022.37.e165
- Tanizaki R, Miyamatsu Y. Zoster sine herpete following BNT162b2 mRNA COVID-19 vaccination in an immunocompetent patient. IDCases. 2022;29:e01563. doi:10.1016/j.idcr.2022.e01563
- Russo R, Cozzani E, Micalizzi C, et al. Chilblain-like lesions after COVID-19 vaccination: a case series. Acta Derm Venereol. 2022;102:adv00711. doi:10.2340/actadv.v102.2076
- Paparella R, Tarani L, Properzi E, et al. Chilblain-like lesions onset during SARS-CoV-2 infection in a COVID-19-vaccinated adolescent: case report and review of literature. Ital J Pediatr. 2022;48(1):93. doi:10.1186/s13052-022-01296-5
- Davido B, Mascitti H, Fortier-Beaulieu M, et al. “Blue toes” following vaccination with the BNT162b2 mRNA COVID-19 vaccine. J Travel Med. 2021;28(4). doi:10.1093/jtm/taab024
- Lopez S, Vakharia P, Vandergriff T, et al. Pernio after COVID-19 vaccination. Br J Dermatol. 2021;185(2):445–447. doi:10.1111/bjd.20404
- Pileri A, Guglielmo A, Raone B, et al. Chilblain lesions after COVID-19 mRNA vaccine. Br J Dermatol. 2021;185(1):e3. doi:10.1111/bjd.20060
- Piccolo V, Bassi A, Argenziano G, et al. BNT162b2 mRNA COVID-19 vaccine-induced chilblain-like lesions reinforces the hypothesis of their relationship with SARS-CoV-2. J Eur Acad Dermatol Venereol. 2021;35:e493–e494. doi:10.1111/jdv.17320
- Pérez-López I, Gil-Villalba A, Ruiz-Villaverde R. Perniosis-like lesions after vaccination with mRNA against COVID-19. Med Clin (Barc). 2022;158:189–190. doi:10.1016/j.medcli.2021.05.016
- Cameli N, Silvestri M, Mariano M, et al. Pernio-like skin lesions after the second dose of Pfizer-BioNTech COVID-19 vaccine. J Eur Acad Dermatol Venereol. 2021;35(11):e725–e727. doi:10.1111/jdv.17500
- Kha C, Itkin A. New-onset chilblains in close temporal association to mRNA-1273 vaccination. JAAD Case Rep. 2021;12:12–14. doi:10.1016/j.jdcr.2021.03.046
- Lesort C, Kanitakis J, Donzier L, et al. Chilblain-like lesions after BNT162b2 mRNA COVID-19 vaccine: a case report suggesting that “COVID toes” are due to the immune reaction to SARS-CoV-2. J Eur Acad Dermatol Venereol. 2021;35:e630–2. doi:10.1111/jdv.17451
- Kaminetsky J, Rudikoff D. New-onset vitiligo following mRNA-1273 (Moderna) COVID-19 vaccination. Clin Case Rep. 2021;9:e04865. doi:10.1002/ccr3.4865
- Militello M, Ambur AB, Steffes W. Vitiligo possibly triggered by COVID-19 vaccination. Cureus. 2022;14:e20902. doi:10.7759/cureus.20902
- Singh R, Cohen JL, Astudillo M, et al. Vitiligo of the arm after COVID-19 vaccination. JAAD Case Rep. 2022;28:142–144. doi:10.1016/j.jdcr.2022.06.003
- Nicolaidou E, Vavouli C, Koumprentziotis I-A, et al. New-onset vitiligo after COVID −19 mRNA vaccination: a causal association? J Eur Acad Dermatol Venereol. 2022. doi:10.1111/jdv.18513
- Flores-Terry MÁ, García-Arpa M, Santiago-Sánchez Mateo JL, et al. [Facial vitiligo after SARS-CoV-2 vaccination]. Actas Dermosifiliogr. 2022;113(7):721. Spanish. doi:10.1016/j.ad.2022.01.030
- Bukhari AE. New-onset of vitiligo in a child following COVID-19 vaccination. JAAD Case Rep. 2022;22:68–69. doi:10.1016/j.jdcr.2022.02.021
- Uğurer E, Sivaz O, Kıvanç Altunay İ, et al. Newly-developed vitiligo following COVID-19 mRNA vaccine. J Cosmet Dermatol. 2022;21(4):1350–1351. doi:10.1111/jocd.14843
- Ciccarese G, Drago F, Boldrin S, et al. Sudden onset of vitiligo after COVID-19 vaccine. Dermatol Ther. 2022;35(1):e15196. doi:10.1111/dth.15196
- López Riquelme I, Fernández Ballesteros MD, Serrano Ordoñez A, et al. COVID −19 and autoimmune phenomena: vitiligo after AstraZeneca vaccine. Dermatol Ther. 2022;35:e15502. doi:10.1111/dth.15502
- Okan G, Vural P. Worsening of the vitiligo following the second dose of the BNT162B2 mRNA COVID-19 vaccine. Dermatol Ther. 2022;35:e15280. doi:10.1111/dth.15280
- Caroppo F, Deotto ML, Tartaglia J, et al. Vitiligo worsened following the second dose of mRNA SARS-CoV −2 vaccine. Dermatol Ther. 2022;35:e15434. doi:10.1111/dth.15434
- Martora F, Villani A, Marasca C, et al. Skin reaction after SARS-CoV −2 vaccines Reply to ‘cutaneous adverse reactions following SARS-CoV −2 vaccine booster dose: a real-life multicentre experience’. J Eur Acad Dermatol Venereol. 2022. doi:10.1111/jdv.18531
- McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85(1):46–55. doi:10.1016/j.jaad.2021.03.092
- Hlaca N, Zagar T, Kastelan M, et al. Pityriasis rubra pilaris following booster dose of mRNA (Pfizer-BioNTech) COVID-19 vaccine [published online ahead of print, 2022 Aug 27]. Dermatol Ther;2022. e15791. doi:10.1111/dth.15791
- Annabi E, Dupin N, Sohier P, et al. Rare cutaneous adverse effects of COVID-19 vaccines: a case series and review of the literature. J Eur Acad Dermatol Venereol. 2021;35:e847–e850. doi:10.1111/jdv.17578
- Hunjan MK, Roberts C, Karim S, et al. Pityriasis rubra pilaris-like eruption following administration of the BNT163b2 (Pfizer-BioNTech) mRNA COVID-19 vaccine. Clin Exp Dermatol. 2022;47:188–190. doi:10.1111/ced.14878
- Fiorillo G, Pancetti S, Cortese A, et al. Leukocytoclastic vasculitis (cutaneous small-vessel vasculitis) after COVID-19 vaccination. J Autoimmun. 2022;127:102783. doi:10.1016/j.jaut.2021.102783
- Avallone G, Quaglino P, Cavallo F, et al. SARS-CoV-2 vaccine-related cutaneous manifestations: a systematic review. Int J Dermatol. 2022;61(10):1187–1204. doi:10.1111/ijd.16063
- Gambichler T, Scheel CH, Arafat Y, et al. Erythrodermic pityriasis rubra pilaris after SARS-CoV −2 vaccination with concomitant COVID −19 infection. J Eur Acad Dermatol Venereol. 2022;36:e675–6. doi:10.1111/jdv.18214
- Nguyen TH, Gabros S, Friefeld S, et al. Generalized granuloma annulare after COVID-19 vaccination. JAAD Case Rep. 2022;25:18–21. doi:10.1016/j.jdcr.2022.05.003
- Martora F, Villani A, Battista T, et al. COVID-19 vaccination and inflammatory skin diseases. J Cosmet Dermatol. 2022. doi:10.1111/jocd.15414
- Camela E, Scalvenzi M, Megna M, et al. Reply to “A case of symmetrical drug-related intertriginous and flexural exanthema-like eruption associated with Pfizer COVID-19 vaccination” by Manaa et al. Dermatol Ther. 2022;35(7):e15881.
- Martora F, Fabbrocini G, Guerrasio G, et al. Reply to ‘A case of pityriasis lichenoides et varioliformis acuta developed after first dose of Oxford-AstraZeneca COVID −19 vaccine’. J Eur Acad Dermatol Venereol. 2022. doi:10.1111/jdv.18646
- Martora F, Fornaro L, Picone V, et al. Herpes zoster and alopecia areata following mRNA BNT162b2 COVID-19 vaccine: controversial immune effects. J Cosmet Dermatol. 2022. doi:10.1111/jocd.15465
- Martora F, Fabbrocini G, Nappa P, et al. Impact of the COVID −19 pandemic on hospital admissions of patients with rare diseases: an experience of a Southern Italy referral center. Int J Dermatol. 2022;61(7):e237–e238. doi:10.1111/ijd.16236
- Villani A, Potestio L, Fabbrocini G, et al. Long-term efficacy of telemedicine for patients with locally advanced basal cell carcinoma during COVID-19 pandemic [published online ahead of print, 2022 Aug 26]. Dermatol Ther;2022. e15786. doi:10.1111/dth.15786
- Ruggiero A, Martora F, Fornaro F, et al. Reply to ‘Impact of the French COVID-19 pandemic lockdown on newly diagnosed melanoma delay and severity’ by R. Molinier et al. J Eur Acad Dermatol Venereol. 2022. doi:10.1111/jdv.18512
- Villani A, Potestio L, Fabbrocini G, et al. New emerging treatment options for advanced basal cell carcinoma and squamous cell carcinoma. Adv Ther. 2022;39(3):1164–1178. doi:10.1007/s12325-022-02044-1
- Scalvenzi M, Villani A, Mazzella C, et al. Vismodegib treatment in a HIV positive patient on antiretroviral therapy. Indian J Dermatol Venereol Leprol. 2018;84(6):758–760. doi:10.4103/ijdvl.IJDVL_92_18
- Villani A, Potestio L, Fabbrocini G, et al. The treatment of advanced melanoma: therapeutic update. Int J Mol Sci. 2022;23(12):6388. doi:10.3390/ijms23126388
- Farr MA, Duvic M, Joshi TP. Teledermatology during COVID-19: an updated review. Am J Clin Dermatol. 2021;22(4):467–475. doi:10.1007/s40257-021-00601-y
- Ruggiero A, Megna M, Fabbrocini G, et al. Video and telephone teledermatology consultations during COVID-19 in comparison: patient satisfaction, doubts and concerns. Clin Exp Dermatol. 2022. doi:10.1111/ced.15286
- Megna M, Camela E, Villani A, et al. Teledermatology: a useful tool also after COVID-19 era? J Cosmet Dermatol. 2022;21(6):2309–2310. doi:10.1111/jocd.14938
- Soiza RL, Scicluna C, Thomson EC. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing. 2021;50(2):279–283. doi:10.1093/ageing/afaa274
- Liu Q, Qin C, Liu M, et al. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis. Infect Dis Poverty. 2021;10(1):132. doi:10.1186/s40249-021-00915-3
- Potestio L, Villani A, Fabbrocini G, et al. Cutaneous reactions following booster dose of COVID-19 mRNA vaccination: what we should know? J Cosmet Dermatol. 2022. doi:10.1111/jocd.15331
