Abstract
Background
Tinea capitis (TC) is a common dermatophyte infection of the scalp that can also involve the eyebrows and eyelashes.
Aim
This study aimed to find the causative fungus responsible for TC in Botswana and determine its association with the clinical types of TC.
Methods
Samples for potassium hydroxide 10% mounts and fungal cultures were collected in a microbiology laboratory at the National Health Laboratory, Gaborone, Botswana. Dermasel agar and Sabouraud dextrose agar were inoculated with the samples. Lactophenol cotton blue mounts were prepared from the culture-positive samples to study the morphological characteristics.
Results
Trichophyton violaceum was found to be the predominant causative organism of TC. Trichophyton tonsurans was isolated from one patient. Both are anthropophilic species.
Conclusion
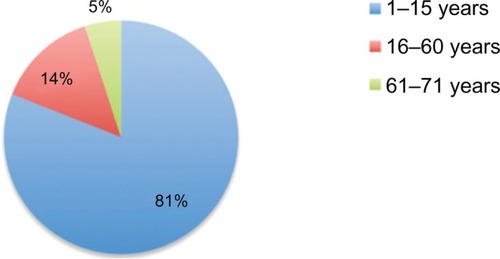
TC was found to be most common in those aged 1–15 years (81%). Of 17 patients in this age group, 16 were younger than 10 years old and one was 14 years old. T. violaceum was the most common dermatophyte species isolated.
Introduction
Tinea capitis (TC) is the most common dermatophyte infection in children, with the highest incidence in children aged 3–7 years old.Citation1 This age predilection may partly result from the fungistatic properties of fatty acids in postpubertal sebum.Citation2
Children of African descent are at increased risk of infection.Citation3 An early diagnosis is very important, because delay in treatment can lead to superadded bacterial infection resulting in cicatrization and permanent baldness. Further, from a public health point of view, it is important to treat the infection because it is contagious. The etiological agent varies from one geographical region to another. The author previously found Trichophyton violaceum to be the most common isolate in Botswana (unpublished research) and wished to formally document this current situation in this country, as she is aware that variations and transitions of responsible organisms have been identified in other centers in other countries.
Materials and methods
From January 2009 to December 2010, 42 patients attended the dermatology clinic of the referral hospital in Gaborone with a clinical diagnosis of TC. The sample consisted of 30 children and 12 adults. The clinical types of TC varied from mild non-inflammatory scaly lesions to inflammatory TC.
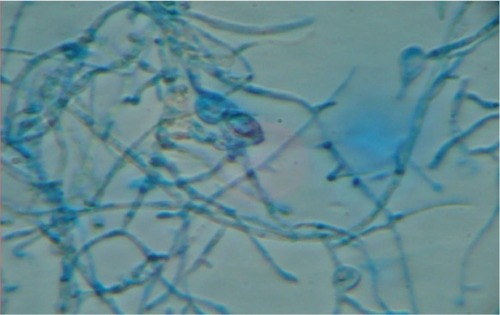
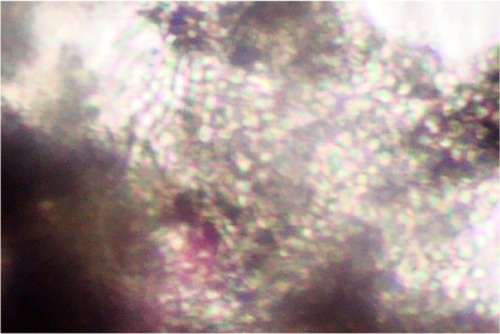
When each patient was referred to the microbiology laboratory, samples were collected after the suspected infected area was cleaned with 70% alcohol. Samples were collected from three suspicious sitesCitation4 using a scalpel blade and the wet swab method. This method was used because a simple scalp scrape alone (previously the standard method) is no longer recommended for confidently ruling out the presence of a dermatophyte.Citation5 Potassium hydroxide 10% mounts were prepared to look for fungal elements and determine the type of hair invasion endothrix ().
Figure 1 Endothrix pattern of hair invasion, with multiple spores present within the hair shaft (“bag of marbles” appearance) (potassium hydroxide 10% mount; Magnification × 400).
The sample collection had two limitations. First, several of the patients referred from different districts had not shampooed their hair and attended having applied petroleum jelly, which masked the clinical signs and interfered with the appropriate sample collection and processing. Second, carriers in the family were not screened: adults or children.
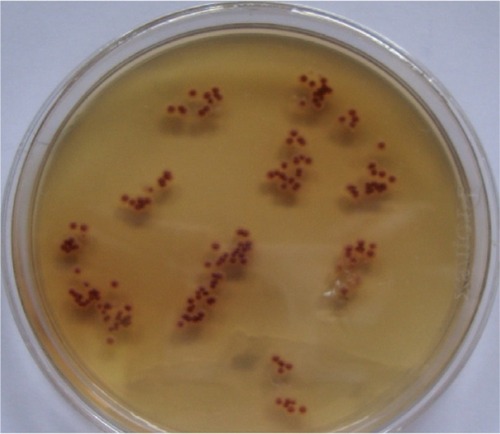
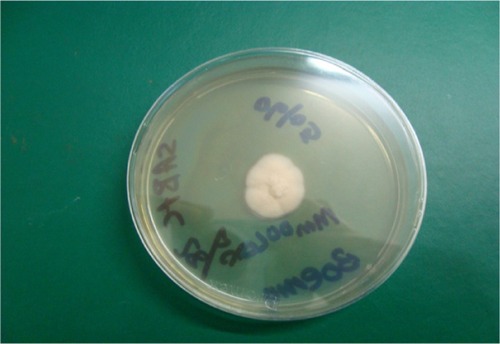
Lactophenol cotton blue mounts were prepared from the positive culture plates. Isolates were identified based on macroscopic and microscopic features compared with standard descriptions in mycological laboratory texts and manuals ( and ).Citation6–Citation9
Results
The majority of patients attending the microbiology department at the National Health Laboratory presented with diffuse scaly dandruff-like lesions. Two female patients in the 1–15 year old age group presented with a pustular type of TC and one with kerion. T. violaceum was found to be the predominant species (n = 20 [95%]) and Trichophyton tonsurans was the causative organism of TC in one case (5%). TC was most common among children aged 1–15 years (n = 17 [81%]) – 16 of the cases in this age group were in children younger than 10 years old. In those aged 16–60 and 61–67 years old, three (14%) and those one (5%), respectively, were found to have TC ( and ). The distribution of the different clinical forms of TC is given in . Some subjects were found to have tinea pedis due to Trichophyton mentagrophyte.
Table 1 Distribution of tinea capitis according to age group and sex
Table 2 Clinical types of tinea capitis identified (n = 42)
Out of the 20 cases TC due to violet pigment-producing T. violaceum was seen in 16 (80%) of the culture-positive samples () and the white variant of T. violaceum in four (20%) ().
Discussion
T. violaceum has been reported as the most common cause of TC in LibyaCitation10 and in Botswana’s neighbor South Africa. In a study of 100 children (mean age of 4.6 years) in Kwa-Zulu/Natal, dermatologists identified T. violaceum in 90% of positive cultures, making it probably the most common cause of TC in South Africa.Citation11T. violaceum has also been reported the most common dermatophyte in India,Citation12 Nepal,Citation13 and Pakistan.Citation14 TC is considered rare in adults,Citation15 but in the present study, it was identified in four adults, one of whom was a 65-year-old female. A reduction in sebum triglycerides may predispose postmenopausal women to the development of TC.Citation16
According to one study, the prevalence of dermatophytosis was four times higher in those infected with the human immunodeficiency virus.Citation17 This may be because manifestations may be atypical and more severe, resulting in extensive lesions, when dermatophytes infect immunocompromised patients.Citation18
Due to limited resources, lack of expertise, and awareness, TC can persist in the local populations of Botswana. A team of well-trained dermatologists and mycologists is needed for the right clinical and laboratory diagnosis of TC. Proper sample collection after head washing is very important for the proper clinical diagnosis and appropriate processing of samples. Since the treatment is of long duration, under- or overdiagnosis is not without complications. Diagnostic techniques must be improved by using two methods of collection and samples must be collected from at least three suspicious areas on the scalp. Asymptomatic carriage seems to be restricted to anthropophilic dermatophytes such as T. tonsurans, T. violaceum, and Microsporum audouinii. These organisms generally lack host inflammatory response and consequently mild signs of infection may escape clinical detection.Citation19
As T. violaceum and T. tonsurans are anthropophilic fungi, potential carriers should also be screened and treated once identified. Children or adults who have neither signs nor symptoms of infection, but from whose scalps causative fungi can be grown, are described as “carriers.” Such asymptomatic carriers at home or at school can shed the fungus, so are potentially important sources of disease transmission.Citation1,Citation20 These carriers should be investigated and treated if needed. However, if there is heavy dermatophyte growth from scalp brushes taken from children with clinically normal scalps they should be treated as if they are infected – that is, with oral therapy.Citation21
Carriers should be treated with adjunctive topical therapy. Selenium sulphide,Citation22 zinc pyrithione, povidone iodide, or ketconazoleCitation23 shampoos as well as fungicidal creams or lotionsCitation24 have been shown to decrease the carriage of viable spores responsible for the disease contagion. Shampoos should be applied to scalp and hair for 5 minutes twice weekly for 2–4 weeksCitation25,Citation26 or three times weekly until the patient is clinically and mycologically cured.Citation27
Some infection control measures should be observed. Brushes and combs as well as other hair accessories should be disinfected after use or discarded.Citation27 Scissors may be placed in an instrument disinfectant – for example, for 5 minutes in a Mucocit-B (Merz Hygiene, Frankfurt, Germany) drill bath.Citation24 Bed linen, towels, and hats should not be shared. According to some experts, school-going/day care-attending children can continue going to school or day care once treatment has been initiated with oral and topical agents although there is still a risk of infecting fellow students.Citation28
Conclusion
T. violaceum was the most common dermatophyte species isolated in our research in Botswana. TC was most prevalent in children aged 1–15 years old, but cases were also found in adults. Due to limited resources, lack of expertise, and awareness, TC can persist in the local populations of Botswana. Doctors, nurses, and microbiology laboratory staff should be trained in the diagnosis and management of TC.
Disclosure
The author declares no conflicts of interest in this work.
References
- ElewskiBETinea capitis: a current perspectiveJ Am Acad Dermatol2000421 Pt 112010607315
- ShyRTinea corporis and tinea capitisPediatr Rev200728516417417473121
- HackettBCO’ConnellKCafferkeyMO’DonnellBFKeaneFMTinea capitis in a paediatric populationIr Med J2006991029429517274169
- HubbardT Wde TriquetJMBrush-culture method for diagnosing tinea capitisPaediatrics1992903416418
- AkbabaMIlkitMSutolukZAtesAZorbaHComparison of hairbrush, toothbrush and cotton swab methods for diagnosing asymptomatic dermatophyte scalp carriageJ Eur Acad Dermatol Venereol200822335636218269603
- LaroneDHMedically Important Fungi: A Guide to Identification3rd edWashington DCAmerican Society for Microbiology (ASM) Press1995112184
- LennetEHBalowsaAHauslerWJJrShadomyHManual of Clinical Microbiology4th edWashington DCASM Press1985500594
- Kwon-ChungKJBennettJEMedical Mycology2nd edPhiladelphia, PALea and Febige1992105170
- RipponJWMedical Mycology: The Pathogenic Fungi and Pathogenic Actinomycetes3rd edPhiladelphia, PASaunders1988169275
- EllabibMSAgajMKhalifaZKavanaghKTrichophyton violaceum is the dominant cause of tinea capitis in children in Tripoli, Libya: results of a two year surveyMycopathologia2001153314514711998877
- MorarNDlovaNCGuptaAKAboobakerJTinea capitis in Kwa-Zulu Natal, South AfricaPaediatr Dermatol2004214444447
- KallaGBergaBSolankiAGoyalABatraAClinicomycological study of tinea capitis in desert district of RajasthanIndian J Dermatol Venereol Leprol199561634234520953016
- JhaBNGargVKAgrawalSKhanalBAgarwallaATinea capitis in eastern NepalInt J Dermatol200645210010216445495
- JahangirMHussainIKhurshidKHaroonTSA clinico-etiologic correlation in tinea capitisInt J Dermatol199938427527810321943
- BarlowDSaxeNTinea capitis in adultsInt J Dermatol19882763883803229881
- KaneJSummerbellRSiglerLKrajdenSLandGLaboratory Handbook of Dermatophytes: A Clinical Guide and Laboratory Handbook of Dermatophytes and Other Filamentous Fungi from Skin, Hair, and NailsBelmont, CAStar1997
- GoodmanDSTeplitzEDWishnerAKleinRSBurkPGHershenbaumEPrevalence of cutaneous disease in patients with acquired immunodeficiency syndrome (AIDS) or AIDS-related complexJ Am Acad Dermatol1987172 Pt 12102202957397
- RayMCGatelyLE3rdDermatologic manifestations of HIV infection and AIDSInfect Dis Clin North Am1994835836057814835
- BennassarAGrimaltRManagement of tinea capitis in childhoodClin Cosmet Investig Dermatol201038998
- IlkitMDemirhindiHAsymptomatic dermatophyte scalp carriage: laboratory diagnosis, epidemiology and managementMycopathologia20081652617118034369
- Health Protection AgencyTinea Capitis in the United Kingdom: A Report on its Diagnosis, Management and PreventionLondonHealth Protection Agency2007
- AllenHBHonigPJLeydenJJMcGinleyKJSelenium sulfide: adjunctive therapy for tinea capitisPaediatrics19826918183
- GreerDLSuccessful treatment of tinea capitis with 2% ketoconazole shampooInt J Dermatol200039430230410809984
- SeebacherCAbeckDBraschJGerman-Speaking Mycological SocietyGerman Dematology SocietyGerman Hospital Hygiene SocietyTinea capitis: ringworm of the scalpMycoses200750321822617472621
- Ginter-HanselmayerGSmolleJGuptaAItraconazole in the treatment of tinea capitis caused by Microsporum canis: experience in a large cohortPediatr Dermatol200421449950215283801
- FullerLCSmithCHCerioRA randomized comparison of 4 weeks of terbinafne vs 8 weeks of griseofulvin for the treatment of tinea capitisBr J Dermatol2001144232132711251566
- ElewskiBETreatment of tinea capitis: beyond griseofulvinJ Am Acad Dermatol1999406 Pt 2S27S3010367913
- HigginsEMFullerLCSmithCHGuidelines for the management of tinea capitis. British Association of DermatologistsBr J Dermatol20001431535810886135