Abstract
Objectives
Provide background for use of acquiring autologous adipose tissue as a tissue graft and source of adult progenitor cells for use in cosmetic plastic surgery. Discuss the background and mechanisms of action of closed syringe vacuum lipoaspiration, with emphasis on accessing adipose-derived mesenchymal/stromal cells and the stromal vascular fraction (SVF) for use in aesthetic, structural reconstruction and regenerative applications. Explain a proven protocol for acquiring high-quality autologous fat grafts (AFG) with use of disposable, microcannula systems.
Design
Explain the components and advantage of use of the patented super luer-lock and microcannulas system for use with the closed-syringe system. A sequential explanation of equipment selection for minimally traumatic lipoaspiration in small volumes is presented, including use of blunt injection cannulas to reduce risk of embolism.
Results
Thousands of AFG have proven safe and efficacious for lipoaspiration techniques for large and small structural fat grafting procedures. The importance and advantages of gentle harvesting of the adipose tissue complex has become very clear in the past 5 years. The closed-syringe system offers a minimally invasive, gentle system with which to mobilize subdermal fat tissues in a suspension form. Resulting total nuclear counting of undifferentiated cells of the adipose-derived -SVF suggests that the yield achieved is better than use of always-on, constant mechanical pump applied vacuum systems.
Conclusion
Use of a closed-syringe lipoaspiration system featuring disposable microcannulas offers a safe and effective means of harvesting small volumes of nonmanipulated adipose tissues and its accompanying progenitor cells within the SVF. Closed syringes and microcannulas are available as safe, sterile, disposable, compact systems for acquiring high-quality AFG. Presented is a detailed, step-by-step, proven protocol for performing quality autologous structural adipose transplantation.
Introduction and background
For many years, cosmetic plastic surgeons have recognized the value of low-pressure lipoaspiration for successful transplantation of adipose tissue for structural augmentation. In the introductory years (1980–1990) of liposuction techniques, autologous fat grafting (AFG) was considered unpredictable. Once bioengineers discovered the actual mechanisms by which lipoaspiration worked, the closed syringe system for gentle harvesting and transplantation was developed and patented. Early belief that effective lipoaspiration was directly related to force of vacuum was replaced by understanding, that, introduction of fluid into the fat layers permitted the adipocyte cells and stroma elements to enter into suspension. This suspension was easily extracted through use of closed syringes, and provided adipose tissues with reduced damage and improved grafting results.Citation1
In the mid-1980s, the importance of tumescent fluid distribution was first appreciated, and more value was placed on extensive pre-tunneling (moving cannula without applying vacuum). This technique better distributes local solution and enhances the ability to mobilize the adipose tissues into a suspension state, which yields more successful and predictable autologous fat grafts (AFG).Citation1
In the early 2000s, appreciation of the potential of adipose tissue and its related stromal elements led to examination of the adipose-derived adult mesenchymal stem cell content (AD-MSC).Citation2,Citation3 Evidence has clearly shown the key importance of the progenitor cells (SVF) and extracellular matrix (ECM) as integral contributors to the tissue maintenance and healing processes.Citation4 Studies of adipocyte replenishment (following normal senescence and cellular death) showed that these attached progenitor cells were activated to form adipocytes and thereby maintain adipose tissue integrity over time.Citation5,Citation6 Since there is easy accessibility and greater availability of multipotent progenitor cells for all mesogenic lines within adipose tissues, utilization of AD-MSC/SVF has become a central focus in optimizing effectiveness of autologous fat acquisition and grafting in cosmetic plastic surgery and clinical regenerative medicine.Citation7–Citation10
Preclinical and clinical applications have been reported in many scientific studies in the biological, bioengineering, and clinical medical literature.Citation11 Cosmetic plastic surgeons initially focused on understanding the mechanisms to achieve safe and effective AFG. It was believed that intact cellular (mature adipocytes) transplantation was the most important goal.Citation12 However, it has become crystal clear that mature adult adipocytes transplanted may be the least important feature producing long-term success, even in structural fat augmentation graft applications. Current beliefs are that success in long-term AFG is actually due to activation of adherent progenitor cells (attached to mature adipocytes, ECM and SVF), and proliferation of those progenitor cells to differentiate into the target cell for replacement.Citation4,Citation13–Citation15
As an example, placement of lipoaspirants into existing adipose tissue favors proliferation and differentiation into adipose cell phenotypes. As understanding of the maintenance (homeostatic) and replenishment of adipose cell cycles in vivo increases, extensive research has been devoted to the study of microenvironment (niche), cell-to-cell/cell-to-matrix factors, and autocrine/paracrine signaling system functions. Since AD-MSC/SVF are capable of differentiation for all mesogenic lines, including: 1) chondrogenic; 2) fibro-muscular (including tendon, ligament, and skeletal and cardiac muscle); 3) osteogenic; and 4) adipogenic cell lines, uses in clinical applications have increased.Citation16–Citation22
Rapidly accumulating clinical data on the safety and efficacy of AD-MSC/SVF in vivo provides clear evidence that adipose tissue grafts possess extensive potentials in wound healing, well beyond the structural augmentation in cosmetic plastic surgical uses.
Understanding these mechanisms results in important application potentials for aesthetic, reconstructive, and regenerative medicine. Reporting of preclinical, early clinical, and controlled studies in animal and human models shows worldwide recognition of the potential uses of these cells in diverse areas of medicine and surgery.Citation23–Citation25
AFG as heterogeneous stromal-stem cell source and living bioscaffolding for use in aesthetic reconstructive surgery
Research and clinical applications have led to appreciation of the existence of a large heterogeneous, undifferentiated nucleated cell populations and extensive native bioactive scaffolding that is an integral component of the total SVF components within the adipose tissues.Citation4
Most reports examining the nature and components of the stromal vascular fraction (SVF) have come from decades of study on bone marrow-derived stem cells (BM-SCs).Citation26 It is now recognized that AD-MSCs have essentially the same capabilities while offering significant advantages over BM-MSCs utilization. These include: 1) very similar progenitor differentiation capabilities; 2) important cellular subsets (such as mesenchymal and perivascular cells) are found in much higher concentrations compared to bone marrow aspirants (>1500X); 3) less expensive to harvest; 4) less invasive (safer); 5) readily available tissue; and 6) less technically demanding than bone marrow penetration and cellular harvest. These features have led to increased uses in many areas of medicine and surgery.Citation27–Citation33
With identification of the near-terminally differentiated cells (pre-adipocytes) attached directly to the cytoplastic surface of all mature adipocytes by Granneman et al, homeostatic mechanisms with adipose metabolism have been better understood.Citation34 With advanced understanding of the processes of homeostatic adipose replacement, many reasons why carefully harvested autologous fat grafts for structural augmentation is effective have become much more predictable and understood than in years past.Citation35–Citation37
Technology is now available to effectively isolate/concentrate these progenitor cells, but such manipulation involving chemical digestion is not permitted for clinical use within the US. Therefore, utilization of non-manipulated AFG/PRP concentrates remains the only option at this time. With recognition that lipoaspiration (versus en bloc excised fat) acquires a bit over 50% of the native multipotent, nucleated cell populations, such isolation and concentration protocols have gained favor in efforts to help restore the multipotent cell numbers to near native levels.Citation38 At this time, such digestion and isolation procedures are prohibited by the regulatory agencies within the US, as they are considered more-than-minimally manipulated tissues and subject to such rules.
Laboratory findings suggest that use of closed-syringe harvesting techniques produces higher nucleated cellular counts as compared with low-pressure wall suction or detuned lipoaspiration mechanical pumps (Alexander RW, Mandle R; Harvard Biosciences Laboratory, unpublished data, 2012) (See ).
Figure 1 Comparison of syringe-harvested isolated adipose-derived mesenchymal stromal cell (AD-MSC) counts (open circles) versus use of low-pressure machine harvest (wall suction or detuned lipoaspiration machine [dark circles]).
Abbreviation: OD, outside diameter.
![Figure 1 Comparison of syringe-harvested isolated adipose-derived mesenchymal stromal cell (AD-MSC) counts (open circles) versus use of low-pressure machine harvest (wall suction or detuned lipoaspiration machine [dark circles]).](/cms/asset/fb64aa63-8dce-4f73-b5a8-44cb9679b99a/dcci_a_40575_f0001_b.jpg)
The patented Tulip™ closed-syringe system (Tulip Medical Systems, San Diego, CA, USA), with its array of microcannulas (small volumes-,100 cc) and standard volume reduction cannulas (for >100 cc liporeduction and contouring), is a recognized and proven lipoaspiration system. Explanation and detailed discussion of a repeatable, effective, and safe protocol for adipose tissue complex harvest for cosmetic plastic surgeons will be provided in this paper.
Materials and methods
Selection of lipoaspiration sites
The lower abdomen and flank areas of both males and females are considered ideal adipose donor sites due to distribution of human adipose tissues and relatively large deposits. Choice of aspiration sites in the medial and lateral thigh/buttocks areas is sometimes favored for lipoaspiration and adipose graft harvesting in female patients due to genetic distribution within the gynoid body type. In patients with very low percentage body fat needing autologous grafts, use of a high-definition ultrasound probe is helpful to determine the thickness and depth of adipose deposits that can be acquired.
Preparation of lipoaspiration sites (donor and recipient)
The patients may be placed in either supine or lateral decubitus position to facilitate the preparation and complete sterile isolation of the proposed donor area(s). It is considered important to follow a standard sterile protocol for both the harvesting and placement sites. Routine operative site asepsis should be maintained in all cases, with patients marked in an upright or standing position to effectively mark the area of available or unwanted adipose tissue deposits.
Microcannula instrumentation
The patented Tulip™ closed-syringe system for lipoaspiration features very smooth cannulas and a “super” luer-lock connection for use with standard luer-lock syringes (). This hub connection is a very important component of the closed-syringe and microcannula system, in that it provides an excellent seal for maintaining even vacuum forces desirable during lipoaspiration (). Since the super-luer lock connection seals at both the internal luer connection and at the outer ring of the standard luer connection, it thereby provides a very stable, rigid base. Using microcannula sized instruments are very flexible compared to standard cannula types. The super luer lok provides substantial stability and control in these cases. Internal female luer-type connectors on some microcannula systems on the market become less efficient when cannulas are redirected, placing a torque on the junction of cannula-syringe barrel, and allowing air leakage into the closed system (particularly in the longer cannula selections). This does not usually prevent aspiration capabilities, but it does decrease the efficiency and may introduce cavitation to the harvest tissues.
Figure 2 Complete closed-syringe microcannula system (Tulip® GEMS™, Tulip Medical Systems, San Diego, CA, USA).
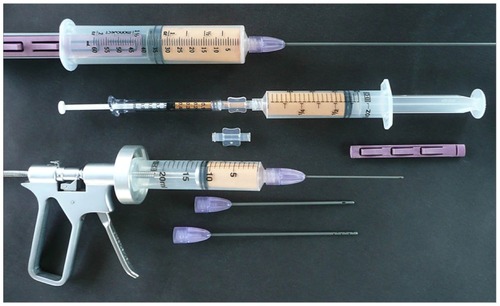
Figure 3 Tulip® super luer-lock connection (Tulip Medical Systems, San Diego, CA, USA).

Two standard options for microcannula selection offered within the Tulip™ system are:
Cell-friendly microcannula option (autoclavable) (). These cannulas are internally polished by microabrasive extrusion process to maximize internal smoothness and reduce adipose tissue damage to the adipocytes, precursor cells, and their accompanying matrix. External cannula anodizing processes provide a smoother surface for ease of passage within the subdermal adipose plane. This is a popular design utilized by cosmetic plastic surgeons for liporeduction as well as harvesting of AFG for structural augmentation procedures. References the closed syringe system instruments are also available for larger volume liporeduction (liposuction) cases and in large volume structural graft cases. In cannulas of less than 3.0 mm, it is important to thoroughly flush with water-prep soap mix, followed by ultrasonic cleaning, and thorough re-flushing with water prior to steam or gas sterilization.
Sterile, coated disposable microcannula option (). Use of disposable microcannulas in small diameters of less than 3.0 mm (range 0.9–2.4 mm outside diameter [OD]) presents a significant challenge to ensure proper and effective cleaning–sterilization cycles. This makes a disposable option attractive, particularly in the smaller diameter cannula group. These are packaged and labeled in a sterile wrap, and can be opened directly onto the sterile field or back table. Featuring the super luer-lock base, these stainless steel cannulas are totally coated internally and externally with a hydrophilic material that provides an extremely smooth coating, which passes through adipose tissues with minimal resistance and trauma. Initially, hydrogel coatings were applied on both internal and external surfaces, but now have been replaced by more efficient and effective coating materials, featuring more than 20 times more lubriciousness than previous coating materials. It is believed that the least cellular and tissue trauma created, the better the quality of the adipose grafts.
Figure 5 Disposable microcannula cannulas for closed-syringe lipoaspiration of small-volume autologous adipose grafting (Tulip® GEMS™, Tulip Medical Systems, San Diego, CA, USA).
Notes: Top: snap-lock option for syringes; middle: coated multiport infiltrator, offset harvester, single-port injector; bottom: clear luer–luer anaerobic transfer.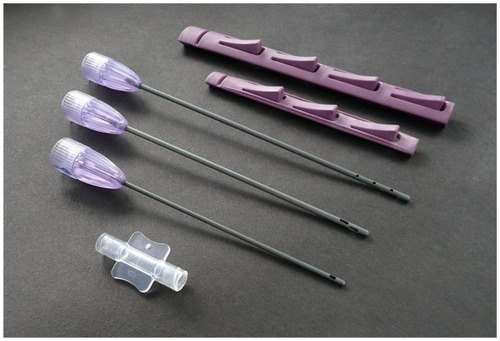
With increased recognition of difficulties in effectively cleaning the nondisposable microcannulas (<3.0 mm), most surgeons and operating facilities are choosing use of completely disposable infiltration, harvesting, and injection cannulas.
Selection of microcannula length and diameters
For small volume applications (<100 cc), it is recommended to use a small, multiport infiltrator cannula for even and thorough distribution of local anesthesia throughout the adipose donor layer. Openings near the tip are multiple and oriented for 360-degree distribution of the tumescent local solution while moving through the subdermal fat layers. It is common for practitioners to use this infiltration cannula in diameters of 2.1–2.4 mm OD and a length of 10–20 cm ( [top]).
Figure 6 Close-up of microcannula openings.
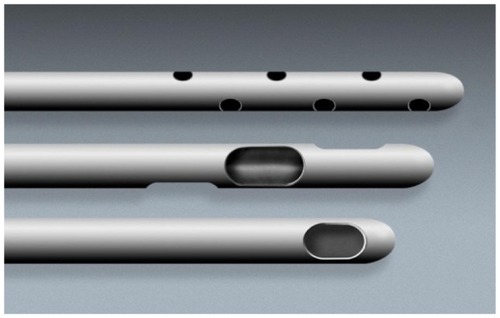
Harvesting cannulas are designed to actually acquire the adipose tissue grafts from the subdermal fat plane, following the same pattern and location of local anesthesia distribution ( [middle]).
The openings on the harvesting cannulas are usually provided as inline or offset openings (meaning in a nonlinear pattern near the tip of the cannula). These vary in diameter from 1.67 mm to 2.4 mm (OD), with a length of 10–20 cm. Selection of a slightly shorter harvesting cannula compared to length of infiltrator makes it somewhat easier to remain within the local anesthesia distribution areas in awake patients.
Closed-system syringe locks are available in two options, an “external” and an “internal” form. The external locks are specifically designed for use on Bectin Dickinson Co. (Franklin Lakes, NJ, USA) or Monoject (Covidien-Kendall, Mansfield, MA, USA) 10/12 and 20 cc luer-lock syringes and 60 cc Toomey tip syringes to hold the syringe plunger in a fully drawn position during the application of vacuum. Before application of vacuum by pulling the syringe plunger to the desired level, it is essential to draw sterile saline fluid into the cannula to completely displace all air within the system. When pulled and twisted into the locked position, the edge of the lock engages the side of the plunger permitting the physician to apply even and gentle vacuum pressure while moving the cannula through the tumesced adipose layer. The internal type lock, called a snap lock, universally fits a range of different syringe sizes from various manufacturers. ().
Figure 7 Closed-syringe lock options.
Anaerobic transfers (luer-to-luer) are utilized to facilitate loading of treatment syringes prior to grafting procedures, and for optional use of additives to the grafts (such as combining platelet concentrates [HD PRP] and adipose grafts into one syringe [ (bottom)]). They are also useful for transferring the treatment mix into syringe sizes of physician preference for injection and avoiding undesirable exposure of the harvested graft to air (). It is considered important to avoid excessive air exposure to grafts due to the potential for contamination by airborne particles or pathogens. Techniques described as helpful in free lipid removal (such as Telfa™ “rolling”; Johnson & Johnson, New Brunswick, NJ, USA) are vulnerable to such contamination.
A controlled aliquot injector gun is useful for placement of controlled 0.5 cc aliquots of graft into the prepared tunnels and locations. When additives are added to the adipose tissue graft, the density of the injection material may be increased (eg, use of HD PRP plus thrombin to provide rapid release of platelet-derived growth factors and signal proteins). This may result in the physician requiring more force to inject into the tissue site, or, to encounter sudden and uneven distribution of desired small aliquots of graft with prepared tunnels associated with adipose matrix density within the graft itself. Single-trigger pull provides exact volumes of solution to be placed with less pressure required by the provider ().
Recommended step-by-step protocol for performing closed system microcannula lipoaspiration
It is recommended that the area of donor and recipient sites be outlined with the patient in upright position and using a skin-marking pencil. This area will then be prepped, draped, and isolated to expose the thickest deposit of palpable fat tissues, and serve as a distribution pattern for local anesthetic infiltration (see for an example).
Figure 10 Example marking of lower abdomen site for harvesting of subdermal adipose tissue.
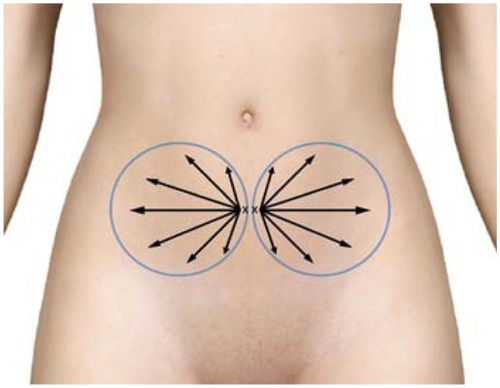
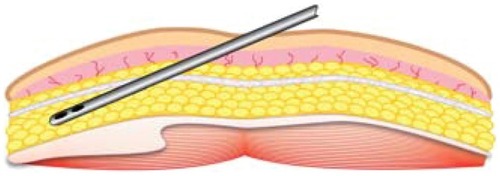
After marking, preparation, and sterile isolation of the donor area, an 18–20 g beveled needle is held such that the side edge is held vertical to the skin. This side edge is utilized to create a small, vertical (slit-like) opening, extending through the epidermis and dermis into the subdermal fat plane of the donor site. It is very important to avoid too large an opening, as closed-syringe-system vacuum depends on maintaining a tight side wall opening to ensure even vacuum application. Use of stab incisions with scalpel blades of #15 or #11 sizes tends to create larger than necessary or desired openings. The opening is made larger by selectively cutting the dermal layer (under the skin surface) with the edge of the needle bevel. In large cannula sizes, use of a tapered stainless sharp trocar (3 mm) is utilized to permit a snug fit of the aspiration cannulas into the desired space for cannulas of >3 mm OD. This allows the introduction of the multiport infiltration cannula through the skin and the subdermal fascia (and in the anterior abdominal wall example, should perforate and remain beneath Scarpa’s fascia) ().
Following entry, the multiport infiltrator cannula should be passed in a horizontal fashion within deeper aspects of the subcutaneous donor fat deposit, above the muscular layer, in a “spokes-of-a-wheel” pattern. Pinching the skin-fat tissues may help in passing the cannula. During movement of the infiltrating cannula, very slow injection of tumescent local anesthesia fluid, during both entry and withdrawal strokes improves even distribution. Placement in layers, beginning in the deeper aspects, and moving to more superficial levels is most effective. The importance of avoiding “pooling” of the tumescent local anesthesia is that evenly distributing liquids improves the efficiency of harvest by providing a suspensory fluid carrier for the adipose graft tissues, as well as excellent patient comfort. If tumescent fluids are rapidly injected or injected only in a few locations within the fat deposits, pooling is often the result. This pooling is recognized by the surgeon by the presence of larger amounts of infranatant fluids seen after gravity decantation or centrifugation.
In typical small volume grafting cases, use of tumescent local anesthesia in a range of 20–30 cc is common. As a general guideline, it is important to place at least 1 cc of tumescent local per 1 cc of anticipated graft to be harvested. For example, if the plan is to aspirate 50 cc of adipose tissues, then use of 50 cc or greater fluid volume is distributed with the adipose layer to provide the fluid carrier for extraction of the grafts. A common example of the component of tumescent solution is to add the contents of a 50 cc multidose vial of local anesthetic (eg, 0.5% to 1.0% xylocaine with or without epinephrine [1:100,000]) to 1 L of sterile saline or balanced salt solution to provide sufficient tumescent fluid for lipoaspiration.
Upon completion of even distribution of tumescent fluid within the proposed donor area, it is recommended that repetitive passage of the infiltrating cannula throughout the tumesced donor area (termed “pre-tunneling”) multiple times is important and very helpful to attain an even and high quality graft. This more thoroughly distributes local anesthetic fluid for patient comfort, and it also provides the needed “carrier fluid” to effectively suspend the adipose tissues prior to harvesting with low pressure and minimal bleeding. This is a very important step that will improve comfort during harvest, plus make extraction more efficient and result in markedly less volume of the unwanted infranatant fluid layer.
In small-volume transfers, most practitioners select a 20 cc luer syringe attached to the harvesting microcannula with a mounted locking device. A very small volume (1–2 cc) of sterile 0.9% saline is drawn into the cannula to displace air from the system prior to insertion into the harvest (donor site). This is termed “charging” the syringe device, and is necessary to eliminate all air from within the cannula and syringe, thereby avoiding cavitation produced when using mechanical pump suction devices (wall suction, detuned lipoaspiration machines, etc).
Once the harvesting cannula is inserted into the locally tumesced and pre-tunneled adipose layer, the syringe plunger is drawn to partial or full extension depending on desired vacuum pressure, and twisted to provide a “lock,” if using an external type, or a ledge, which snaps to hold the plunger in one of three positions. After application of vacuum, the physician is free to move the harvesting cannula in a forward and back series of passages. It is important that these passages are within the same plane and of the same pattern used during the placement of tumescent solution. Adipose return, at first, will be somewhat slower, as the graft tissue must be in suspension to be able to be easily extracted. Continuing these movements with vacuum applied will yield adipose tissues with minimal bleeding in most patients.
During the displacement of air, it is recommended that 4 inch by 4 inch sterile gauze should be held over the harvesting tip openings to avoid spraying contents. Occasionally, in cases where there is a larger volume of infranatant fluid (the layer immediately below the fat tissues), simply express the liquid portion, reinsert the harvesting cannula into the harvest site, lock the plunger, and gather more graft tissue. In the event of vacuum pressure loss during the harvesting process, it is sometimes necessary to completely remove the harvesting cannula from the donor site, carefully expressing all air from within the cannula. When this is completed, reinsertion of the cannula is performed, and vacuum is re-established by withdrawing (pulling) the plunger and re-locking it.
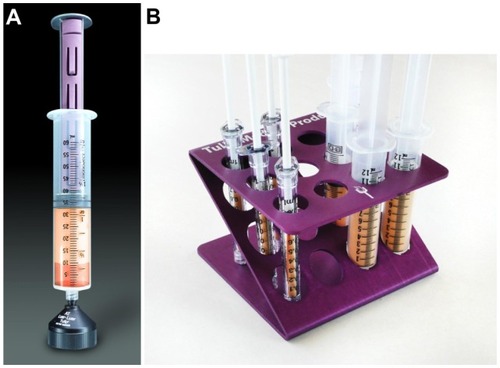
The yellow adipose grafts will quickly gravity separate from the underlying (infranatant) fluid, resulting in the graft floating on top of the small fluid volume within the syringe system. Test tube/syringe stands or decanting stands are available to facilitate this initial gravity separation ().
Remember that a common cause of increased infranatant volume in the decanted syringe is inadequate distribution of local fluid, creating a pooling effect, which reduces efficiency of adipose harvest. It is for this reason that extensive pre-tunneling is highly recommended prior to application of any vacuum to the tissues. Upon completion of aspiration of the desired graft volume, the harvester cannula is removed, and the syringe end-capped and placed in a vertical position in a standard test tube rack or directly mounted on a luer base of a decanting stand to allow gravity to separate three distinct layers within the harvesting syringe. This usually requires decantation for approximately 2–3 minutes or centrifugation (discussed below) at 1000 g for 3–4 minutes. After layer separation, practitioners expel the unwanted liquid layer (infranatant liquid) on which the fat graft floats, which is expressed into sterile containers for disposal.
Following removal of the infranatant fluid layer, if additional graft is needed, it is possible to reinsert the harvester into the donor site to acquire more graft tissue (adipocytes and adipose-derived tissue stromal vascular fraction), additional decanting/centrifugation, followed by anaerobic transfer loading of the graft into the treatment syringes of choice.
When the desired volume is attained, lipoaspiration is completed, and the small opening is covered with a triple antibiotic ointment and a Coverlet (BSN Medical, Charlotte, NC, USA) bandage (adhesive on all sides).
In the event of loss of vacuum (evidenced by a hissing sound), the cannula is simply removed from the adipose tissues, fluid advanced into the harvesting cannula to eliminate air from the system, cannula reinserted, and plunger pulled open and locked to restart the aspiration process.
Ideally, the more thorough removal of unwanted fluids from the graft yields a more dense cellular graft. It is important to effectively avoid the top layer (free lipid layer) from being included in the final AFG transplantation. In addition, the free lipid layer (clear yellow liquid above the harvested graft) should be avoided, as it is an irritant and prolongs the healing of the graft tissues in that it must be removed during the process by macrophages, etc, over time.
Centrifugation option in AFG (recommended)
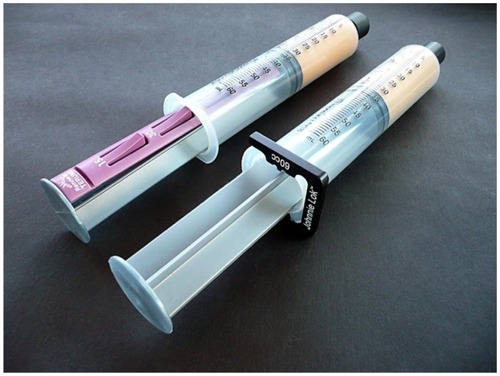
The desire to produce the best compact grafts with minimal remaining fluid excess has led many to include use of optimal centrifugation (1000 g for 3–4 minutes) to effectively compress the graft materials, help to more precisely separate fluid from harvested graft, and facilitate removal of the unwanted free lipid component ()Citation39 “Several providers of tissue processing syringes offer the ability to more easily remove the lipid layer from the graft tissues desired (see ). Whether by disk or weighted plunger, the free lipids are allowed to pass out of the upper graft tissues and remain in the harvesting syringe itself. The ability to thoroughly remove unnecessary fluid from the graft provides the surgeon an opportunity to avoid the mandatory “overcorrection” needed due to the amount of remaining carrier fluids within the graft. Further, use of a disposable separator disk, within the centrifuge processing syringe, ensures more complete separation of the free lipid free lipid layer. Its removal prevents the resulting irritation and inflammatory response when removing the unwanted additional free lipid from within a recipient site ().
Figure 13 The SmartPRep®2 APC+™centrifuge, which forms part of the AdiPRep™ Adipose Transfer System (Harvest-Terumo, Plymouth, MA, USA).

Figure 14 Close-up of a disposable processing syringe containing extracted tissue that has separated.

Some practitioners perform one to two rinses with sterile saline to help reduce any residual local anesthetic solution and red blood cells.Citation40
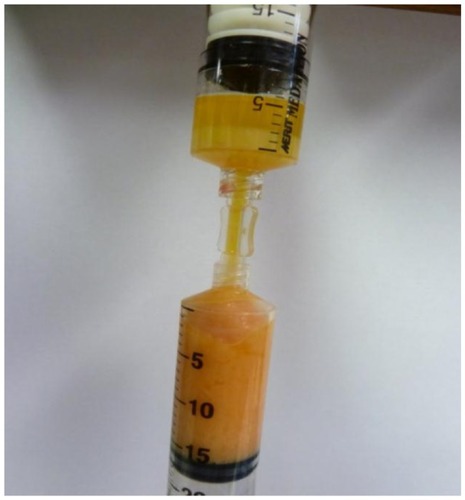
After decantation and/or centrifugation steps, the graft preparation is ready for placement into treatment syringes of physician’s choice. It is important to use clear anaerobic transfers (luer-to-luer connectors) to load the individual application syringes from the prepared, compressed graft (). They are advantageous in that they prevent external air exposure and potential for contamination and permit use of additives, such as high-density platelet-rich plasma (HD PRP) concentrates. Use of HD PRP concentrates are believed to further enhance the healing, graft acceptance, and provide high concentrations of helpful growth factors and signal proteins.
Figure 15 Anaerobic transfer from disposable processing syringe (above) to adipose fat graft syringe (below).
Treatment-standard luer syringes are then mounted with the desired injection cannulas, using coated, single-port cannulas or the surgeons, preferred injector needles. Use of blunt-tip, coated cannulas is recommended, particularly within the facial recipient areas to lower the risk of embolism caused by inadvertent injection of the adipose graft intravascularly. The injection cannulas are available in a variety of lengths and diameters (ranging from 0.9 mm to 1.47 mm OD), to accommodate the surgeon’s preference and specific areas to be grafted. Some elect to inject with sharp needles ranging in size from 18 g to 25 g ().
Figure 16 Tulip® GEMS™ (Tulip Medical Systems, San Diego, CA, USA) single-port injection cannulas (top) and close-up of the tip of one of these (bottom).

The typical graft recipient bed is prepared and developed by pre-tunneling to create a “potential” space, which is subsequently filled in small aliquots and in layers as the injection cannula is being withdrawn.
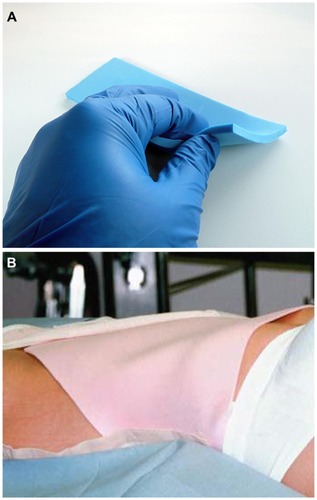
It is recommended that the donor sites be dressed in a proper fashion. Use of small, sterile absorbing gauze dressing placed over the skin opening created to place the tumescent fluids into the subdermal tissues. Further, use of compression and closed cell, medical grade foam (TenderFoam™; T&N Industries, San Diego, CA, USA) will eliminate or minimize post-harvest bruising of the donor area. It is believed that use of foam and compression reduces the lymphatic dispersion of small amounts of red blood cells or breakdown pigments remaining within the surgical harvest site in the form of bruising or discoloration. Firm compression of the gauze and TenderFoam for 24–48 hours is typically effective ().
Discussion
When the mechanisms involved in liposuction technologies were recognized in the mid–late 1980s, the ability to provide small- and large-volume liposuction via the closed-syringe system was proven safe and more predictable and effective. By use of low vacuum pressure and avoiding air within the system, the closed syringe system improved the results of liporeduction surgery and autologous fat grafting success. This included removal of significantly larger volumes in a single session and reduction of deposits within the superficial plane. The ability to safely and effectively reduce unwanted fat deposits, coupled with improved skin redraping resulted in improved surgical outcomes. Besides volume implications, closed-syringe lipoaspiration launched the beginning of more consistent and predictable autologous fat grafting procedures, with improved safety, efficacy, and predictability within aesthetic surgical applications. Structural fat grafting, utilizing the exact techniques described herein, has been completed many thousands of times by many cosmetic plastic surgeons ().
Figure 18 Clinical examples. Lips (A) pre- and (B) 1-year post-augmentation (autologous fat grafting [AFG] plus high-density platelet-rich plasma [HD PRP] [upper lip 3 cc; lower lip 2 cc]). (C) Pre- and (D) postoperative (20 months) AFG plus HD PRP grafting to lips, cheeks and nasolabial folds (lips: upper 2.5 cc; lower 2 cc; cheeks: 5 cc bilateral, malar and sub-malar placement; nasolabial folds: 3 cc bilateral). Cheeks (E and F) pre- and (G and H) 2 years post-AFG plus HD PRP (bilateral cheeks; total volume grafted, 5 cc bilateral malar and sub-malar) (Photos with permission). (I) Pre- and (J) postoperative (4 years) large-volume augmentation of both breasts (closed-syringe technique, cell-friendly cannulas) using AFG plus HD PRP concentrate; right, 300 cc; left, 325 cc).
![Figure 18 Clinical examples. Lips (A) pre- and (B) 1-year post-augmentation (autologous fat grafting [AFG] plus high-density platelet-rich plasma [HD PRP] [upper lip 3 cc; lower lip 2 cc]). (C) Pre- and (D) postoperative (20 months) AFG plus HD PRP grafting to lips, cheeks and nasolabial folds (lips: upper 2.5 cc; lower 2 cc; cheeks: 5 cc bilateral, malar and sub-malar placement; nasolabial folds: 3 cc bilateral). Cheeks (E and F) pre- and (G and H) 2 years post-AFG plus HD PRP (bilateral cheeks; total volume grafted, 5 cc bilateral malar and sub-malar) (Photos with permission). (I) Pre- and (J) postoperative (4 years) large-volume augmentation of both breasts (closed-syringe technique, cell-friendly cannulas) using AFG plus HD PRP concentrate; right, 300 cc; left, 325 cc).](/cms/asset/bbd7ced5-d61b-41aa-bd1e-bfb10064d16e/dcci_a_40575_f0018_c.jpg)
Scientific research has now provided important information to help explain the homeostatic and transplant acceptance mechanisms accomplished by autologous fat. As appreciation of the biocellular nature of the adipose tissue complex increases, the importance and value of the SVF has gained major attention.Citation4
The authors believe that high-quality, dense cellular grafts are very important to the effectiveness of structural fat grafting procedures. To that end, ensurance of complete layer separation of the harvested grafts provided by optimized centrifugation is considered advantageous. In addition to use of such centrifugation, the use of a disposable processing syringe (with separator disk) further ensures that free lipids are effectively removed during graft preparation. The AdiPRep™ system (Harvest-Terumo, Plymouth, MA, USA) provides such a system using the same centrifuge as used for producing HD PRP concentrates (SmartPRep II™; Harvest-Terumo, Plymouth, MA, USA). Use of closed systems for the harvesting and preparation of high-quality grafts is considered of great importance.
It is becoming mainstream knowledge that the actual transplanted mature adipocytes are gradually lost, while serving an important role in their own replacement from attached near-terminally differentiated cells (pre-adipocytes). For several years, leading practitioners sought to achieve pure, adipocyte grafts, without regard to the SVF components and the effects of the local microenvironment available with the recipient fat tissues.
The importance of signaling and growth factor secretion associated with certain paracrine effects, have changed the treatment paradigm of small volume structural grafting. While it is now clear that the loss of most of the mature adipocytes is inevitable, it is appreciated that they still play an important integral role, as they participate in stimulation of the microenvironment, within the associated stromal vascular fraction. The near-terminally differentiated pre-adipocytes are activated to initiate their final differentiation into adipocytes and contribution to lipid metabolism and volume storage. Further, it is now known that the cellular and extracellular matrix (ECM) associated within the stromal elements serves an equally important role, serving as a living bio-scaffold (attachment to adipocytes and SVF) that is critical in encouraging available multipotent cells to effectively activate and proliferate. Both the AFG and additive effects are further enhanced in the surgically damaged recipient tissues through complex “signaling” mechanisms of autocrine and paracrine pathways in vivo.Citation4,Citation36
Conclusion
This paper presents a simple and effective method of lipoaspiration with which to harvest adipocytes and their accompanying progenitor and stromal elements using a disposable, closed syringe and microcannula system. Effective for lipoaspiration of small and large volumes, the use of a closed-syringe system and its accessories offers a full range of options by which to fulfill all AFG requirements. The safety and efficacy of using closed-syringe systems has evolved to the use of a coated, disposable microcannula system specifically designed for use in structural autologous fat harvest and transfer, which currently serves as the most complete and effective gold standard for all closed-syringe systems.
Acknowledgment
All patients consented for use of photographs.
Disclosure
The authors report no conflicts of interest in this work. There are no financial conflicts of interest with Tulip Medical ™, Johnson & Johnson (Telfa™), or T&N Industries (TenderFoam™). Dr Harrell is currently the Chief of Cellular Science and Education, Harvest-Terumo, Plymouth, MA, USA.
References
- AlexanderRWLiposculpture in the superficial plane: closed syringe system for improvements in fat removal and free fat transferAmerican Journal of Cosmetic Surgery199211127134
- ZukPAZhuMMizunoHMultilineage cells from human adipose tissue: implications for cell-based therapiesTissue Eng20017221123811304456
- ZukPAZhuMAshjianPHuman adipose tissue is a source of multipotent stem cellsMolBiol Cell2002131242794295
- AlexanderRWUnderstanding adipose-derived stromal vascular fraction (AD-SVF) cell biology and use on the basis of cellular, chemical, structural and paracrine components: a concise reviewJournal of Prolotherapy201241e855e869
- AldermanDAlexanderRWAdvances in regenerative medicine: high-density platelet-rich plasma and stem cell prolotherapyJournal of Prolotherapy2011104990
- SugaHEtoHAoiNAdipose tissue remodeling under ischemia: death of adipocytes and activation of stem/progenitor cellsPlastReconstr Surg2010126619111923
- EtoHSugaHInoueKAdipose injury-associated factors mitigate hypoxia in ischemic tissues through activation of adipose-derived stem/progenitor/stromal cells and induction of angiogenesisAm J Path201117852322233221514444
- KatoHShort- and long-term cellular events in adipose tissue remodeling after non-vascularized graftingPaper presented at the International Federation for Adipose Therapeutics and Science Miami 2011 9th Annual Symposium on Adipose Stem Cells and Clinical Applications of Adipose Tissue2011 Nov 4–6Miami, Florida, USA
- DoiKCellular origin in adipose tissue remodeling after transplantation: host or donor?Paper presented at the International Federation for Adipose Therapeutics and Science Miami 2011 9th Annual Symposium on Adipose Stem Cells and Clinical Applications of Adipose Tissue2011 Nov 4–6Miami, Florida, USA
- SadatiKSCorradoACAlexanderRWPlatelet-rich plasma (PRP) utilized to promote greater graft volume retention in autologous fat graftingAmerican Journal of Cosmetic Surgery2006234627631
- GimbleJMKatzAJBunnellBAAdipose-derived stem cells for regenerative medicineCirc Res200710091249126017495232
- ColemanSRStructural lipoaugmentationNarinsRSSafe Liposuction and Fat TransferNew York, NYMarcel Dekker2003409423
- MaumasMPeyrafitteJAD’AngeloRNative human adipose stromal cells: localization, morphology and phenotypeIntl J Obes (Lond)201135911411153
- StremBMHicokKCZhuMMultipotential differentiation of adipose-derived stem cellsKeio J Med200554313214116237275
- ParkerAMKatzAJAdipose-derived stem cells for the regeneration of damaged tissuesExpert OpinBiolTher200666567578
- GuilakFCohenDMEstesBTGimbleJMLiedtkeWChenCSControl of stem cell fate by physical interactions with the extracellular matrixCell Stem Cell200951172619570510
- GuilakFEstesBTDiekmanBOMoutosFTGimbleJM2010Nicolas Andry Award: Multipotent adult stem cells from adipose tissue for musculoskeletal tissue engineeringClinOrthopRelat Res2010468925302540
- AlbanoJAlexanderRWAutologous fat grafting as a mesenchymal stem cell source and living bioscaffold in a patellar tendon tearAm J Sports Med2011214359361
- Alexander RobertWAuthor Textbook, Use of PRP In Autologous Fat GraftingAutologous Fat Grafting, TextbookShiffmanMSpringerBerlin20101487112
- AldermanDDAlexanderRWHarrisGRStem cell prolotherapy in regenerative medicine: background, research and protocolsJournal of Prolotherapy201133689708
- AstoriGVignatiFBardelliS“In vitro” and multicolor phenotypic characterization of cell subpopulations identified in fresh human adipose tissue stromal vascular fraction and in the derived mesenchymal stem cellsJ Translat Med2007555110
- GimbleJMBunnellBABuilakFSmithSRKatzAJIsolation and growth of stem cellsPalluaNSuscheckCVTissue Engineering: From Lab to ClinicBerlinSpringer-Verlag201193111
- MizunoHAdipose-derived stem and stromal cells for cell-based therapy: current status of preclinical studies and clinical trialsCurrOpinMolTher2010124442449
- NixonAJDahlgrenLAHauptJLYeagerAEWardDLEffect of adipose-derived nucleated cell fractions on tendon repair in horses with collagenase-induced tendinitisAm J Vet Res200869792893718593247
- YuGFloydZEWuXHalvorsenYDGimbleJMIsolation of human adipose-derived stem cells from lipoaspiratesMethods Mol Biol2011702172721082391
- BlackLLGaynorJGahringDEffect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: a randomized, double-blinded, multicenter, controlled trialVet Ther20078427228418183546
- UysalACMizunoHTendon regeneration and repair with adipose derived stem cellsCurr Stem Cell Res Ther20105216116719941450
- ProckopDJMarrow stromal cells as stem cells for nonhematopoietic tissuesScience1997276530971749082988
- AlexanderRWFat transfer with platelet-rich plasma for breast augmentationShiffmanMABreast Augmentation: Principles and PracticeBerlinSpringer2009451470
- AustLDevlinBFosterSYield of human adipose-derived adult stem cells from liposuction aspiratesCytotherapy20046171414985162
- YoshimuraKShigeuraTMatsumotoDCharacterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspiratesJ Cell Physiol20062081647616557516
- TraktuevDOMerfeld-ClaussSLiJA population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networksCirc Res20081021778517967785
- CrisanMYapSCasteillaLA perivascular origin for mesenchymal stem cells in multiple human organsCell Stem Cell20083330131318786417
- GrannemanJGLiPLuYTilakJSeeing the trees in the forest: selective electroporation of adipocytes within adipose tissueAm J PhysiolEndocrinolMetab20042873E574582
- SpaldingKLArnerEWestermarkPODynamics of fat cell turnover in humansNature2008453719678378718454136
- AbuzeniPZAlexanderRWEnhancement of autologous fat transplantation with platelet rich plasmaAmerican Journal of Cosmetic Surgery20011825970
- AlexanderRWAutologous fat grafts as mesenchymal stromal stem cell source for use in prolotherapy: a simple technique to acquire lipoaspirantsJournal of Prolotherapy201133680688
- YoshimuraKSatoKAoiNKuritaMHirohiTHariiKCell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cellsAesthPlast Surg20083214855
- KuritaMMatsumotoDShigeuraTInfluences of centrifugation on cells and tissues in liposuction aspirates: optimized centrifugation for lipotransfer and cell isolationPlastReconstr Surg2008121310331041
- AlexanderRWAutologous fat grafting: a study of residual intracellular adipocyte lidocaineShiffmanMAAutologous Fat Grafting: Art, Science, and Clinical PracticeBerlin and HeidelbergSpringer-Verlag2010445450