Abstract
Genital herpes is the manifestation of a herpes simplex virus 2 infection. Standard treatment uses both local and systemic approaches. Here, we report on the results of a local therapy approach with 31 female patients at a gynecological practice. In the here-described approach, established genital herpes infection was treated with the medical device Herpotherm®, with or without virostatic drugs. Herpotherm® is a certified medical device operating on the basis of local heat application. Parameters evaluated during the approach were (i) subjective patient assessments and (ii) objective assessment of the physician. In the described therapy approach a positive effect in terms of nature and severity in the course of the disease using Herpotherm® could be demonstrated. It could be shown that Herpotherm® can also be used for genital herpes and that it is well tolerated. In relation to other therapies using topical treatment for genital herpes, an extremely rapid reduction of pain and herpetic symptoms could be observed. Intolerances or discontinued use as a result of complications were not observed.
Keywords:
Introduction
Herpes simplex virus (HSV) and human herpes virus (HHV) infections are very common among the general population. With a prevalence of up to 95%, HSV type 1 infection is more common than HSV type 2 infection. The latter is the principal pathogenic cause of genital herpes, which has a prevalence of approx. 3%–60%, depending on the patient cohort examined.Citation1–Citation3 In industrial nations, however, genital herpes infection is increasingly triggered by HSV type 1 infection.
HSV is transmitted by droplet infection, via micro-lesions. It is then absorbed into epithelial cells, where the virus replicates. By destroying the host cells, virions are released and the disease is spread. Once the virus reaches the nerve endings of sensory neurons, it is endocytosed and transported to the cell body via cellular transport mechanisms.Citation4 The virus persists lifelong in the ganglia, and even after symptoms have completely disappeared it can trigger a relapse of the HSV infection through factors such as impairment of the immune system due to febrile infection.Citation5
Initial infection may be symptom-free. Over the course of time blisters and ulcers can appear, indicating tissue destruction and an inflammatory reaction. In immunosuppressed patients and occasionally in healthy patients, the viruses can spread via the lymphatic system and affect organs, which is often fatal.Citation3 The exudate from the blisters contains an extremely high concentration of the virus.Citation6
A relapse of genital herpes can manifest itself clinically in the form of unnoticed, small red spots up to extremely painful blistering of genital skin and mucous membranes. The blisters are typically round in shape, vary in their size, and appear either alone or in groups.Citation3 The overall healing time for a genital herpes relapse has a large interindividual variability with 5 to 10 days on average.Citation3,Citation7 The affected skin and mucous membrane areas are particularly stressed during the various stages of the blister-forming process (blistering/vesicular phase, wound/ulceration phase, incrustation and healing).
Up to now, treatment of genital herpes involves the systemic administration of antiviral substances such as aciclovir, famciclovir or valaciclovir.Citation8–Citation12 Since it was shown that Herpotherm® can shorten the duration of lip herpes and prevent recurrences when used early in the course of the disease,Citation13 a local therapy approach was conducted at a gynecological practice in order to see whether this holds true for genital herpes infections also.
The treatment of herpes with Herpotherm® takes place with the help of a concentrated heat in the range of 51°C–53°C which, when applied at the proper time, prevents the formation of the blisters and allows further symptoms (prickly sensation, burns, itchiness, feeling of tightness, pain) to subside quickly. Through the heating of the cells infected with the herpes virus, a total or partial denaturing of the enzymes or proteins can be achieved. The reproduction of the virus is thereby prevented and the development of the lip blisters can be stopped. In dermatological testing the formation of lip blisters was totally prevented in the test subjects by means of the correctly timed use of Herpotherm®. When used later, Herpotherm® causes a less serious cycle and/or an earlier subsidence of the symptoms.Citation13
Patients and methods
The local therapy approach was conducted at a single medical practice (Dr EN Kondrateva, Russia) from January 2007 to December 2008. Thirty-one female patients with established HSV infection aged 23–49 years (mean 35) were treated in the therapy. All patients had, at baseline, significant clinically detectable genital herpes symptoms. Twenty-one (68%) patients received Herpotherm® (RIEMSER Pharma GmbH, Greifswald, Germany) treatment plus virostatic medication (aciclovir, [Valenta Pharmaceutica JSC, Russia] and Cycloferon, [NTFF Polysan, St Petersburg, Russia]). Ten (32%) patients were treated with Herpotherm® only. Treatment was based on the decision of the attending physician and assessed using questionnaires filled in by patients and the physician in each visit. Five visits were individually scheduled in order to assess the genital herpes outbreaks over time ().
Figure 1 Five visits were individually scheduled.
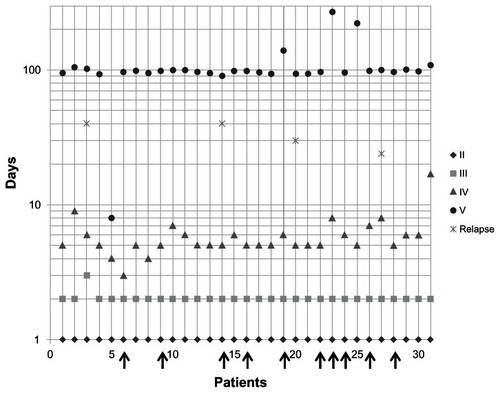
Therapy starting point was defined by initial application of the Herpotherm® after physical examination on day 1 (visit 2). Herpotherm® was used either once or several times a day. Treatment (Herpotherm® and virostatic medication) was self-administered until condition had improved, or for about 5 days.
The mode of action of Herpotherm® is based on a local and time-limited thermal stress of a defined skin area, by applying a microprocessor controlled temperature of 51°C–53°C for 4 seconds.
Results
Diagnosis of the genital herpes was confirmed during the first visit (day 0). Vesicles and/or ulcers of varying intensity were already evident in all of the patients at that time. Accordingly, the disease had already broken out. Treatment was started irrespective of disease stage.
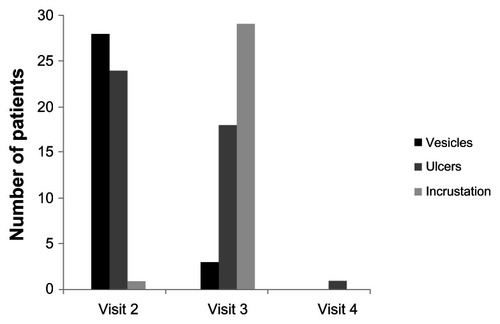
The second visit took place one day after the first visit (day 1). At this time, 28 patients (90%) had vesicles, 24 (77%) had ulcers, and one patient (3%) had incrustation. Treatment began with this visit.
At the third visit (day 2–3), vesicles had already broken down in 25 of the patients (81%), tissue infiltration was reduced in two patients (6%) and exudate was reduced in one patient (3%). Incrustation was already evident in 29 patients (94%) and ulcers in 18 patients (58%). Twenty-nine patients reported a significant reduction of pain, and one patient reported relief of an unpleasant feeling. One patient provided no information.
At the fourth visit (day 3 to 17), pink consolidation in 30 patients (97%) was evident where the vesicles/ulcers had been, to be equated with wound-healing. One patient (3%) treated with Herpotherm® and cycloferon still had scabs (). Thirty patients said that they were no longer suffering from any complaints and only one patient reported continued discomfort.
Figure 2 Improvement of herpetic symptoms from visit 2 to 4.
Visit five took place at day 8 to 270. Relapse occurred in only four patients (13%), three of them were treated with Herpotherm® plus virostatic medication and one with Herpotherm® only. All other patients were symptom-free at visit five ().
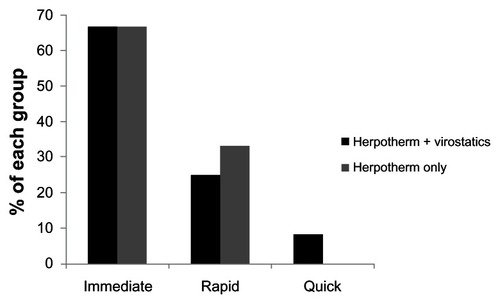
Subjective assessment through patients using Herpotherm® plus virostatics revealed that eight patients (67%) observed immediate, three (25%) rapid, and one (8%) quick improvement of HPV symptoms. Patients treated with Herpotherm® only reported in four cases (67%) an immediate and in two cases (33%) a rapid improvement of herpetic symptoms. Thirteen patients provided no information ().
Figure 3 Onset of improvement of herpetic symptoms.
Only nine patients (29%) reported adverse events; four (13%) reported pain when they first started using Herpotherm®, and the other five (16%) a general feeling of pain. The remaining 22 patients (71%) reported no adverse events.
The physician rated treatment with Herpotherm® in 28 (90%) patients as good and in the remaining three patients (10%) as satisfactory. Two of these three patients were treated with Herpotherm® plus virostatic medication and one with Herpotherm® only, all three of these patients suffered from a relapse of symptoms.
Discussion
In previous studies it could be shown that the medical device Herpotherm® can prevent recurrences of lip herpes (appearance of prodromes) and have a positive effect on further symptoms (tingling, burning, itching, tightness, pain) when used at an early stage of the disease. It was shown that herpetic symptoms could be attenuated and disease duration shortened from typically 9–9.7 days to 1.85 days when applying local heat.Citation13
The present local therapy approach indicates that Herpotherm® can also be used for genital herpes and that it is well tolerated. In relation to other therapies using topical treatment for genital herpes, an extremely rapid reduction of pain on day 1 after the start of treatment as opposed to day 3–4Citation14,Citation15 can be highlighted.
The statements of the affected patients on the efficacy of Herpotherm® support the conclusion that symptoms can be reduced; even at the stage of an existing relapse. According to the physician, the healing process of the patients treated with Herpotherm® only was not protracted or subject to discomfort. The patients receiving virostatic therapy in addition to Herpotherm® treatment appeared to have no therapeutic advantage.
In general, shortening the course of the disease was not expected, since even an antiviral therapy can be expected to shorten the duration of illness by only one day on average (from 6 to 5 days) when administered after an outbreak.Citation10–Citation12
In principle, it can be assumed that the application of Herpotherm® upon the onset of prodromes and before symptoms appear, achieves results similar to those of lip herpes treatment, as both the disease course and the pathophysiology of genital herpes matches that of lip herpes.
The underlying molecular biological mechanism of the physical therapy with Herpotherm® remains unclear. Typical for herpes is the fact that relapse always occurs within the same areas of skin/mucosa innervated by the virus-infested nerves.Citation3 The application of a local treatment therefore makes sense. Possible explanations for the mechanism of action refer, for example, to the activation of heat shock proteins (eg, HSP60), which are involved in regulating a number of proteins, including immunomodulatory proteins.Citation16 It is thought that heat therapy possibly leads to accelerated local immune reactions, which counteract the intensity of a HSV relapse. It is also conceivable that, through heat therapy, a certain degree of denaturation in the affected cells prevents the formation of new virions.
Based on the existing approach, further studies should be conducted in order to substantiate the results obtained from using Herpotherm® in genital herpes.
Acknowledgments
We thank Florian Losch for revising the manuscript and Franziska Voß for expert assistance in preparing the manuscript.
Disclosure
G Schlippe and W Voss have received honoraria for consulting activities from RIEMSER Pharma GmbH. LC Brenn is an employee of RIEMSER Pharma GmbH. The authors report no other conflicts of interest in this work.
References
- HellenbrandWThierfelderWMüller-PebodyBHamoudaOBreuerTSeroprevalence of herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) in former East and West Germany, 1997–1998Eur J Clin Microbiol Infec Dis20052413113515692814
- HaliouaBMalkinJEEpidemiology of genital herpes – recent advancesEur J Dermatol1999917718410210781
- GuptaRWarrenTWaldAGenital herpesLancet20073702127213718156035
- ConnollySAJacksonJOJardetzkyTSLongneckerRFusing structure and function: a structural view of the herpesvirus entry machineryNat Rev Microbiol2009936938121478902
- SteinerIKennedyPGHerpes simplex virus latent infection in the nervous systemJ Neurovirol1995119299222339
- DanielsCLegoffSNotkinsAShedding of infectious virus/antibody complexes from vesicular lesions of patients with recurrent herpes labialisLancet197530652452851344
- GrossGHerpes-simplex-Virus infektionenHautarzt200455818830 German15316634
- PatelRAldersonSGerettiAEuropean guideline for the management of genital herpes, 2010International Journal of STD and AIDS222011110
- Clinical Effectiveness Group (British Association for Sexual Health and HIV)National Guideline for the Management of Genital Herpes2007BASSHCheshire, UK Available at http://www.bashh.org/documents/115/115.pdfAccessed May 9, 2012
- ChosidowODrouaultYGarraffoRVeyssierPValaciclovir as a single dose during prodrome of herpes facialis: a pilot randomized double-blind clinical trialBr J Dermatol200314814214612534609
- ChosidowOFamciclovir vs aciclovir in immunocompetent patients with recurrent genital herpes infections: a parallel-groups, randomized, double-blind clinical trialBr J Dermatol200114481882411298543
- BavaroJBDroletteLKoelleDMOne-day regimen of valacyclovir for treatment of recurrent genital herpes simplex virus 2 infectionSex Transm Dis20083538338618362859
- SchlippeGVossWBayerMBungeINeue Behandlungsmethode bei einem Herpes-labialis-RezidivNew method of treatment for a herpes labialis recurrencederm Praktische Dermatologie201117340342 German
- KinghornGRTurnerEBBartonPotterCWBurkeCAFiddianAPEfficacy of topical acyclovir cream in first and recurrent episodes of genital herpesAntiviral Res198332913016670855
- CoreyLBenedettiJKCritchlowCWDouble-blind controlled trial of topical acyclovir in genital herpes simplex virus infectionsAm J Med1982733263347048919
- QuintanaFJCohenIRThe HSP60 immune system networkTrends Immunol201132899521145789