Abstract
Introduction
Vitiligo is an acquired skin pigmentation disorder, the cause of which is poorly understood. Researchers in this field are dedicated to exploring novel treatments for achieving re-pigmentation.
Methods
Mice were randomly selected and divided into control, model, and model+laser groups. Evaluate the impact of different levels of carbon dioxide laser irradiation on tyrosinase activity, melanocyte viability, and melanin content.
Results
In this study, it was found that the cell viability and melanin content were significantly enhanced in human melanocytes after treatment with different energy densities of fractional carbon dioxide laser. In addition, laser-treated vitiligo mouse models showed mild pathological changes.
Discussion
Therefore, we believe that fractional carbon dioxide laser may be a potential adjunctive modality for treating vitiligo.
Introduction
Vitiligo is an acquired cutaneous pigmentation disorder characterized by chalky white macules or patches caused by the loss of functioning melanocytes in the skin.Citation1 The pathophysiologic theory of vitiligo remains unclear. Various pathogenetic concepts have been proposed and supported by numerous studies. There are three major hypotheses for the pathogenesis of vitiligo: biochemical / cytotoxic, neural and autoimmune.Citation2 Traditional therapeutic options include topical or oral corticosteroids, topical immunomodulators, phototherapy, depigmentation therapy, and skin grafting. However, approximately 10 to 30% of patients do not respond to the treatment and remain refractory; thus, new therapies such as hair transplantation, Janus kinase inhibitors (tofacitinib citrate and ruxolitinib), platelet-rich plasma injection (PRP), oral antioxidant, and fractional laser are being investigated.Citation3–5 To date, multiple clinical trials have proposed the benefits and safety of adding fractional carbon dioxide (CO2) laser in the treatment of vitiligo. However, little is known about the direct effects of CO2 laser irradiation on normal human melanocytes at the cellular and molecular levels.Citation6,Citation7
Melanocytes are specialised pigment cells found in the skin, originating from neurons and synthesizing the pigment melanin in response to physical stimuli such as ultraviolet (UV) radiation or hormones like α-melanocyte stimulating hormone (α-MSH). Tyrosinase (TYR) is a copper-dependent enzyme that catalyzes the conversion of L-3,4-dihydroxy-phenylalanine (L-DOPA), which is the rate-limiting stage in melanin synthesis. It is possible that tyrosinase-related protein-1 (TRP-1) plays a role in the activation and stabilization of tyrosinase.Citation8,Citation9 Microphthalmia-associated transcription factor (MITF) plays a central role in melanin synthesis, as well as in the biogenesis and transport of melanosomes.
Although many treatments are available, a significant proportion of vitiligo lesions are resistant to current treatments. Therefore, we urgently need new treatments for vitiligo. Laser treatment is widely used in dermatology and can effectively treat various skin-related diseases. In recent years, carbon dioxide lasers have been introduced as an adjunct to vitiligo treatment.Citation10 This is a new skin repair method based on the theory of photothermal decomposition. Carbon dioxide laser therapy not only reduces the size of vitiligo lesions, but also stimulates the secretion of cytokines and various growth factors during wound healing, acting as a mitogen for melanocytes adjacent to normal skin and hair follicles. Additionally, lattice laser treatment enhances the penetration of ultraviolet light and local drugs through the epidermis, thereby augmenting the therapeutic effect.Citation11,Citation12 However, the effect of carbon dioxide lattice lasers on melanogenesis in human melanocytes has not been thoroughly studied. Therefore, this study aims to explore the effect and mechanism of carbon dioxide lattice lasers on human melanocytes, as well as the protective effect of carbon dioxide lattice lasers on melanocytes in the mouse vitiligo model.
Materials and Methods
Materials
Dispase II, Growth medium M-254, Human Melanocyte Growth Supplement-2 (HMGS-2) and L-DOPA were purchased from Sigma Chemical Co (St.Louis, MO, USA). Cell Counting Kit‐8 (CCK-8) was purchased from Dojindo (Kumamoto, Japan). Carbon dioxide laser therapeutic apparatus was purchased from Huagong (Wuhan Huagong Laser Medical Co., Ltd., HGL-MC30). All cells were cultured in M-254 medium supplemented with 1% HMGS-2 (Gibco, Carlsbad, CA), 100 U/mL penicillin and 0.1 mg/mL streptomycin. And all cells were maintained in an incubator under humid atmosphere at 37°C with 5% CO2. C57BL/6 mice were purchased from SLAC (Shanghai, China) and housed at the animal facility of Wenzhou Medical University. All experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the Wenzhou Medical University, China, and followed in a national standard of China (GB/T35892-2018).
Isolation and Culture of Melanocytes from Human Foreskins
These methods have been previously described.Citation13,Citation14 In short, the freshly removed prepuce was washed and sterilized in cold buffered penicillin (500 IU/mL) and streptomycin (500 mg/mL). Then, the subcutaneous fat and deep dermis were carefully removed. The prepuce with only the dermis and epidermis was further cut into small pieces and digested with Dispase II (2 mg/mL) at 4 °C for 16 hours. After that, the skin tissues were mechanically separated. The removed epidermis was then immersed in 0.25% trypsin / EDTA solution and digested continuously at 37 °C for 15 minutes. Since then, after the termination of digestion, the cell suspension was repeatedly blown to further disperse the cell mass. It ws then centrifuged at the rotational speed of 1000 rpm for 5 minutes and discarded the supernatant. The obtained cells were re-suspended in M-254 medium supplemented with 1% HMGS-2. Finally, the cells were placed in a tissue culture flask at the density of 1×105 cells/mL and cultured at 37 °C with the concentration of 5% CO2. The medium was changed after 24 hours, and then every 2–3 days. The cells were passaged with 0.25% trypsin-EDTA solution at a density of 80–90%.
Identification of Melanocytes by DOPA Staining
Melanocytes were identified through L-DOPA staining. The specific steps are as follows. The first step was to prepare melanocyte climbing tablets. After washing them with PBS solution, the climbing tablets were fixed with 4% paraformaldehyde at room temperature for 10 minutes, and then washed again with PBS solution. The above operation was repeated 3 times. The slices were incubated in L-DOPA staining solution (Shanghai Sangon Biological Engineering Co., Ltd, Shanghai, China) at 37 °C for 4 hours, and then rinsed with water for 5 minutes. The sections were observed under transmission electron microscope.
Cell Proliferation Was Detected by Cell Counting Kit-8 (CCK-8)
Cell proliferation was measured by CCK-8 assay. To put it simply, the cells were inoculated into a 96-well flat-bottomed microplate at a density of 106 per well, cultured in a growth medium supplemented with M-254, and cultured at 37 °C and 5% CO2 for 48 hours. After removing most of the medium from the microwell plate, melanocytes (0–60 mJ) were irradiated by carbon dioxide laser with different energy, and then further cultured for 24 hours, 48 hours, 3 days, 5 days and 7 days respectively. After reaching the end of the experiment, 10 μL of CCK-8 reagent was added to each well of the microplate containing 100 μL of medium, resulting in a final solution concentration of 10 μL / 100 μL. Then incubated the microplate at 37 °C for 4 hours with 5% CO2. The absorbance was repeatedly determined by an automatic enzyme labeling instrument (Bio-Rad, USA) at 450 nm. The control group (cells without CO2 laser treatment) was normalized to 100% for each experimental group, and the outcome of the experimental group was expressed as a percentage to the control. All experiments were repeated three times, and data were collected when the OD value of the control group approached 1.0.
Determination of Melanin Content
Melanin content is measured as mentioned earlier.Citation15 Human melanocytes were incubated in a T25 culture flask at the density of 1×105 cells. After 48 hours, the cells were exposed to a CO2 laser. After 7 days of incubation, the cells were isolated using trypsin / EDTA, centrifuged at 1000 rpm for 5 minutes, and the supernatant was discarded. The cells were then precipitated and re-suspended in PBS solution for cell counting. Subsequently, the aforementioned cell suspension was transferred into the Eppendorf tube and centrifuge the cell suspension under the same conditions, remove the supernatant, dissolve it in 100 μL mmol/L NaOH at 80 °C for 1 hour, and centrifuge at 16000 xg for 20 minutes. The supernatant was placed in a 96-well microplate and the absorbance of melanin was measured at a wavelength of 405 nm. The ratio of melanin absorbance to the number of cells in the control group (cells without CO2 laser treatment) was normalized to 100% for each test, and the result of the experimental group was expressed as a percentage to the control group.
Cellular Tyrosinase Activity Assay
The cells were cultured in a T25 flask at a density of 1×105 cells. After 48 hours, the cells were exposed to a carbon dioxide laser. After 7 days of incubation, the cells were lysed with phosphate buffer (pH 6.8) containing 1% TritonX-100. The cells were lysed by freeze-thawing and the lysate was clarified by 10000 g centrifugal for 5 minutes. After quantifying the protein level using lytic buffer and adjusting the concentration, 100 μL of each protein solution was placed in a 96-well plate. 40 μL L-DOPA (2 mg/mL) solution was added at 37 °C to start the enzymatic hydrolysis experiment. The absorbance was measured every 10 minutes for a minimum of 1.5 hours at a wavelength of 475 nm using an enzyme labeling instrument. Tyrosinase activity was expressed as a percentage of the control group (melanocytes treated without CO2 laser irradiation).
Mice Vitiligo Models
The female SPF C57BL/6 mice, aged 4 weeks, were purchased from the Shanghai Animal Center of the Chinese Academy of Sciences. The animal care and use procedures are in accordance with the guidelines for the care and use of experimental animals of the National Institutes of Health and approved by the Animal Care and Use Committee of Wenzhou Medical University. Vitiligo mice were induced according to the above method.Citation16 In short, 40% monobenzone cream (4-benzyloxyphenol, Sigma) was applied to the 2×2 cm, hair-removed part of the mouse abdomen. Use a spatula to massage topical ointment thoroughly. The ointment was applied for 50 days and observed until the 65th day. The mice in the control group were topically treated with Vaseline for 50 days.
Experimental Animal Design
The mice were randomly divided into 3 groups of 10 mice each. Normal control group (CL), vitiligo model group and vitiligo laser irradiation group were established respectively. In the laser irradiation group, the vitiligo mice were exposed to carbon dioxide laser (20–60 mJ) once every two weeks. The mice were placed under the condition of a constant temperature of 20 ± 2 °C, relative humidity of 50 ± 10% and light / dark cycle for 12 hours.
Histological Analysis
The decolorized skin far from the topical monobenzone ointment was obtained and fixed in 4% paraformaldehyde at 4 °C for 24 hours. After that, the sample was dehydrated by alcohol gradient, removed, and embedded in paraffin blocks. The paraffin sections of 6 mm were stained with hematoxylin and eosin (H-E).
Statistical Analysis
In order to verify the statistical significance, the mean (M) and standard deviation (SD) of the three independent measurements were calculated. Analysis of variance (ANOVA) was used to compare the experimental data between the experimental group and the control group. The value of P < 0.05 (*) is considered to be statistically significant.
Results
Morphology of Human Melanocytes and Observation of Melanocytes by DOPA Staining
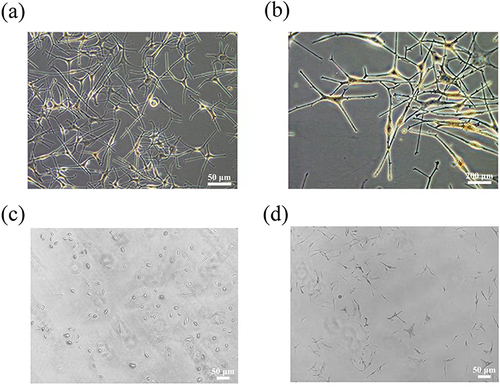
Cells from normal skin tissues mainly include keratinocytes, melanocytes and fibroblasts. Microscopically, melanocytes are observed as bipolar and dendritic cells, which can be seen more clearly in and . After DOPA staining, melanocytes ( and ) exhibited a dendritic structure stained by DOPA, but no staining of keratinocytes and fibroblasts was found.
Effects of Carbon Dioxide Laser on Cell Viability
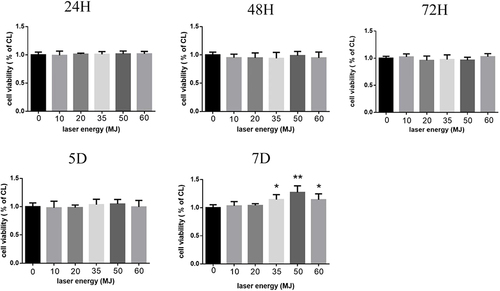
In order to study the effects of carbon dioxide laser on the survival and migration of human melanocytes, human melanocytes were treated with a CO2 laser using different energy levels (0–60 mJ) and different duration (24 hours, 48 hours, 3 days, 5 days, 7 days). The activity of the cells was assessed using CCK-8 and further analysis was carried out. As shown in , the control group without laser irradiation was set at 100%. The result illustrated that the proliferation rate of melanocytes is the highest when the laser intensity is 50 mJ, and significant changes in cell activity were observed after 7 days of treatment. On this basis, we used a carbon dioxide laser with an energy of 50 mJ to treat cells in order to observe its effect on melanogenesis of melanocytes.
Effect of Carbon Dioxide Laser on Melanin Production by Human Melanocytes
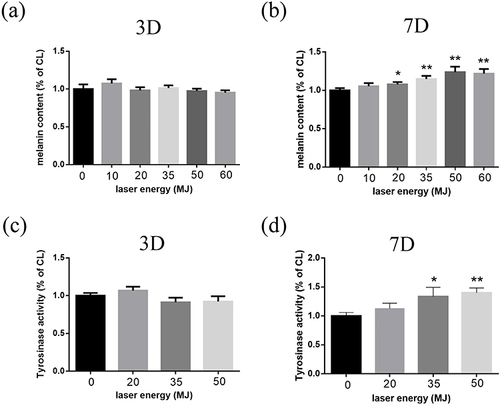
To investigate the effect of carbon dioxide laser on melanogenesis in human melanocytes, we used a common culture medium as a control to measure the level of melanin produced by human melanocytes treated with different intensity (0–60 mJ) carbon dioxide laser for 24 hours. As shown in and , under more than 20 mJ intensity, the carbon dioxide laser significantly increases the melanin content. Furthermore, 50 mJ could induce the most distinctive promotion in melanogenesis.
Effect of Carbon Dioxide Laser on Tyrosinase Activity of Human Melanocytes
Tyrosinase is involved in the first two key steps of melanogenesis. Therefore, we measured the activity of intracellular tyrosinase before and after laser treatment to clarify the mechanism of stimulating melanin synthesis. Untreated human melanocytes were used as the control, while cells treated with 50 mJ carbon dioxide laser for 24 hours were used as the experimental group. As shown in and , compared with the control cells, carbon dioxide laser stimulated tyrosinase activity. The results illustrated that melanin content significantly increased after carbon dioxide laser treatment, suggesting that it might stimulate tyrosinase activity in melanocytes.
The Treatment Effect of Carbon Dioxide Laser in Vitiligo Mice Model
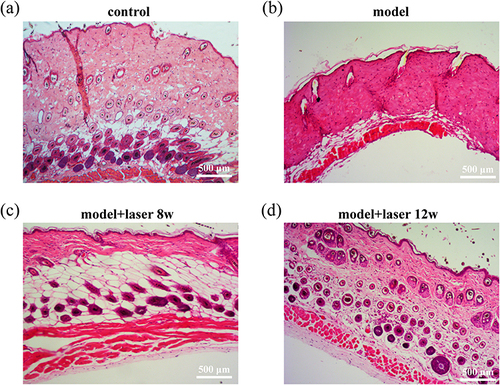
After the topical application of monobenzone cream, small white plaques initially appeared at the exposed site, and then the area of depigmented patches gradually expanded. After long-term treatment, some mice showed distant white plaques on the torso, ears and tail, indicating decolorization in the unexposed areas. The white plaque gradually expanded over time, which might eventually result in severe discoloration of most parts of the body. The mice in the control group were only given Vaseline for external use, and there was no skin leukoplakia in the process of modeling. The mice in the laser group were exposed to a 35 mJ carbon dioxide laser every two weeks, while the mice in the control group and model groups did not receive any laser irradiation. The decolorized skin sections far from the monobenzone smear site were obtained in the model group and laser group, and the non-decolorized skin was obtained in the control group. As shown in , H-E staining shows that the dorsal epidermis of normal mice is very thin and lacks melanocyte distribution. The dermis and subcutaneous tissue in mice are similar to those in humans, with the difference is that there are many hair follicles in the skin of mice, and there is a dense distribution of melanin around the hair follicles. In mice with vitiligo, there were no melanocytes in the basal layer. Compared to normal mice, the number of hair follicles decreased and there was no melanin distribution around the hair follicles. The number of hair follicles in the laser group was significantly higher than that in the vitiligo group, and the distribution of melanin around the hair follicles was obvious.
Discussion
Vitiligo is an acquired skin depigmentation disease characterized by white spots or plaques on the skin. The pathogenesis of vitiligo is unclear, but the destruction of melanocytes and subsequent depigmentation in skin lesions are considered to be the key events.Citation17,Citation18 Melanin synthesis in the skin plays an essential evolutionary role in the treatment of hypopigmentation. Melanin biosynthesis is catalyzed by three melanocyte-specific enzymes: TYR, TRP-1 and TRP-2. TYR is a rate-limiting enzyme in melanogenesis, which catalyzes the hydroxylation of tyrosine to DOPA and oxidizes DOPA to quinone.Citation17
UV treatment such as PUVA, NB-UVB, and 308 nm excimer lasers not only stimulate melanin synthesis in residual viable melanocytes but also promote their proliferation, effectively facilitating pigment regeneration within vitiligo lesions.Citation17,Citation19 However, due to intense ultraviolet radiation, the skin is at risk of deep tanning, burns and skin aging. An increasing number of reports have emphasized the value of CO2 lattice laser in the treatment of vitiligo.Citation20 Carbon dioxide laser has the advantage of selective application and can prevent unnecessary exposure to uninvolved skin. Therefore, this treatment is a reasonable option for localized vitiligo. The CO2 laser does not affect the entire epidermis but leaves intact skin between the lattice holes, promoting the process of skin healing and minimizing side effects. However, the application of carbon dioxide laser is restricted due to contraindications in patients with a predisposition to scarring or impaired coagulation function. Moreover, post-laser surgery care necessitates wound dryness and protection against sun exposure to prevent ultraviolet radiation-induced skin pigmentation. The mechanism of CO2 lattice laser in the treatment of vitiligo has the following aspects. Firstly, it induces immediate tissue contraction, which reduces the size of vitiligo lesions. Secondly, the secretion of cytokines and various growth factors during wound healing can serve as mitogens of melanocytes in adjacent normal skin and hair follicles. Lastly, lattice laser can promote the penetration of ultraviolet light and topical drugs through the epidermis and enhance its effect. In addition, laser ablation treatment extending from the edge to adjacent normal skin may cause melanocytes to migrate from normal skin to lesions. This migration is vital for pigmentation in areas with low hair density, such as limb skin.
The results of our in vitro experiments have demonstrated that the CO2 laser can enhance melanin synthesis and promote the survival of melanocytes by increasing their activity and stimulating tyrosinase expression. Additionally, histological analysis using HE staining on vitiligo mice treated with Monobenzone cream further confirmed that the laser treatment group exhibited a significantly higher number of hair follicles compared to the vitiligo group, along with a significantly greater distribution of melanin around these follicles. Similarly, previous studies by Weshahy R et al have demonstrated the efficacy of combining laser therapy with 5-fluorouracil for the treatment of acrovitiligo.Citation21 However, it should be noted that the mouse model utilized in this study does possess certain limitations. Although the histological features of monobenzone-induced white spots and vitiligo patients exhibit a high degree of similarity, it is important to acknowledge that the decolorization observed in these cases arises from direct melanocyte destruction caused by decolorizing agents, independent of killer T cells. This differs from the involvement of multiple CD8+ T cells observed in vitiligo patients.Citation22
Conclusion
To sum up, carbon dioxide laser at an intensity of 35 mJ can up-regulate melanin synthesis in human melanocytes by enhancing tyrosinase activity and promoting cell proliferation at the cellular level. In addition, carbon dioxide lattice laser also has a certain therapeutic effect on vitiligo mice. Therefore, it is possible for the carbon dioxide lattice laser to become an adjunct treatment for clinical treatment of vitiligo.
Disclosure
The authors have declared that there is no conflict of interest in this work.
Acknowledgments
This study was supported by the National Science Foundation of China (82073453), the Zhejiang Provincial Natural Science Foundation of China (LY20H110002), the General Project Funds from the Health Department of Zhejiang Province (2020KY446), the outstanding Young People Fund in Zhejiang Provincial People’s Hospital (ZRY2018C004).
References
- Alikhan A, Felsten LM, Daly M, Petronic-Rosic V. Vitiligo: a comprehensive overview Part I. Introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65(3):473–491. doi:10.1016/j.jaad.2010.11.061
- Speeckaert R, van Geel N. Vitiligo: an Update on Pathophysiology and Treatment Options. Am J Clin Derma. 2017;18(6):733–744. doi:10.1007/s40257-017-0298-5
- Vachiramon V, Chaiyabutr C, Rattanaumpawan P, Kanokrungsee S. Effects of a preceding fractional carbon dioxide laser on the outcome of combined local narrowband ultraviolet B and topical steroids in patients with vitiligo in difficult-to-treat areas. Las Surg Med. 2016;48(2):197–202. doi:10.1002/lsm.22389
- Mobasher P, Guerra R, Li SJ, Frangos J, Ganesan AK, Huang V. Open-label pilot study of tofacitinib 2% for the treatment of refractory vitiligo. Br J Dermatol. 2020;182(4):1047–1049. doi:10.1111/bjd.18606
- Aziz Jalali M, Jafari B, Isfahani M, Nilforoushzadeh MA. Treatment of segmental vitiligo with normal-hair follicle autograft. Med J Islam Repub Iran. 2013;27(4):210–214.
- Orringer JS, Rittié L, Baker D, Voorhees JJ, Fisher G. Molecular mechanisms of nonablative fractionated laser resurfacing. Br J Dermatol. 2010;163(4):757–768. doi:10.1111/j.1365-2133.2010.09998.x
- Kim JE, Won CH, Bak H, et al. Gene profiling analysis of the early effects of ablative fractional carbon dioxide laser treatment on human skin. Dermatolog Surg. 2013;39(7):1033–1043. doi:10.1111/dsu.12170
- Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84(4):1155–1228. doi:10.1152/physrev.00044.2003
- Schallreuter KU, Kothari S, Chavan B, Spencer JD. Regulation of melanogenesis - controversies and new concepts. Experim Dermat. 2008;17(5):395–404. doi:10.1111/j.1600-0625.2007.00675.x
- Zou BY, Zheng WY, Pan HJ, Yang B, Liu ZF. Research trends and hotspot analysis of fractional carbon dioxide laser: a bibliometric and visualized analysis via Citespace. J Cosmet Dermatol. 2022;21(11):5484–5499. doi:10.1111/jocd.15267
- Hu YX, Qi XY, Hu YQ, et al. Effects of CO2 fractional laser therapy on peripheral blood cytokines in patients with vitiligo. Dermatologic Therapy. 2019;32(4):e12992. doi:10.1111/dth.12992
- Afify AA, Zuelfakkar NM, Eshafi MA. Fractional CO2 laser, platelet rich plasma and narrow band ultraviolet B in the treatment of Vitiligo (A randomized clinical trial). Lasers Med Sci. 2021;36(7):1479–1486. doi:10.1007/s10103-020-03195-9
- Ding XX, Mei E, Hu MR, et al. Effect of puerarin on melanogenesis in human melanocytes and vitiligo mouse models and the underlying mechanism. Phytother Res. 2019;33(1):205–213. doi:10.1002/ptr.6218
- Godwin LS, Castle JT, Kohli JS, et al. Isolation, culture, and transfection of melanocytes. Current Protocol Cell Biol. 2014;63(1):1.8.1–1.8.20. doi:10.1002/0471143030.cb0108s63
- Munshi R, Joshi S, Talele G, Shah R. An in-vitro assay estimating changes in melanin content of melanoma cells due to ultra-dilute, potentized preparations. Homeopathy. 2019;108(3):183–187. doi:10.1055/s-0039-1678541
- Azzolino V, Zapata L, Garg M, et al. Jak inhibitors reverse vitiligo in mice but do not deplete skin resident memory t cells. J Invest Dermatol. 2021;141(1):182–+. doi:10.1016/j.jid.2020.04.027
- Zhang L, Wang XX, Chen SJ, et al. Comparison of efficacy and safety profile for home NB-UVB vs. outpatient NB-UVB in the treatment of non-segmental vitiligo: a prospective cohort study. Photode Photoim Photomed. 2019;35(4):261–267. doi:10.1111/phpp.12462
- Fritz K, Salavastru C. The 308 nm Excimer laser. Treatment of vitiligo and hypopigmentation. Hautarzt. 2018;69(1):44–47. doi:10.1007/s00105-017-4097-y
- Lai XL, Wichers HJ, Soler-Lopez M, Dijkstra BW. Structure and Function of Human Tyrosinase and Tyrosinase-Related Proteins. Chemistry. 2018;24(1):47–55. doi:10.1002/chem.201704410
- Kim HJ, Hong ES, Cho SH, Lee JD, Kim HS. Fractional Carbon Dioxide Laser as an “Add-on”Treatment for Vitiligo: A Meta-analysis with Systematic Review. Acta Dermato-Venereologica. 2018;98(2):180–184. doi:10.2340/00015555-2836
- Weshahy R, Abdelhamid MF, Sayed KS, El Desouky ED, Ramez SA. Efficacy and safety of combined fractional ablative CO2 laser and 5 fluorouracil in the treatment of acral vitiligo: an open, uncontrolled study. J Cosmet Dermatol. 2022;21(11):5636–5641. doi:10.1111/jocd.15116
- Fukuda K. Networks of CD8< SUP>+</SUP> T Cell Response Activation in Melanoma and Vitiligo. Front Immunol. 2022;13866703. doi:10.3389/fimmu.2022.866703