Abstract
Purpose
Porcine-based dermal injectable collagen is effective for nasolabial fold correction. In the present study, a new dermal injectable collagen, incorporating a novel cross-linking technology and premixed with lidocaine, was introduced. The study aimed to determine the efficacy of the new dermal injectable collagen in improving bilateral nasolabial fold wrinkles, and reducing pain during injection.
Patients and Methods
This prospective, double-blind, multicenter, parallel-group, randomized trial enrolled participants with moderate-to-severe bilateral nasolabial fold wrinkles from February 2019 to March 2021. Participants were randomly assigned to the test group (new dermal injectable collagen with lidocaine featuring a novel cross-linking technology) or control group (traditionally cross-linked dermal injectable collagen with lidocaine). Participants were monitored for adverse events (AEs), and for pain using the Thermometer Pain Scale (TPS) and a visual analog scale (VAS). Efficacy was measured using the Wrinkle Severity Rating Scale (WSRS) and the Global Aesthetic Improvement Scale (GAIS).
Results
On the poor or better sides, the 2 groups exhibited a significant decrease in WSRS scores at 4, 12, 24, and 36 weeks after treatment, compared to baseline WSRS scores (all, p < 0.05). Compared to the control group, the test group had a greater decrease in WSRS score (poor or better sides) at 12, 24, 36, and 52 weeks after treatment (all, p < 0.05). A similar observation was also found in the WSRS response rate and GAIS score of the 2 groups. VAS and TPS scores were not significantly different between the 2 groups (p > 0.05), indicating that pain reduction was similar in the 2 groups. All AEs were anticipated AEs associated with facial aesthetic injections, and most recovered within 0 to 30 days without sequelae. There were no differences in AEs between the 2 groups (all, p > 0.05).
Conclusion
The new dermal injectable collagen with lidocaine exhibited better efficacy for correcting nasolabial fold wrinkles compared to the control group. Both relieved pain and produced only transient and tolerable AEs.
Introduction
Skin aging and obvious wrinkles can lead to low self-esteem and affect quality of life psychologically and socio-culturally.Citation1 It has been demonstrated that fragmented collagen fibers and reduced collagen synthesis in the dermal layer lead to a thinner dermis and the appearance of static wrinkles.Citation2 This suggests that collagen supplementation can be used to correct static wrinkles. As such, collagen filler is an ideal option for treating collagen deficiency and the wrinkles associated with aging skin.Citation3,Citation4
The Food and Drug Administration (FDA) has approved a number of injectable collagen fillers for filling and repairing imperfections such as wrinkles, acne vulgaris scars, and minor trauma scars. These collagen fillers were made of materials from different sources: bovine-based collagen fillers (eg, Zyderm and Zyplast), porcine-based collagen fillers (eg, Evolence), and human-based collagen fillers (eg, Cosmoderm, Cosmoplast).Citation5,Citation6 However, due to zoonosis, allergic reaction, high management costs, or its susceptibility to enzymatic degradation,Citation7–9 collagen fillers such as Zyderm, Zyplast, Evolence, Cosmoderm, and Cosmoplast have been withdrawn and are no longer available in the United States.Citation10–13 Consequently, there is an urgent need to develop a new collagen filler that offers better safety, increased longevity, and greater volume correction of wrinkles and other facial imperfections. Due to this, several commercial porcine collagen-based products are available and are used principally for facial contouring.Citation14–16
Sunmax Biotechnology has extensive experience in developing porcine-derived collagen materials and medical devices such as dermal injectable collagen. The first dermal injectable collagen product launched by Sunmax was approved by Taiwan Food and Drug Administration (2006, TFDA) for correction of facial skin defects and wrinkles. The second generation of the dermal injectable collagen product line is a device incorporating slightly cross-linked collagen using conventional cross-linking agents to produce moderate resistance to proteases and is stable at the implant site for a longer period of time, thereby improving its efficacy.
Recent studies have reported that pain is a common complaint of people receiving dermal filler injections.Citation5,Citation17,Citation18 In 2010, Weinkle et alCitation19 compared 2 methods for bilateral nasolabial folds treatment: a premix of Dermicol-P35 27G and 0.3% lidocaine, and placement of a local anesthetic patch followed by injection of Dermicol-P35 27G, and shows that patients who received the premix of Dermicol-P35 and 0.3% lidocaine experienced significantly less pain without compromising efficacy.
Based on the aforementioned study, a cross-linked dermal injectable collagen was developed to deliberately incorporate lidocaine, in order to provide a local anesthetic effect and directly relieve pain during injection. This third generation of dermal injectable collagen was approved by TFDA in 2014. Subsequently, a novel dermal injectable collagen was developed using an innovative cross-linking technology, with the addition of lidocaine. The safety and longevity of the new injectable collagen have been assessed based on ISO 10993 standards, and the characteristics of the extensively cross-linked collagen have been studied and compared with its predecessors.
Thus, the purpose of this study was to further determine the effectiveness of the new dermal injectable collagen in improving bilateral nasolabial folds and reducing pain during injection due to the addition of lidocaine to the injectable.
Methods
Study Design and Participants
This was a prospective, randomized, double-blind, multicenter (Linkou Chang-Gung Memorial Hospital, Tri-Service General Hospital, and National Taiwan University Hospital) clinical trial in Taiwan. Randomization codes were generated using SAS® version 9.2 software, with a blocking size of 4. The study was designed in compliance with Consolidated Standards of Reporting Trials (CONSORT) guidelines. The study design is shown in . The inclusion criteria were as follows: 1) Male or female 20 to 65 years old; 2) Bilateral defects in the bilateral nasolabial folds of grade 3 or 4 (moderate-to-severe) on the Wrinkle Severity Rating Scale (WSRS);Citation20 3) Willingness to receive soft tissue augmentation for wrinkle treatment; 4) Willingness to avoid other facial aesthetic therapy during the clinical trial; 5) Healthy facial skin without any disorder that interferes with the assessment of skin aging (eg, facial nerve disorders); 6) Provided written informed consent for participation in the trial. Exclusion criteria were as follows: 1) A history of anaphylactoid reaction, auto-immune disease, or allergies to collagen and lidocaine; 2) Coagulation disorder, 3) Females with a positive pregnancy test at screening, plan a pregnancy, or are breastfeeding; 4) Infection, severe skin disease, inflammation, or related symptoms on the nasolabial folds, or keloid; 5) Severe cardiac, renal, hepatic, or respiratory diseases; 6) A clinically diagnosed mental illness; 7) Alcohol use disorder (According to American National Institute of Health standards); 8) Use of immunosuppressive drugs, chemotherapy, systemic steroids, anticoagulant treatment, or non-steroidal anti-inflammatory drugs (NSAIDs); 9) Permanent implants in the nasolabial folds area; 10) Prior nasolabial fold augmentation treatment or correctional procedure.
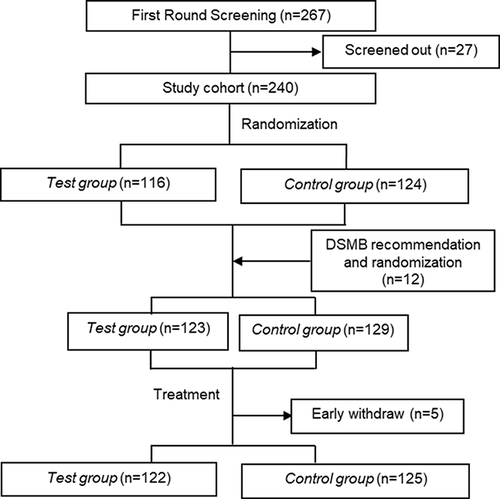
Between February 2019 and March 2021, 267 participants were screened (), and 27 were excluded for not meeting the inclusion criteria. After randomization, 116 participants were assigned to the test group (Sunmax FULLSGEN with lidocaine), and 124 to the control group (Sunmax FACIALGAIN collagen implant with lidocaine). Upon the recommendation of the Data and Safety Monitoring Board (DSMB), 12 additional participants were added; 7 were randomly assigned to the test group and 5 were assigned to the control group. Thus, a total of 252 participants were included in the Full Analysis Set (FAS) cohort; 123 in the test group and 129 in the control group. Of these participants, 122 in the test group and 125 in the control group completed the treatment and follow-up, and were included in the per-protocol (PP) cohort.
Ethical Considerations
The study complied with the Declaration of Helsinki. The study was approved by the Institutional Review Board or Research Ethics Committee of Tri-Service General Hospital (IRB No. 1–107-03-001), Chang Gung Medical Foundation (IRB No. 201801242A0), and National Taiwan University Hospital (REC No. 201809007DSA) and registered in ClinicalTrials.gov (NCT03844529), written informed consent was obtained from patients for all patients.
Injection Procedure and Dose
All injection depths were mid to deep in the dermis. Injections were performed with a 25G blunt needle through a small opening. Based on physicians’ assessment, the injection methods include linear injection (96 cases in test group and 98 cases in control group), fan-shaped injection (37 cases in test group and 39 cases in control group), fishbone (fern-shaped) injection (4 cases in test group and 1 case in control group), and others (1 case in control group). The test group was injected with 1.9 ± 0.74 mL Sunmax FULLSGEN with lidocaine (Sunmax Biotechnology Co., Ltd., Tainan City, Taiwan) in each nasolabial fold, while control group was injected with 2.1 ± 0.73 mL Sunmax FACIALGAIN with lidocaine (Sunmax Biotechnology Co., Ltd., Tainan city, Taiwan) in each nasolabial fold.
Outcome Measures
The primary efficacy endpoint was the Wrinkle Severity Rating Scale (WSRS) response rate (defined below) at the 24th week after injection, as assessed by the evaluators. The secondary efficacy endpoint were 1) WSRS response rate at the 4th, 12th, 36th, and 52nd week after injection as assessed by the evaluators; 2) WSRS score at the 4th, 12th, 24th, 36th, and 52nd week as assessed by the evaluators; 3) Comparison of the difference between the baseline score and the WSRS score at the 4th, 12th, 24th, 36th, and 52nd week after injection as assessed by the evaluators; 4) Global Aesthetic Improvement Scale (GAIS) score improvement at the 4th, 12th, 24th, 36th, and 52nd week after injection as assessed by the participants; 5) GAIS score improvement at the 4th, 12th, 24th, 36th, and 52nd week as assessed by the evaluators; 6) Visual Analogue Scale (VAS) pain score within 30 mins after injection as assessed by the participants; and 7) Thermometer Pain Scale (TPS) score within 30 mins after injection as assessed by the investigator. Scores used for outcome assessment must have been agreed to by at least 2 evaluators. When the results of the 3 evaluators were not consistent, re-assessment was required until the scores of at least 2 evaluators were consistent.
The severity of nasolabial fold wrinkles was divided into 5 grades based on the WSRS scale, with grade 1 being the mildest and grade 5 being the most severe. Wrinkles were defined as effectively improved (WSRS response rate) if the improvement in score was ≥1. The degree of GAIS improvement was divided into 5 grades, 1 was defined as very much improved, and 5 as worse than before. By investigator’s evaluation, the “poor side” was defined as the side with the worst result at the 24th week after treatment, and the “better side” was defined as the side with greater improvement in score at the 24th week after treatment. The WSRS response rate was calculated using the formula:
Assessment of Adverse Effects
The adverse effects (AEs), including adverse reactions such as nodule, pain, swelling, tenderness, contusion, erythema, pruritus, and skin exfoliation,Citation21 were evaluated in the 2 groups within 52 weeks after treatment. The severity of AEs was assessed according to the following criteria: level 1 (mild), the adverse events that did not affect the daily activity of the subjects; level 2 (moderate), the adverse events impacted the daily activity of the subjects but they do not need any medical intervention; level 3 (severe), the events affected the daily activity of the subjects and they need the medical intervention.
Statistical Analysis
The Z-test with continuity correction was used to compare the WSRS response rate of the 2 groups. Descriptive statistics were used to analyze the mean and distribution trends of VAS and TPS scores, and the t-test was used to analyze the other secondary endpoints. If the data were not normally distributed, the Mann–Whitney U-test was used to compare the scores for the 2 groups. However, the comparison of the response rates between the 2 groups was still performed using the chi-squared test with continuity correction. All statistical assessments were 2-tailed, and p-values <0.05 were considered statistically significant. Statistical analyses were conducted using SAS statistical software (Version 9.4, SAS Institute, Cary, North Carolina, USA).
Results
Patient Characteristics
The characteristics of the FAS cohort (n = 252) are summarized in . The mean age of both groups was 45 years, and the majority of participants in both groups were female. The baseline WSRS score on the better side was 3.5 ± 0.50 in the test group and 3.4 ± 0.49 in the control group. The baseline WSRS score on the poor side was 3.4 ± 0.50 in the test group and 3.4 ± 0.49 in the control group. Demographic and other characteristics were similar between the test group and the control group (all, p > 0.05). There was no significant difference in the baseline severity scores (WSRS, GAIS, VAS, and TPS) of the poor side or the better side between the 2 groups (all, p > 0.05).
Table 1 Participant Characteristics
Outcomes of Dermal Injectable Collagen Therapy
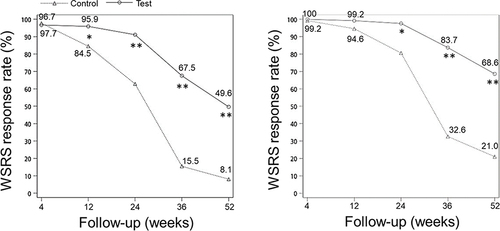
The study used the FAS cohort to examine the primary and secondary endpoints. On the poor and better sides, there was a significant decrease of WSRS scores at 4, 12, 24, 36, and 52 weeks after treatment compared to baseline WSRS scores (all, p < 0.05, ). However, the control group had no significant decrease of WSRS score at 52 weeks after treatment compared to the baseline WSRS score on poor side (p > 0.05, ). The test group had a greater decrease of WSRS score of the poor side at 12, 24, 36, and 24 weeks after treatment than the control group, and the test group had a greater decrease of WSRS score of the better side at 4, 12, 24, 36, and 52 weeks after treatment (all, p < 0.05, ). Furthermore, compared to the control group, the test group had a high WSRS response rate of the poor side at 12, 24, 36, and 52 weeks after treatment (all, p < 0.05, ). In the test group, the WSRS response rate of the better side was significantly increased at 24, 36, and 52 weeks after treatment (all, p < 0.05, ). At 52 weeks after treatment, the WSRS response rate on the better side was 68.6% in the test group and 21.0% in the control group, while the response rate on the poor side was 49.6% in the test group and 8.1% in the control group.
Table 2 Differences Between the Baseline WSRS Score and Scores at the 4th, 12th, 24th, 36th, and 52nd Week
Figure 2 WSRS response rate assessed by the evaluators Left panel, Poor side. Right panel, Better side. *p<0.05, **p<0.001.
The GAIS score improvement on the poor side and better side as assessed by the evaluator are shown in . On the poor side, there was a significant difference between the 2 groups starting at 4, 12, 24, 36, and 52 weeks after treatment (all, p < 0.05). The mean GAIS score improvement in the test group at the above times points was 1.7, 2.0, 2.3, 2.6, and 2.8 and in the control group the mean improvement was 1.8, 2.4, 2.9, 3.3, and 3.7, respectively. On the better side, there was a significant difference between the 2 groups starting at 12, 24, 36, and 52 weeks after treatment. The mean GAIS score improvement in the test group at the above time points was 1.9, 2.2, 2.6, and 2.7 and in the control group was 2.3, 2.8, 3.2, and 3.5, respectively (all, p < 0.05). However, there was no significant difference between the 2 groups at 4 weeks post-treatment (p = 0.062).
Table 3 GAIS Score Improvement at the 4th, 12th, 24th, 36th, and 52nd Week
The VAS pain score assessed by the participants and the TPS score assessed by the investigator within 30 minutes after treatment were compared between the 2 groups. As shown in , the mean VAS pain score in the test group was 3.2 and in the control group was 2.9 (p > 0.05). A similar result was observed for the TPS score.
Table 4 Pain Score Within 30 Minutes After Injection
Safety
summarized the adverse events reported within 52 weeks after treatment, with a total of 217 people reported with AEs (112 in test group and 105 in control group). The total count of AE was 1021, including 535 in test group and 486 in control group. There were no significant differences between the 2 groups with respect to the number and type of AEs (all p > 0.05), most AEs were mild. Within the AEs, only 63.6% in test group and 58.8% in control group were related or possibly related to the device. Most of these medical device-related adverse events resolved within 0 to 30 days without sequelae (99.7% in test group and 100% in control group). Many of AEs were common complications in dermal filler treatment, which were happened around the injection site, such as erythema, swelling, pain, tenderness, contusion, pruritus, nodule, and rash. Besides, there were no severe adverse reaction happened in this trial.
Table 5 Adverse Events in the 2 Group
Discussion
Porcine collagen is widely used to treat various conditions, such as gynecologic surgery,Citation22 orthopedic surgery,Citation23 and abdominal wall surgeryCitation24 due to its high biocompatibility with human tissue. Additionally, porcine-based dermal collagen is effective for the treatment of facial wrinkles, including the tear-trough deformityCitation25 and nasolabial fold wrinkles.Citation26 Notably, Lorenc et alCitation27 showed that porcine collagen filler required a lower extrusion force and yield point compared with hyaluronic acid-based fillers, and these properties allow clinicians to inject the filler more comfortably and precisely.
The new dermal injectable collagen with lidocaine is a porcine-derived collagen device that has been newly developed by Sunmax Biotechnology Corporation. It is composed of cross-linked collagen fibers employing a novel cross-linking technology, and the novel properties make it an effective and convenient dermal filler. Using cross-linking agents, such as the well-known cross-linking agent – glutaraldehyde, in the dermal filler manufacturing process increases the stability of the molecular structure of the collagen matrix.Citation28 Cross-linking technology also enhances the mechanical properties of a conventional collagen-based filler. There are studies showed the durability and safety of second generation dermal injectable collagen has been demonstrated in animal modelsCitation29 and patientsCitation30 to address the importance of cross-linking technology. As a result, the novel cross-linking technology led to a better performance of the new dermal injectable collagen in WSRS and GAIS scores in the present study, compared to the control group. Further, because porcine collagen does not trigger a chronic inflammatory responseCitation31 and is associated with infrequent and mild allergic reactions,Citation32 a skin test does not need to be performed before treatment with the new dermal injectable collagen with lidocaine. Thus, aesthetic procedures can be performed immediately after diagnosis.
Discomfort during aesthetic procedures may be a concern for some patients desiring facial rejuvenation.Citation5,Citation17,Citation18 Busso et alCitation33 reported that mixing calcium hydroxyapatite with lidocaine before injection reduced patient discomfort without affecting the physical properties of the dermal filler. Additionally, Weinkle in 2010Citation19 showed that Dermicol-P35 27G premixed with lidocaine provided substantially better pain relief than Dermicol-P35 27G injected after application of a topical anesthetic. Currently, no further study has investigated the effects of collagen-based dermal filler with lidocaine in aesthetic procedures. In this study, patients treated with the new dermal injectable collagen with lidocaine had a mean VAS pain score of 3.2 and a mean TPS score of 3.1 within 30 minutes after injection. Previous studies suggest a VAS score of 3 after dermal injection with hyaluronic acid (HA)Citation34 and a TPS score of 2 after dermal injection with Dermicol-P35 27G premixed with lidocaineCitation19 represent mild pain. Thus, our results indicate that the new dermal injectable collagen with lidocaine treatment is associated with only minimal pain.
Factors associated with skin aging can be classified into 2 categories, intrinsic and extrinsic.Citation35 Regardless of reason, wrinkles and reduced elasticity are typical phenomena of skin aging and the result of progressive atrophy of the dermis.Citation36 One of the main mechanisms of dermal atrophy is thought to be a reduction in the amount of extracellular matrix (ECM), particularly collagen in the dermis.Citation37 In the formation of wrinkles in aged skin, the production of collagen decreases and its degradation increases, which leads to an overall reduction in collagen amount.Citation38 The decreased synthesis and replacement rate causes collagen matrix loss, and thus skin collapse and loss of elasticity, which in turn leads to the appearance of wrinkles, folds, and facial contour changes,Citation39 Most antiaging approaches target and aim to reverse this process.Citation40 Due to this, several commercial porcine collagen-based products are available, and are used principally for facial contouring, such as for the nasolabial folds,Citation14,Citation16,Citation41 lips,Citation41 glabellar groove,Citation15 post-rhinoplasty dorsal irregularities,Citation15,Citation42 depressed acne scars,Citation15,Citation43 and augmentation.Citation43 In the present study, the results of WSRS and GAIS evaluation demonstrated that a porcine collagen-based dermal filler is effective for the treatment of facial wrinkles, including moderate-to-severe bilateral nasolabial folds. According to these findings, we suggest that further investigation should be performed to determine the efficacy of this dermal filler for treating other facial contour changes (eg, lips, glabellar groove, post-rhinoplasty dorsal irregularities, depressed acne scars and augmentation).
Compared to the baseline WSRS score, the scores on the poor and better sides at 4, 12, 24, 36, and 52 weeks after were significantly decreased in participants receiving either the test group or the control group. Notably, the test group resulted in a high WSRS response rate at 12, 24, 36, and 52 weeks, indicating that cross-linked porcine collagen prolongs the effect of the collagen filler. However, we still observed a declining trend in WSRS score difference when comparing the score at 52 weeks to the baseline score. A similar observation was also found in the WSRS response rates and the GAIS scores of the 2 groups. This may be due to degradation of the collagen-based matrix by in vivo collagenase.Citation14 Previous studies have shown that the majority of patients require touch-up injections approximately every 3–12 months.Citation44 Nevertheless, patients with moderate-to-severe bilateral nasolabial folds who received the test group were able to obtain a WSRS response rate of 68.6% on the better side, and 49.6% on the poor side at 52 weeks after treatment.
HA fillers are widely accepted due to their properties, such as an excellent risk–benefit ratio, simplicity of use, long-lasting effects, versatility, and reversibility.Citation45 Other reported adverse effects associated with HA-based dermal fillers include surface irregularities, Tyndall effect and late onset nodules,Citation46,Citation47 which were not observed in the present study of collagen-based dermal filler. Collagen-based dermal filler has been reported to reduce downtime, bruising, and pain during injection, and restore the lost structural components to aging skin.Citation47
As with any medical procedure, dermal filler injections come with risks. Most adverse reactions associated with injectable fillers occur at an early stage after injection and usually resolve within a few days. According to information published by the US FDA on the risks associated with soft tissue fillers, some adverse reactions may occur weeks, months, or years after the injection. All injectable fillers may lead to short-term and long-term side effects, or both. Common adverse effects include contusion, redness, swelling, pain, tenderness, pruritus, and rash. Less common adverse effects include lumps (nodules or granulomas) that form under or inside the skin that may need surgical removal, infection, open wounds that require drainage, sores at the injection site, allergic reactions, and necrosis of tissue.Citation21 Results of the present study showed that the incidence of adverse reactions in the test group was 81.3% and that in the control group was 72.9%, and the difference was not significant. At the end of the trial, most adverse reactions had resolved or were under control. In addition, no serious adverse reactions or allergic adverse reactions occurred in either group. These results suggest that the new dermal injectable collagen employing the novel cross-linking technology and premixed with lidocaine has a good safety profile. However, a previous study reported that a cross-linked porcine-based collagen filler should not be injected into the lips due to the high incidence of nodule formation.Citation48 For this reason, further investigation of the safety of the new dermal injectable collagen with lidocaine used in other facial areas is necessary.
A limitation of this study was that touch-up treatments with either the test group or the control group were not offered to the participants. Previous studies have indicated that repeated treatments with dermal fillers may be needed for optimal correction.Citation48–50 Thus, the inclusion of touch-up injections in this study may have increased the effectiveness of the two dermal injectable collagen devices and participant satisfaction.
Conclusion
The new dermal injectable collagen, featuring a novel cross-linking technology and premixed with lidocaine, exhibited significant superiority at the primary efficacy endpoint, and later follow-up time points, as compared with the traditionally cross-linked injectable collagen, for correcting nasolabial fold wrinkles. Although the new dermal injectable collagen with lidocaine showed a slight increase in pain and mild to moderate adverse events after treatment, these results indicate that the devices studied may be worthwhile options for patients seeking both convenient and long-lasting aesthetic treatment.
Disclosure
The authors report no conflicts of interest in this work.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Additional information
Funding
References
- Scarano A, Mortellaro C, Mavriqi L, Di Cerbo A. Evaluation Effectiveness of the Voltaic Arc Dermabrasion in Perioral Rhytides Eradication. J Craniofac Surg. 2016;27(5):1205–1208.
- Manaloto RM, Alster TS. Periorbital rejuvenation: a review of dermatologic treatments. Dermatol Surg. 1999;25(1):1–9.
- Fagien S. Facial soft-tissue augmentation with injectable autologous and allogeneic human tissue collagen matrix (autologen and dermalogen). Plast Reconstr Surg. 2000;105(1):362–373.
- Ma J, Liu M, Wang Y, et al. Quantitative proteomics analysis of young and elderly skin with DIA mass spectrometry reveals new skin aging-related proteins. Aging (Albany NY). 2020;12(13):13529–13554.
- Lucey P, Goldberg DJ. Complications of collagen fillers. Facial Plast Surg. 2014;30(6):615–622.
- Rostan E. Collagen fillers. Facial Plast Surg Clin North Am. 2007;15(1):55–61, vi.
- Buck DW 2nd, Alam M, Kim JY. Injectable fillers for facial rejuvenation: a review. J Plast Reconstr Aesthet Surg. 2009;62(1):11–18.
- Lynn AK, Yannas IV, Bonfield W. Antigenicity and immunogenicity of collagen. J Biomed Mater Res B Appl Biomater. 2004;71(2):343–354.
- Parenteau-Bareil R, Gauvin R, Berthod F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials. 2010;3(3):1863–1887.
- Cheng L-Y, Sun X-M, Tang M-Y, Jin R, Cui W-G, Zhang Y-G. An update review on recent skin fillers. Plast Aesthetic Res. 2016;3:92–99.
- Ginat DT, Schatz CJ. Imaging features of midface injectable fillers and associated complications. AJNR Am J Neuroradiol. 2013;34(8):1488–1495.
- Luebberding S, Alexiades-Armenakas M. Safety of dermal fillers. J Drugs Dermatol. 2012;11(9):1053–1058.
- Requena L, Requena C, Christensen L, Zimmermann US, Kutzner H, Cerroni L. Adverse reactions to injectable soft tissue fillers. J Am Acad Dermatol. 2011;64(1):1–34; quiz 35–6.
- Lee JH, Choi YS, Kim SM, Kim YJ, Rhie JW, Jun YJ. Efficacy and safety of porcine collagen filler for nasolabial fold correction in Asians: a prospective multicenter, 12 months follow-up study. J Korean Med Sci. 2014;29 Suppl 3(Suppl 3):S217–21.
- Solomon P, Sklar M, Zener R. Facial soft tissue augmentation with Artecoll((R)): a review of eight years of clinical experience in 153 patients. Can J Plast Surg. 2012;20(1):28–32.
- Sparavigna A, Tateo A, Inselvini E, Tocchio M. Anti-Age Activity and Tolerance Evaluation of Collagen Micro-Injection Treatment Associated to Topical Application of a Cosmetic Formulation (Investigator-Initiated Multicentre Trial). J Clin Exp Dermatol Res. 2017;8:1–8.
- Cho KH, Uthaman S, Park IK, Cho CS. Injectable Biomaterials in Plastic and Reconstructive Surgery: a Review of the Current Status. Tissue Eng Regen Med. 2018;15(5):559–574.
- John A, Lakshmanan P. Collagen: animal Sources and Biomedical Application. J Appl Pharm Sci. 2015;5(3):123–127.
- Weinkle S. Efficacy and tolerability of admixing 0.3% lidocaine with Dermicol-P35 27G for the treatment of nasolabial folds. Dermatol Surg. 2010;36(3):316–320.
- Day DJ, Littler CM, Swift RW, Gottlieb S. The wrinkle severity rating scale: a validation study. Am J Clin Dermatol. 2004;5(1):49–52.
- FDA. Dermal Fillers (Soft Tissue Fillers). FDA. Available from: https://www.fda.gov/medical-devices/aesthetic-cosmetic-devices/dermal-fillers-soft-tissue-fillers. Accessed June 18, 2024.
- Alperin M. Collagen scaffold: a treatment for large mesh exposure following vaginal prolapse repair. Int Urogynecol J. 2014;25(11):1597–1599.
- Giannotti S, Ghilardi M, Dell’osso G, et al. Study of the porcine dermal collagen repair patch in morpho-functional recovery of the rotator cuff after minimum follow-up of 2.5 years. Surg Technol Int. 2014;24:348–352.
- Patel KM, Nahabedian MY, Gatti M, Bhanot P. Indications and outcomes following complex abdominal reconstruction with component separation combined with porcine acellular dermal matrix reinforcement. Ann Plast Surg. 2012;69(4):394–398.
- Goldberg DJ. Correction of tear trough deformity with novel porcine collagen dermal filler (Dermicol-P35). Aesthet Surg J. 2009;29(3 Suppl):S9.
- Narins RS, Brandt FS, Lorenc ZP, Maas CS, Monheit GD, Smith SR. Twelve-month persistency of a novel ribose-cross-linked collagen dermal filler. Dermatol Surg. 2008;34 Suppl 1:S31–S39.
- Lorenc ZP, Nir E, Azachi M. Characterization of physical properties and histologic evaluation of injectable Dermicol-p35 porcine-collagen dermal filler. Plast Reconstr Surg. 2010;125(6):1805–1813.
- Paul RG, Bailey AJ. Chemical stabilisation of collagen as a biomimetic. ScientificWorldJournal. 2003;3:138–155.
- Lin SD, Huang SH, Lin YN, et al. Injected Implant of Uncultured Stromal Vascular Fraction Loaded Onto a Collagen Gel: in Vivo Study of Adipogenesis and Long-term Outcomes. Ann Plast Surg. 2016;76 Suppl 1:S108–S116.
- Wen MH, Cheng PW, Liao LJ, Chou HW, Wang CT. Treatment outcomes of injection laryngoplasty using cross-linked porcine collagen and hyaluronic acid. Otolaryngol Head Neck Surg. 2013;149(6):900–906.
- Gurney TA, Kim DW. Applications of porcine dermal collagen (ENDURAGen) in facial plastic surgery. Facial Plast Surg Clin North Am. 2007;15(1):113–21, viii.
- Shoshani D, Markovitz E, Cohen Y, Heremans A, Goldlust A. Skin test hypersensitivity study of a cross-linked, porcine collagen implant for aesthetic surgery. Dermatol Surg. 2007;33 Suppl 2:S152–S158.
- Busso M, Voigts R. An investigation of changes in physical properties of injectable calcium hydroxylapatite in a carrier gel when mixed with lidocaine and with lidocaine/epinephrine. Dermatol Surg. 2008;34(Suppl 1):S16–23.
- Levy PM, De Boulle K, Raspaldo H. Comparison of injection comfort of a new category of cohesive hyaluronic acid filler with preincorporated lidocaine and a hyaluronic acid filler alone. Dermatol Surg. 2009;35(Suppl 1):332–336.
- Farage MA, Miller KW, Elsner P, Maibach HI. Intrinsic and extrinsic factors in skin ageing: a review. Int J Cosmet Sci. 2008;30(2):87–95.
- Lapiere CM. The ageing dermis: the main cause for the appearance of ‘old’ skin. Br J Dermatol. 1990;122(Suppl 35):5–11.
- Kohl E, Steinbauer J, Landthaler M, Szeimies RM. Skin ageing. J Eur Acad Dermatol Venereol. 2011;25(8):873–884.
- Varani J, Dame MK, Rittie L, et al. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol. 2006;168(6):1861–1868.
- Fisher GJ, Varani J, Voorhees JJ. Looking older: fibroblast collapse and therapeutic implications. Arch Dermatol. 2008;144(5):666–672.
- Shin JW, Kwon SH, Choi JY, et al. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int J Mol Sci. 2019;20(9):67.
- Solish NJ. Assessment of recovery time for the collagen products Dermicol-P35 27G and 30G. J Am Acad Dermatol. 2010;62(5):824–830.
- Cassuto D. The use of Dermicol-P35 dermal filler for nonsurgical rhinoplasty. Aesthet Surg J. 2009;29(3 Suppl):548.
- Smith KC. Repair of acne scars with Dermicol-P35. Aesthet Surg J. 2009;29(3 Suppl).
- Klein AW. Skin filling. Collagen and other injectables of the skin. Dermatol Clin. 2001;19(3):491–508, ix.
- Gold M. The science and art of hyaluronic acid dermal filler use in esthetic applications. J Cosmet Dermatol. 2009;8(4):301–307.
- Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337(20):1419–1428.
- Bauman L. CosmoDerm/CosmoPlast (human bioengineered collagen) for the aging face. Facial Plast Surg. 2004;20(2):125–128.
- Braun M, Braun S. Nodule formation following lip augmentation using porcine collagen-derived filler. J Drugs Dermatol. 2008;7(6):579–581.
- Klein AW. Collagen substances. Facial Plast Surg Clin North Am. 2001;9(2):205–18, viii.
- Narins RS, Brandt F, Leyden J, Lorenc ZP, Rubin M, Smith S. A randomized, double-blind, multicenter comparison of the efficacy and tolerability of Restylane versus Zyplast for the correction of nasolabial folds. Dermatol Surg. 2003;29(6):588–595.