Abstract
Introduction
This study observed the effectiveness of ustekinumab and reactivation risk of concurrent latent tuberculosis infection (LTBI) and inactive hepatitis B virus (HBV) infection in Chinese mainland psoriasis patients on ustekinumab treatment.
Methods
This retrospective, multicenter, observational study was conducted in three centers in China. Adult patients with moderate to severe plaque psoriasis were treated with ustekinumab for 28 weeks. The effectiveness endpoint included 75% and 90% improvement in Psoriasis Area Severity Index (PASI75/90) response rate, percentage of PASI improvement, change of absolute PASI score and body surface area involvement (BSA) score, absolute PASI ≤1/3 and Physicians’ Global Assessment (PGA)=0/1, as well as Dermatology life quality index (DLQI)=0/1 response rate at week 4, 16 and 28. Screening of tuberculosis and hepatitis were performed at baseline and week 28.
Results
A total of 82 patients were enrolled between March 2021 and May 2023 and the number of patients combined with LTBI and inactive HBV infection was 20 and 21 respectively. The PASI75 and PASI90 response rate at week 28 was 95.1% and 81.7% respectively. The mean PASI score decreased from 14.93 ± 12.07 at baseline to 0.78 ± 1.86 at week 28, and the mean BSA score decreased from 21% ± 18% at baseline to 1% ± 2% at week 28 (both P<0.001 compared with baseline). DLQI 0/1 response rate at week 28 was 73.2%. No reactivation of LTBI and inactive HBV infection and also no new-onset tuberculosis and hepatitis B occurred in patients without LTBI and inactive HBV infection at baseline.
Conclusion
Ustekinumab demonstrated great effectiveness in Chinese plaque psoriasis patients and good safety in psoriasis concurrent with LTBI and inactive HBV infection under the real-world setting.
Introduction
Psoriasis is a chronic, recurrent, inflammatory and systematic skin disease which affects around 0.47% of Chinese people and causes large impairments to the quality of life of patients; 89.1% of Chinese psoriasis patients suffer from mental health problems caused by psoriasis.Citation1,Citation2 The most common subtype of psoriasis is plaque psoriasis (PsO), which accounts for 84.5% of Chinese psoriasis patients and is characterized by well demarcated, red and raised plaques covered by silvery scales.Citation3 In recent years, the improvements of anti-interleukin biologics included IL12/23i, IL-17Ai and IL-23i brought great improvement for the treatment of PsO.Citation4 However, the infection risk of biologics still requires special attention in patients combined with HBV and tuberculosis infection, especially in countries with high HBV and tuberculosis burden such as China. Nearly half of Chinese population are infected with mycobacterium tuberculosis and the infection rate ranking top 2 globally.Citation5 About 70 million people are chronic HBV infection patients in China.Citation6 With the high skin lesion clearance rate of new anti-interleukin biologics, more and more physicians pay attention to the quality of life of psoriasis patients. Several trials used patient-reported outcomes such as DLQI as an important endpoint to evaluate the impact of biologics on patients’ quality of life.Citation7–9
Ustekinumab, a fully human anti-IL12/23 monoclonal antibody that binds to the P40 subunit shared by IL-12 and IL-23 therefore inhibits the downstream inflammatory signal pathway, is the first approved anti-interleukin biologic for the treatment of plaque psoriasis which was approved by FDA in 2009.Citation10 In 2017, ustekinumab was approved for the treatment of Chinese adult moderate to severe plaque psoriasis patients and in 2023 it was approved for the treatment of Chinese 6–17 year old pediatric plaque psoriasis patients weighing 60–100 kg.Citation7,Citation11,Citation12 Previous genome-wide association studies found that IL-12B and IL-23R are susceptibility genes of Chinese psoriasis patients.Citation13,Citation14 A randomized, double-blind, placebo-controlled study also indicated that ustekinumab demonstrated great efficacy and quality of life improvement in Chinese plaque patients with no new safety signal was found,Citation7 in which the efficacy response rate was numerically higher than that of Phase 3 trials conducted in Western countries.Citation8,Citation9 Several real-world studies also proved that psoriasis patients combined with latent tuberculosis infection and inactive HBV infection treated with ustekinumab were safe.Citation15–17 However, no real-world data of ustekinumab in Chinese plaque psoriasis has been published up to now, especially after Ustekinumab had been incorporated into medical insurance system from 2022 in China, vast growth of application in clinical practice brought great attention and interest of real-world effectiveness and safety, especially infection risk of Ustekinumab to Chinese Dermatologists. In addition, another widely-used anti-17A biologic secukinumab in China has been proved safe in Chinese patients combined with LTBI or inactive HBV infection in a real-world study,Citation18 but there is a lack of evidence for Ustekinumab in terms of safety in patients combined with LTBI or inactive HBV infection, especially previous mechanism research display inconsistent outcome, two studies pointed out interferon inhibited by Ustekinumab play positive role in anti-tuberculosis and hepatitis,Citation19,Citation20 and another demonstrated that Ustekinumab improves skin lesion without affecting T cell immune response,Citation21 which brought much confusion to Chinese dermatologists. Compared to randomized-controlled trial, real-world data can reflect the effectiveness and safety of a biologic in daily clinical practice taking account of different complications and concomitant medications,Citation22 especially the heavy burden of HBV and tuberculosis infection in Chinese patients,Citation5,Citation6 so it is essential to identify the real-world effectiveness and safety of Ustekinumab in Chinese psoriasis patients.
This observational study aimed to assess the real-world effectiveness, quality of life improvement and safety of ustekinumab in Chinese plaque psoriasis patients, as well as the safety profile of ustekinumab in Chinese psoriasis combined with latent tuberculosis and inactive HBV infection.
Materials and Methods
Study Design and Patients
This retrospective, multicenter, observational real-world study was conducted in three centers(The No. 1 Affiliated Hospital of University of Science and Technology of China, The Lu’an Hospital Affiliated to Anhui Medical University, The No. 1 Affiliated Hospital of Anhui University of Traditional Chinese Medicine) in Anhui province, China between March 2021 and May 2023. Eligible patients were aged at least 18 years old, and were diagnosed as moderate to severe plaque psoriasis meeting one of the following criteria: i) Body surface area involvement (BSA) ≥3%; and ii) Psoriasis area and severity index (PASI) ≥3 according to “Guideline for the diagnosis and treatment of psoriasis in China (2023 edition)”.Citation2 All patients were screened for tuberculosis and Hepatitis B infection at baseline, active tuberculosis infection as well as active Hepatitis B infection were excluded. Patients combined with LTBI and inactive HBV infection were permitted to participate in this study and underwent preventive treatment in accordance with the “Guidelines for the treatment of psoriasis with biologic agents in China (2021)”, namely isoniazid alone or isoniazid combined with rifampicin programme for patients with LTBI, anti-hepatitis B viral drug entecavir for patients combined with inactive HBV infection.Citation23 Enrolled patients received a standard dose of ustekinumab treatments (45 mg at week 0 and week 4, followed by 45 mg every 12 weeks administrated subcutaneously for patients weighing 100 kg or less; 90 mg at week 0 and week 4, followed by 90 mg every 12 weeks administrated subcutaneously for patients weighing more than 100 kg) for 28 weeks. Hepatitis B were monitored every three months using serological examination and tuberculosis were monitored at baseline and week 28 using T-spot and chest-imaging.Citation23 Baseline demographics and clinical characteristics of patients were recorded.
Diagnosis and Reactivation Criterion of LTBI and Inactive HBV Infection
Patients with positive outcome of T-spot, no symptoms of active tuberculosis and active tuberculosis focus showed on chest-X ray/CT were defined as LTBI. The manifestations of coughing for more than 2 weeks, fever, weight loss, night sweats was were defined as reactivation of LTBI; abnormal chest-imaging manifestation indicating active tuberculosis and positive microbiological finding.Citation24,Citation25
Inactive HBV infection was defined as HBsAg positive with HBV-DNA negative (<104/mL) and normal liver function or isolated HBcAb positive with HBV-DNA negative and normal liver function.Citation23 Reactivation of HBV was defined as meeting with at least one of the following criteria: HBV DNA load increase more than 100-fold compared with baseline; detectable HBV DNA in patients previously with undetectable DNA; positive HBsAg in patients previously with negative HBsAg as well as liver dysfunction defined as threefold increase in ALT compared with baseline or absolute value of ALT level more than 100 U/L.Citation26
Study Outcomes
The effectiveness outcome included PASI75/90/100 and PASI improvement at week 4, 16 and 28; change of absolute PASI score and BSA score during the whole treatment period; percentage of patients achieved absolute PASI≤1, absolute PASI≤3 and PGA=0/1. DLQI 0/1 response rate was used to evaluate the quality of life improvement reported by patients. The safety outcome were the reactivation or new-onset of active tuberculosis and hepatitis B as well as any adverse event during the 28-week treatment.
Statistical Analysis
The data was analysed with the statistical software R (Version 4.2.2) and the quantitative data was presented as mean ± SD and median (interquartile range), the categorical data was presented as frequency and percentage. Statistical difference of absolute PASI and BSA between each visit (week 4, 12 and 28) and baseline was analysed with Bonferroni because of biased distribution. Two-tailed P<0.05 was considered as significantly different.
Results
Baseline Demographics and Clinical Characteristics
A total of 82 patients was enrolled between March 2021 and May 2023, and the numbers of patients with LTBI and inactive HBV infection was 20 and 21 respectively. Six patients were combined with LTBI and inactive HBV infection simultaneously. The average age of all the patients was 43.33 ± 14.18 years old. The mean weight and BMI were 69.07 ± 11.90 kg and 24.02 ± 3.32 kg/m2. 67.1% of patients were male and 43.9% of patients had family history. The mean disease duration was 14.68 ± 9.65 years and 7.1% of patients previously received biologic treatment. The average absolute PASI score and BSA score was 14.93 ± 12.0 and 20.65 ± 18.06 respectively. The percentage of patients with PGA marked or severe (PGA≥4) was 23.2%. The mean DLQI score was 14.50 ± 7.23 ( and ).
Table 1 Baseline Demographics and Clinical Characteristics of the Patients
Table 2 Tuberculosis and Hepatitis B Screening Outcomes at Week 28 of Patients Combined with LTBI or Inactive HBV Infection at Baseline
Clinical Response of Ustekinumab in Chinese Psoriasis Patients in the Real-World Setting
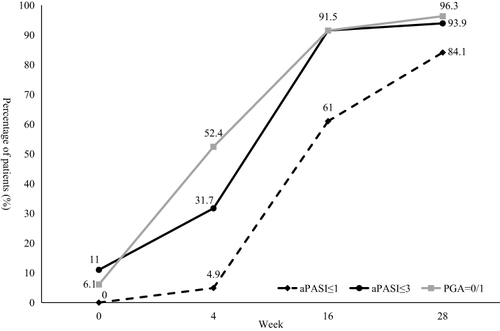
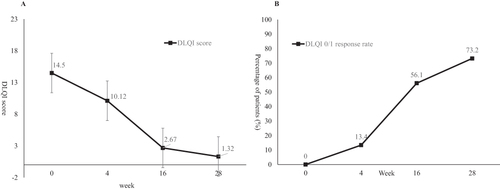
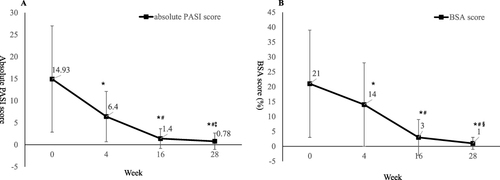
Ustekinumab showed effectiveness as early as the first visit at week 4, the PASI75 response rate was 12.2% and mean PASI improvement was 49.89% at week 4. The percentage of patients achieved PASI75 and PASI90 at week 16 was 87.8% and 63.4%, and the response rate at week 28 was 95.1% and 81.7% respectively. The mean PASI improvement at week 28 was 94.27% (). The mean PASI score decreased from 14.93 ± 12.07 at baseline to 0.78 ± 1.86 at week 28, the mean BSA score decreased from 21% ± 18% at baseline to 1% ± 2% at week 28 (both P<0.001 compared with baseline, ). In consideration of disease severity at baseline, the absolute PASI and BSA score no more than 1 or 3 was also calculated, as well as PGA=0/1. At week 28, the percentage of patients achieved aPASI≤3, aPASI≤1 and PGA=0/1 was 93.9%, 84.1% and 96.3% respectively (). The men DLQI score decreased from 14.50 ± 7.23 at baseline to 1.32 ± 2.35 at week 28, and the DLQI 0/1 response rate was 73.2% at week 28 ().
Figure 1 PASI 75 and PASI 90 response rate (A) and percentage of PASI improvement (B) during the 28-week treatment period.
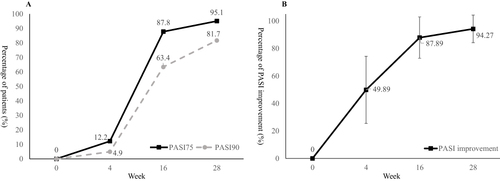
Figure 2 Change of absolute PASI score (A) and BSA score (B) during the 28-week treatment period. *P<0.001 compared with baseline; #P<0.001 compared with week 4; ‡P<0.001 compared with week 16; §P=0.001 compared with week 16.
Reactivation Risk of LTBI and Inactive HBV Infection in Chinese Patients Treated with Ustekinumab
During the whole 28 week treatment period, there was no reactivation of LTBI and inactive HBV infection under the preventive treatment against tuberculosis and hepatitis B according to the Chinese guideline in patients combined with LTBI and inactive HBV infection at baseline. There was also no new-onset tuberculosis and hepatitis in patients without tuberculosis and hepatitis B at baseline. Blood biochemical examination included alanine aminotransferase and aspartate aminotransferase did not transit to be abnormal at week 28 in all patients. The screening outcomes of patients at baseline and week 28 were respectively described in . Also, no other adverse event including fungal infection, malignancy, severe infection, inflammatory bowel disease or major cardiovascular events happened during the study.
Discussion
As high disease burden of LTBI and HBV infection in China mainland,Citation5,Citation6 as well as lack of effectiveness and safety real world data of ustekinumab in moderate to severe plaque psoriasis patients in China mainland, in which situation more complicated condition such as complications and concomitant medications were taken into consideration,Citation22 especially after been incorporating into national medical insurance of China, real-world effectiveness and infection risk of Ustekinumab aroused great interest and attention to Chinese dermatologists. This first retrospective, observational study verified the effectiveness of ustekinumab in Chinese psoriasis patients and safety of ustekinumab in Chinese psoriasis patients combined with LTBI and inactive HBV infection in the real-world setting. The PASI75 and PASI90 response rate at week 28 was up to 95.1% and 81.7%. The quality of life was also obviously improved with 73.2% of patients achieved DLQI 0/1 response at week 28. No reactivation was observed in 20 LTBI patients and 21 inactive HBV infection patients, as well as no adverse event was observed throughout the 28-week treatment of ustekinumab.
A multicenter, randomized, double-blind and placebo-controlled phase 3 study investigated the efficacy and safety of ustekinumab in Chinese moderate to severe psoriasis showed that 91.5% of patients achieved PASI75 and 80.4% of patients treated with ustekinumab achieved PASI90 at week 28.Citation7 Our study showed similar response rate compared with the study. Proportion of patients previously received biologic in both studies were comparable and relatively low, which is considered as a predictor of good response.Citation8 Two randomized controlled phase 3 trials enrolled moderate to severe plaque psoriasis patients mainly from Europe and North America showed lower PASI 90 response rate at week 28, 49.2% of 45 mg group and 55.6% of 90 mg group in PHOENIX 1 and 44.8% of 45 mg group and 54.3% of 90 mg group in PHOENIX 2 respectively, effectiveness results of these two trials were lower than our study, which may be interpreted by higher weight (90.3 kg to 93.8 kg across different groups) and higher proportion of biologic-experienced patients (36.5% to 52.5% across different groups) in the two trials.Citation8,Citation9 Another explanation may be genetic difference, IL-12B and IL-23R are susceptibility genes in Chinese psoriasis patients and ustekinumab binds to IL-12 and IL-23 may display better effectiveness in these genetic susceptible patients.Citation13,Citation14 Patient-reported outcomes are becoming more and more important in evaluating the severity and efficacy of treatment. Several guidelines use DLQI as one of the major endpoints in assessing the severity and treatment goal of psoriasis.Citation2,Citation27,Citation28 Several randomized controlled trials demonstrated similar DLQI 0/1 response rate at week 28 compared with our study.Citation7–9 And consistent with previous study, DLQI 0/1 response rate kept increasing with the continuously improvement of PASI or BSA, indicating the importance of high skin lesion clearance in improving the patients’ quality of life.Citation29
In terms of safety of tuberculosis and HBV infection, IL-12 and Th1 cytokine interferon induced by it were considered to play a positive role in clearing virus, thus biologic bind to IL-12 arouse concern for immunosuppression and increased infection risk.Citation19,Citation20 Kenshiro Tsuda et al demonstrated that ustekinumab improves psoriasis without affecting the T cell immune response compared with controls,Citation21 several Phase 2/3 trials also verified no significant risk for infection of ustekinumab in psoriasis patients,Citation30 several other trials confirmed the safety of ustekinumab in psoriasis combined with LTBI and inactive HBV infection,Citation15–17 so as several retrospective studies demonstrated safety of other biologics including IL-17 inhibitors and IL-23 inhibitors in patients combined with LTBI or inactive HBV infection,Citation31–34 but none of them was conducted in China where the tuberculosis and hepatitis burden are high. An observational study confirmed the safety of another widely used biologic in China, IL-17A inhibitor secukinumab in 43 Chinese patients combined with HBV infection or LTBI,Citation18 and we firstly confirmed the safety of IL-12/23 inhibitor ustekinumab in Chinese psoriasis patients combined with inactive HBV infection and LTBI. Apart from hepatitis B and tuberculosis, other concerned safety events of biologic such as fungal infection, malignancy, severe infection, inflammatory bowel disease and major cardiovascular events was also not observed in our study, confirmed the safety of ustekinumab in Chinese mainland psoriasis patients. A greater sample size and longer follow-up duration of the effectiveness and safety of ustekinumab in Chinese mainland moderate to severe plaque psoriasis patients is still under observed.
In conclusion, ustekinumab demonstrated great skin lesion clearance ability and brought great improvement of quality of life to Chinese plaque psoriasis patients under a real-world setting. The safety of ustekinumab in psoriasis concurrent with LTBI and inactive HBV infection were also confirmed with no reactivation or new-onset throughout the whole 28-week treatment. Also no new safety signal was observed during the study.
Ethics Approval
This study was conducted in accordance of Helsinki and the protocol was approved by the ethics committee of all participating centers including The No.1 Affiliated Hospital of University of Science and Technology of China, The Lu’an Hospital Affiliated to Anhui Medical University and The No.1 Affiliated Hospital of Anhui University of traditional Chinese medicine.
Consent to Participate
All patients provided written informed consent.
Disclosure
The authors have no potential conflicts of interest or financial disclosures that are pertinent to this article.
Acknowledgments
Special thanks to all the authors for their efforts and contribution to this study, and thanks Jian Xu for scientific communication.
Data Sharing Statement
The data of this study can be available from Dr Wen-Sheng Lu ([email protected]) for reasonable request.
Additional information
Funding
References
- Chen XL, Zheng LY, Zhang H, et al. Disease burden and quality of life in patients with psoriasis: an internet-based questionnaire survey. Chin J Dermatol. 2019;52(11):791–795 doi:10.35541/cjd.20190247.
- Zhang XJ, Wang G, Zhang FR, Zhang XB, Li B. Guideline for the diagnosis and treatment of psoriasis in China (2023 edition). Chin J Dermatol. 2023;56(7):573–625 doi:10.35541/cjd.20220839.
- Yang Z, Yao X, Wang M, Li H, Li R. Updates in psoriasis diagnosis and treatment status in China: results from the National Psoriasis Center Registry. Chin Med J. 2023;136(23):2874–2876 doi:10.1097/CM9.0000000000002563.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–1072. doi:10.1016/j.jaad.2018.11.057
- Bao CD, Chen YH, Gu JR, Gao MQ, Huang F. Expert position paper on tuberculosis prevention and management in tumor necrosis factor antagonist application. Chin J Rheumatol. 2013;17(8):508–512.
- Wang GQ, Wang FS, Zhuang H, Li TS, Zheng SJ. Guidelines for the prevention and treatment of chronic hepatitis B (version 2019). J Clin Hepatol. 2019;35(12):2648–2669.
- Zhu X, Zheng M, Song M, et al. Efficacy and safety of ustekinumab in Chinese patients with moderate to severe plaque-type psoriasis: results from a phase 3 clinical trial (LOTUS). J Drugs Dermatol. 2013;12(2):166–174.
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (Phoenix 2). Lancet. 2008;371(9625):1675–1684. doi:10.1016/S0140-6736(08)60726-6
- Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (Phoenix 1). Lancet. 2008;371(9625):1665–1674. doi:10.1016/S0140-6736(08)60725-4
- Ghoreschi K, Balato A, Enerback C, Sabat R. Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet. 2021;397(10275):754–766. doi:10.1016/S0140-6736(21)00184-7
- Philipp S, Menter A, Nikkels AF, et al. Ustekinumab for the treatment of moderate-to-severe plaque psoriasis in paediatric patients (>/= 6 to <12 years of age): efficacy, safety, pharmacokinetic and biomarker results from the open-label CADMUS Jr study. Br J Dermatol. 2020;183(4):664–672. doi:10.1111/bjd.19018
- Landells I, Marano C, Hsu MC, et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study. J Am Acad Dermatol. 2015;73(4):594–603. doi:10.1016/j.jaad.2015.07.002
- Tang H, Jin X, Li Y, et al. A large-scale screen for coding variants predisposing to psoriasis. Nat Genet. 2014;46(1):45–50. doi:10.1038/ng.2827
- Zhang XJ, Huang W, Yang S, et al. Psoriasis genome-wide association study identifies susceptibility variants within LCE gene cluster at 1q21. Nat Genet. 2009;41(2):205–210. doi:10.1038/ng.310
- Navarro R, Vilarrasa E, Herranz P, et al. Safety and effectiveness of ustekinumab and antitumour necrosis factor therapy in patients with psoriasis and chronic viral hepatitis B or C: a retrospective, multicentre study in a clinical setting. Br J Dermatol. 2013;168(3):609–616. doi:10.1111/bjd.12045
- Cho SI, Kang S, Kim YE, Lee JY, Jo SJ. Ustekinumab does not increase tuberculosis risk: results from a national database in South Korea. J Am Acad Dermatol. 2020;82(5):1243–1245. doi:10.1016/j.jaad.2019.12.033
- Siegel SAR, Winthrop KL, Ehst BD, Ortega Loayza A. Ustekinumab use in patients with severe psoriasis co-infected with hepatitis B and/or C. Br J Dermatol. 2019;180(5):1232–1233. doi:10.1111/bjd.17444
- Liu S, He Z, Wu W, Jin H, Cui Y. Safety of secukinumab in the treatment of patients with axial spondyloarthritis and concurrent hepatitis B virus infection or latent tuberculosis infection. Clin Rheumatol. 2023;42(9):2369–2376. doi:10.1007/s10067-023-06630-8
- Wu JF, Wu TC, Chen CH, et al. Serum levels of interleukin-10 and interleukin-12 predict early, spontaneous hepatitis B virus e antigen seroconversion. Gastroenterology. 2010;138(1):165–72 e1–3. doi:10.1053/j.gastro.2009.09.018
- Narinyan W, Poladian N, Orujyan D, Gargaloyan A, Venketaraman V. Immunologic role of innate lymphoid cells against mycobacterial tuberculosis infection. Biomedicines. 2022;10(11):2828. doi:10.3390/biomedicines10112828
- Tsuda K, Yamanaka K, Kondo M, et al. Ustekinumab improves psoriasis without altering T cell cytokine production, differentiation, and T cell receptor repertoire diversity. PLoS One. 2012;7(12):e51819. doi:10.1371/journal.pone.0051819
- Porzsolt F, Eisemann M, Habs M, Wyer P. Form follows function: pragmatic controlled trials (PCTs) have to answer different questions and require different designs than randomized controlled trials (RCTs). Z Gesundh Wiss. 2013;21(3):307–313. doi:10.1007/s10389-012-0544-5
- Wang G, Zhang XJ, Chen AJ, Gao XH, Gu H. Guidelines for the treatment of psoriasis with biologic agents in China (2021). Chin J Dermatol. 2021;54(12):1033–1047.
- Haas MK, Belknap RW. Diagnostic tests for latent tuberculosis infection. Clin Chest Med. 2019;40(4):829–837. doi:10.1016/j.ccm.2019.07.007
- Gong W, Wu X. Differential diagnosis of latent tuberculosis infection and active tuberculosis: a key to a successful tuberculosis control strategy. Front Microbiol. 2021;12:745592. doi:10.3389/fmicb.2021.745592
- Shi Y, Zheng M. Hepatitis B virus persistence and reactivation. BMJ. 2020;370:m2200.
- Amatore F, Villani AP, Tauber M, Viguier M, Guillot B; Psoriasis Research Group of the French Society of D. French guidelines on the use of systemic treatments for moderate-to-severe psoriasis in adults. J Eur Acad Dermatol Venereol. 2019;33(3):464–483. doi:10.1111/jdv.15340
- Saeki H, Terui T, Morita A, et al. Japanese guidance for use of biologics for psoriasis (the 2019 version). J Dermatol. 2020;47(3):201–222. doi:10.1111/1346-8138.15196
- Alpalhao M, Duarte J, Diogo R, et al. Lower limbs are the most difficult-to-treat body region of patients with psoriasis: pooled analysis of CLEAR and CLARITY studies of secukinumab versus ustekinumab by body region. BioDrugs. 2022;36(6):781–789. doi:10.1007/s40259-022-00558-2
- Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168(4):844–854. doi:10.1111/bjd.12214
- Gargiulo L, Pavia G, Valenti M, et al. Safety of biologic therapies in patients with moderate-to-severe plaque psoriasis and concomitant viral hepatitis: a monocentric retrospective study. Dermatol Ther. 2022;12(5):1263–1270. doi:10.1007/s13555-022-00726-w
- Mastorino L, Dapavo P, Trunfio M, et al. Risk of reactivation of latent tuberculosis in psoriasis patients on biologic therapies: a retrospective cohort from a tertiary care centre in Northern Italy. Acta Derm Venereol. 2022;102:adv00821. doi:10.2340/actadv.v102.1982
- Torres T, Chiricozzi A, Puig L, et al. Treatment of psoriasis patients with latent tuberculosis using IL-17 and IL-23 inhibitors: a retrospective, multinational, multicentre study. Am J Clin Dermatol. 2024;25(2):333–342. doi:10.1007/s40257-024-00845-4
- Ibba L, Gargiulo L, Vignoli CA, et al. Safety of anti-IL-23 drugs in patients with moderate- to-severe plaque psoriasis and previous tuberculosis infection: a monocentric retrospective study. J Dermatol Treat. 2023;34(1):2241585. doi:10.1080/09546634.2023.2241585