Abstract
Purpose
Coronavirus disease (COVID-19) may trigger the reactivation of the latent varicella-zoster virus and may be a risk factor for herpes zoster (HZ). However, the causal relationship between COVID-19 and varicella-zoster infections remains controversial. This study aimed to estimate the causal inferences between COVID-19 and HZ.
Methods
This study used a two-sample Mendelian randomization (MR) design. The inverse variance-weighted method was used as the primary method and sensitivity analyses were conducted, including the MR-Egger regression, weighted median and weighted mode. We searched at https://gwas.mrcieu.ac.uk/ using the keywords “COVID-19” for exposure data and “zoster” for outcome datasets.
Results
We got 26 COVID-19 datasets and five zoster datasets. We used 26 COVID-19 datasets as exposure data corresponding to each zoster dataset for the MR analysis. There were nine datasets of COVID-19 where the number of SNPs was fewer than three in the MR analysis of the risk of HZ, varicella zoster virus (VZV) glycoprotein E and I antibody levels, anti-VZV IgG seropositivity, and post-zoster neuralgia. In addition, there were 10 datasets of COVID-19 where the number of SNPs was less than three in the MR analysis of anti-VZV IgG levels. The results of the MR analysis showed that all p-values were greater than 0.05. Sensitivity analysis revealed no evidence of horizontal pleiotropy in most two sample MR analyses.
Conclusion
Our results indicate that there is no causal relationship between COVID-19 and varicella-zoster infection, HZ progression, and postherpetic neuralgia.
Introduction
Herpes zoster (HZ) is induced by the reactivation of the latent varicella-zoster virus (VZV) and is characterised by a unilateral vesicular rash on the face, head, or trunk.Citation1 Clinically significant reactivation of HZ occurs in 10–20% of the naturally infected population, and with age, especially after the age of 50 years, the possibility of HZ increases proportionally.Citation2 Postherpetic neuralgia (PHN) is the most serious complication of HZCitation3 and decreases the quality of life.
During the COVID-19 pandemic, many researchers studied the association between COVID-19 and HZ. COVID-19 may the trigger reactivation of latent VZV and may be a risk factor for HZ.Citation4 However, the causal relationship between COVID-19 and HZ remains unclear. In a review, Algaadi held the view that there is a causal relationship between COVID-19 and HZ, according to the available data.Citation5 However, Diez-Domingo et al believed that the existing evidence is insufficient to determine whether COVID-19 increases the risk of HZ.Citation6
Mendelian randomization (MR) is an epidemiological method, that uses genetic variants for instrumental variable estimation to replace the exposure variable of interest and study the impact of exposure on specific outcomes.Citation7 Owing to the random allocation of single nucleotide polymorphisms (SNPs) during pregnancy, they are less likely to be influenced by confounding factors.Citation8 The MR design can reduce residual confusion and reverse causality, thus strengthening the causal inference of exposure result correlation. There have been few articles on MR analysis of COVID-19 and HZ. In this study, we conducted a two-sample MR analysis to study the relationship between COVID-19 and varicella-zoster infection, HZ progression and PHN.
Materials and Methods
Study Design
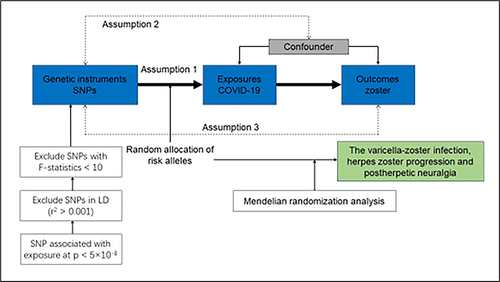
This study used a two-sample MR design to estimate causal inferences between COVID-19 and HZ. The MR study is based on the following three assumptions: First, the genetic variants selected as instrumental variants (IVs) were strongly associated with exposure (COVID-19). Second, the IVs were not associated with any confounders. Third, the IVs directly affected the outcomes through exposure rather than other pathways. A flow chart of the analysis is shown in .
Data Sources
We downloaded the summary data from open access genome-wide association studies data sets at https://gwas.mrcieu.ac.uk/. We searched for “COVID-19” and “zoster”. There were 31 datasets for COVID-19 and five datasets for zoster.
Instrumental Variants (IVs) Selection
SNPs associated with COVID-19 were selected using the conventional genome-wide association significance threshold (p < 5×10−8). Linkage disequilibrium (LD) clumping was used to identify and exclude SNPs in LD (R2 > 0.001 or within ± 10,000 kilobase (kb) distance 10,000 Genomes European-ancestry Reference Panel). The mean F-statistics were calculated to test for weak instruments as previously described.
Statistical Analysis
The inverse variance-weighted (IVW) method was used as the primary method, and sensitivity analyses were conducted including the MR-Egger regression, weighted median, and weighted mode. The MR analysis was performed with R version 4.2.3 using the “TwoSampleMR” package.
Results
We conducted an MR analysis between COVID-19 (31 datasets) and HZ (five datasets). Five COVID-19 datasets were eliminated because their populations were not the same as those of the outcome data. We used 26 COVID-19 datasets as exposure data corresponding to each zoster dataset for the MR analysis. There were five datasets of HZ, including “Zoster (herpes zoster)”, “Varicella zoster virus glycoproteins E and I antibody levels”, “anti-varicella zoster virus IgG levels”, “Anti-varicella zoster virus IgG seropositivity”, and “postzoster neuralgia”. Detailed information on the exposures and outcomes is provided in the Supplementary Materials (Tables S1 and S2). The results of the MR and sensitivity analyses for the five outcome indicators are summarized in the Supplementary Materials (Tables S3-S7, Figures S1-S5).
There were nine datasets of COVID-19 in which the number of SNPs was fewer than three in the MR analysis of the risk of HZ, varicella-zoster virus glycoproteins E and I antibody levels, anti-varicella zoster virus IgG seropositivity, and the risk of post-zoster neuralgia. In addition, there were 10 COVID-19 datasets in which the number of SNPs was less than three in the MR analysis with anti-varicella zoster virus IgG levels.
These results suggest a non-causal association between COVID-19 and varicella-zoster infection, HZ progression, and PHN. COVID-19 was not genetically associated with varicella-zoster infection, HZ progression, and PHN. The results of the MR-Egger, weighted median, and weighted mode methods are consistent with those from the IVW analysis. The results of the MR analysis show that most p-values are greater than 0.05. No pleiotropy was identified in the sensitivity test, and no SNPs independently drove the results in leave-one-out sensitivity in the majority of our analyses, indicating the reliability of the causal effect estimates. Two scatter plots are shown ( and ) following.
Discussion
To the best of our knowledge, no study has performed MR analysis of COVID-19 and HZ. In our analysis, the p-values in the four MR methods were greater than 0.05, and we found that COVID-19 was not causally involved in varicella-zoster infection, HZ progression, and PHN. These results demonstrate that COVID-19 itself has no causal effect on VZV infection or the progression of HZ.
Although several researchers have studied the relationship between COVID-19 and HZ, no consensus has yet been reached. It is widely believed that there is an association between COVID-19 and HZ. However, the exact nature of this association remains to be elucidated.Citation9 Due to the intracellular pathogen transmission of VZV, cell-mediated immunity plays a more important role than humoral immunity.Citation10 COVID-19 has been shown to be associated with a persistent decrease in lymphocytes during the disease,Citation10 and it is thought that the lymphocyte reduction caused by COVID-19, especially CD3+ CD8+ lymphocytes, may activate latent VZV.Citation11–13 All 10 patients in one case seriesCitation14 developed HZ within 3 weeks of being diagnosed with COVID-19 infection, which suggested the important role of COVID-19 in the occurrence of HZ. In one study, the results indicated that the risk of HZ is significantly increased in patients over 50 years of age diagnosed with COVID-19, and COVID-19 infection may trigger VZV reactivation.Citation4 In another article, the authors suggested that varicella-like disease and HZ may be cutaneous symptoms in patients with COVID-19.Citation15 Once affected by COVID-19, cell-mediated immunity is suppressed, and a cytokine storm may occur.Citation16–18 Increased psychological stress may also put patients at risk of VZV reactivation.Citation17,Citation19 Shingles in patients with COVID-19 are caused by multiple factors.
We also analysed COVID-19 and VZV antibodies (“varicella-zoster virus glycoprotein E and I antibody levels”, “anti-varicella zoster virus IgG levels”, and “anti-varicella zoster virus IgG seropositivity”). In addition to cell-mediated immune responses, VZV infection can induce strong humoral immunity.Citation2 Antibodies can last for a lifetime and provide protection against re-infection.Citation20 VZV-specific IgG has a 50-year functional half-life without enhancement, and its presence indicates a history of contact or immunity to re-infection.Citation2,Citation21 In cells infected with VZV, glycoprotein E (gE) is the most abundant noncovalently linked glycoprotein I (gI).Citation22 VZV gE is the most immunogenic VZV glycoprotein and an essential target for the host immune reaction.Citation23 We can infer that IgG is probably induced by VZV gE because VZV gE has been proven to bind to the Fc fragment of IgG.Citation22 In our study, there is no causal relationship between COVID-19 or VZV IgG levels and seropositivity.
Chronic pain may occur after shingles and seriously influence the quality of life. PHN is now difficult to distinguish from acute herpetic pain and these two types of pain may coexist in certain clinical situation.Citation24,Citation25 Early relief of acute pain may reduce the risk of chronic pain.Citation24,Citation26 In the present study, COVID-19 had no causal effect on the risk of developing PHN. According to the existing literature, age is the most significant risk factor for chronic pain.Citation3,Citation27 Although age was also associated with COVID-19 infection, the occurrence of chronic pain was not directly associated with COVID-19.
Generally, the association between COVID-19 and HZ may occur via a response of the immune system. However, we cannot demonstrate that COVID-19 leads to an increase of HZ infection and a poor prognosis.
Conclusion
These results suggested a non-causal association between COVID-19 and varicella-zoster infection, HZ progression, and PHN. Our results indicate no causal relationship between COVID-19 and varicella-zoster infection, HZ progression, or chronic pain.
Ethics Approval and Consent to Participate
According to Article 32 of the Measures for Ethical Review of Life Science and Medical Research Involving Human Beings adopted by the National Science and Technology Ethics Committee of the People’s Republic of China, ethical review can be exempted because the data used in this study do not cause any harm to human beings, do not involve any sensitive personal information or commercial interests, and the databases selected are open and legal. Therefore, approval from a new ethics review committee is not required.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Kennedy PGE, Gershon AA. Clinical features of varicella-zoster virus infection. Viruses. 2018;10(11):609. doi:10.3390/v10110609
- Freer G, Pistello M. Varicella-zoster virus infection: natural history, clinical manifestations, immunity and current and future vaccination strategies. New Microbiol. 2018;41(2):95–105.
- Gershon AA, Breuer J, Cohen JI, et al. Varicella zoster virus infection. Nat Rev Dis Primers. 2015;1:15016. doi:10.1038/nrdp.2015.16
- Bhavsar A, Lonnet G, Wang C, et al. Increased risk of herpes zoster in adults ≥50 years old diagnosed With COVID-19 in the United States. Open Forum Infect Dis. 2022;9(5):ofac118. doi:10.1093/ofid/ofac118
- Algaadi SA. Herpes zoster and COVID-19 infection: a coincidence or a causal relationship? Infection. 2022;50(2):289–293. doi:10.1007/s15010-021-01714-6
- Diez-Domingo J, Parikh R, Bhavsar AB, Cisneros E, McCormick N, Lecrenier N. Can COVID-19 increase the risk of herpes zoster? a narrative review. Dermatol Ther. 2021;11(4):1119–1126. doi:10.1007/s13555-021-00549-1
- Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23(R1):R89–98. doi:10.1093/hmg/ddu328
- Sanderson E, Smith GD, Windmeijer F, Bowden J. Corrigendum to: an examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. Int J Epidemiol. 2020;49(3):1057. doi:10.1093/ije/dyaa101
- Katz J, Yue S, Xue W. Herpes simplex and herpes zoster viruses in COVID-19 patients. Ir J Med Sci. 2022;191(3):1093–1097. doi:10.1007/s11845-021-02714-z
- Elsaie ML, Nada HA. Herpes zoster (shingles) complicating the course of COVID19 infection. J DermatolTreat. 2022;33(2):1123–1125. doi:10.1080/09546634.2020.1782823
- Shors AR. Herpes zoster and severe acute herpetic neuralgia as a complication of COVID-19 infection. JAAD Case Rep. 2020;6(7):656–657. doi:10.1016/j.jdcr.2020.05.012
- Sil A, Bhanja DB, Sarkar AD, Chandra A. Herpes zoster involving trigeminal nerve: an early cutaneous predictor of COVID-19-a case series and review of the literature. J Cosmet Dermatol. 2022;21(4):1371–1373. doi:10.1111/jocd.14679
- Tartari F, Spadotto A, Zengarini C, et al. Herpes zoster in COVID-19-positive patients. Int J Dermatol. 2020;59(8):1028–1029. doi:10.1111/ijd.15001
- Narasimhan M, Ramakrishnan R, Durai PCT, Sneha B. Association between COVID-19 infection and herpes zoster: a case series. J Family Med Prim Care. 2023;12(10):2516–2519. doi:10.4103/jfmpc.jfmpc_2112_22
- Patil A, Goldust M, Wollina U. Herpes zoster: a review of clinical manifestations and management. Viruses. 2022;14(2):192. doi:10.3390/v14020192
- Xu R, Zhou Y, Cai L, et al. Co-reactivation of the human herpesvirus alpha subfamily (herpes simplex virus-1 and varicella zoster virus) in a critically ill patient with COVID-19. Br J Dermatol. 2020;183(6):1145–1147. doi:10.1111/bjd.19484
- Pona A, Jiwani RA, Afriyie F, Labbe J, Cook PP, Mao Y. Herpes zoster as a potential complication of coronavirus disease 2019. Dermatol Ther. 2020;33(6):e13930. doi:10.1111/dth.13930
- Xu B, Fan CY, Wang AL, et al. Suppressed T cell-mediated immunity in patients with COVID-19: a clinical retrospective study in Wuhan, China. J Infect. 2020;81(1):e51–e60. doi:10.1016/j.jinf.2020.04.012
- Xiao S, Luo D, Xiao Y. Survivors of COVID-19 are at high risk of posttraumatic stress disorder. Glob Health Res Policy. 2020;5:29. doi:10.1186/s41256-020-00155-2
- Morell A, Barandun S. Prophylactic and therapeutic use of immunoglobulin for intravenous administration in patients with secondary immunodeficiencies associated with malignancies. Pediatr Infect Dis J. 1988;7(5 Suppl):S87–91.
- Laing KJ, Ouwendijk WJD, Koelle DM, Verjans G. Immunobiology of varicella-zoster virus infection. J Infect Dis. 2018;218(suppl_2):S68–S74. doi:10.1093/infdis/jiy403
- Arvin AM. Varicella-zoster virus. Clin Microbiol Rev. 1996;9(3):361–381. doi:10.1128/CMR.9.3.361
- Liu J, Lin J, Cai L, et al. Immunogenicity of varicella zoster virus DNA vaccines encoding glycoprotein E and immediate early protein 63 in mice. Viruses. 2022;14(6):1214. doi:10.3390/v14061214
- Iseki M, Yamamoto T, Ogawa Y, et al. Efficacy and safety of intravenous fosphenytoin for patients with acute herpes zoster-associated pain: a placebo-controlled randomized trial. J Dermatol. 2024;51(2):234–242. doi:10.1111/1346-8138.17054
- Johnson RW. Zoster-associated pain: what is known, who is at risk and how can it be managed? Herpes. 2007;14(Suppl 2):30–34.
- Dworkin RH, Perkins FM, Nagasako EM. Prospects for the prevention of postherpetic neuralgia in herpes zoster patients. Clin J Pain. 2000;16(2 Suppl):S90–100. doi:10.1097/00002508-200006001-00016
- Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med. 1965;58(1):9–20.