Abstract
Background
Non-scarring hair loss (NSHL) is a global health concern with increasing prevalence due to lifestyle changes and an aging population. It can cause psychological distress and affect quality of life.
Objective
This study aimed to identify the associations between NSHL and immune cell phenotypes using a two-sample Mendelian randomization (MR) analysis, offering insights for future immune-based therapies for NSHL.
Methods
We obtained immunocyte data from the IEU Open GWAS Project and NSHL data from the same database and used MR analysis to evaluate the causal association between each immunophenotype and NSHL. Three statistical methods were employed: the MR-Egger regression, weighted median estimation, and inverse variance weighting (IVW).
Results
The MR resonance imaging identified 31 immunocyte phenotypes associated with NSHL. Among these, 19 immunocyte phenotypes were negatively associated with NSHL, indicating their protective effects. The remaining 12 immunocyte phenotypes were positive association. Sensitivity analyses suggested the robustness of all MR findings.
Conclusion
These findings highlight a clear correlation between NSHL and immunity, demonstrating the significant role of certain immune cell phenotypes. This study offers a new direction for immune-based therapies in the treatment of NSHL.
Introduction
Hair loss (HL) is a global issue and its prevalence continues to increase with human lifespan and lifestyle changes.Citation1 In particular, non-scarring hair loss (NSHL) causes great distress and has an adverse psychological impact on many individuals.Citation2 Currently, the main methods for treating NSHL are medications, such as minoxidil and finasteride, and surgical interventions, such as hair transplantation. However, these methods have potential side effects, including rashes, headaches, and sexual dysfunction.Citation3,Citation4 In addition to genetic factors, changes in human lifestyle are believed to be among the main contributors to the increased prevalence of hair loss. Factors, such as poor dietary habits, lack of exercise, and significant psychological stress, are associated with hair loss. Furthermore, on a global scale, environmental factors, such as air pollution and exposure to ultraviolet radiation, have been found to be closely correlated with the incidence of hair loss.Citation5,Citation6 Understanding the etiology and mechanisms of illness is crucial for disease treatment. Changes in lifestyle habits and stress can lead to alterations in human immune function.Citation7,Citation8 Studies are currently examining the relationship between hair loss and the immune system.Citation9,Citation10 This provides a new direction for the future implementation of immunotherapy for the treatment of NSHL. However, a key prerequisite for successful immunotherapy is the identification of immune cells associated with NSHL.
Mendelian randomization (MR) is a methodology used to assess causality in observational studies. This method is based on Mendelian inheritance principles, which suggest that the inheritance of genetic variation occurs randomly. Consequently, the genetic variations associated with specific diseases may have influenced the variables under consideration. The MR investigates the correlation between exposure and outcomes by contrasting populations with distinct genetic variations. This approach provides superior control over potential confounding factors compared with conventional observational studies, thereby enhancing the reliability of the conclusions.Citation11
Therefore, our study aimed to examine the association between NSHL and immune cells using two-sample MR analysis.
Materials and Methods
Acquired Data
We acquired immunocyte data from the Ieu Open Gwas Project (http://gwas.mrcieu.ac.uk), a genome-wide association studies database.Citation12 These data were used as exposure data in this study. The identification numbers for the data ranged from GCST0001391 to GCST0002121. In this study, we conducted a genome-wide association analysis of 629 blood immune cell-related traits in 272,100 individuals from the European general population. The study included 731 immunophenotypes.Citation13 We obtained the NSHL data[ID: finn-b-L12_HAIRLOSSNONSCAR, Year:2021, Population: European, Sex: Males and Females, n(case):81, n (control): 211,139, Number of SNPs: 16,380,450] as the outcome using the same method.
MR Analysis
Following data acquisition, we conducted separate evaluations for two associations: 1) between SNPs and exposure, and 2) between each SNP and outcome. Subsequently, an MR analysis was performed to evaluate the causal association between each immunophenotype and NSHL.
Three statistical methods were used to investigate the relationship between immunophenotypes and NSHL: MR-Egger regression, weighted median estimation, and inverse variance weighting (IVW). MR-Egger regression, employed in MR studies, is a statistical method used to detect and account for pleiotropic effects. Pleiotropy refers to the phenomenon in which a genetic variant influences outcomes through multiple pathways. The MR-Egger approach incorporates instrumental variables to estimate the causal effect of exposure on the outcome while accounting for potential pleiotropic effects.Citation14 The weighted median estimator is a statistical technique used to estimate the representative value of a population by calculating the median score of a weighted sample. In this method, individual data points in a sample are assigned weights that reflect their relative importance or influence the overall estimation of population parameters. This approach is particularly advantageous when dealing with outliers in data distribution, as it offers a more robust measure of central tendency than conventional methods, such as the arithmetic mean.Citation15 IVW is a statistical methodology frequently used in meta-analyses to combine the effect size estimates obtained from multiple studies. The weight assigned to each study in IVW was determined based on the inverse of its variance, which represented the precision of the effect estimate. By considering both sample size and variability in effect sizes across studies, the IVW approach generates a pooled effect size that incorporates information from the entire body of evidence.Citation16
To ensure the accuracy of MR analysis, it is crucial that the genetic variation of the instrumental variables chosen exhibit a strong correlation with the risk factors. Therefore, we set the significance level at p<5x10−8 as the criterion for screening instrumental variables.Citation13,Citation17
Sensitivity Analysis
The IVW method was used to calculate the Q valueCitation18 to assess the heterogeneity between SNPs, and the Q P-value was calculated to determine heterogeneity. Additionally, we conducted a “leave-one-out” analysis to investigate the potential influence of individual SNPs on the causal association. Furthermore, we applied the MR-Egger regression tests to monitor the presence of potential horizontal pleiotropy effects.
Statistical Analysis
MR analysis was conducted using the “TwoSampleMR” package in R (V4.2.3).Citation19 Calculation of the causal association between each immunophenotype and NSHL is reported as an odds ratio (OR) with a corresponding 95% confidence interval (CI). The threshold for statistical significance was set at p<0.05.
Results
MR
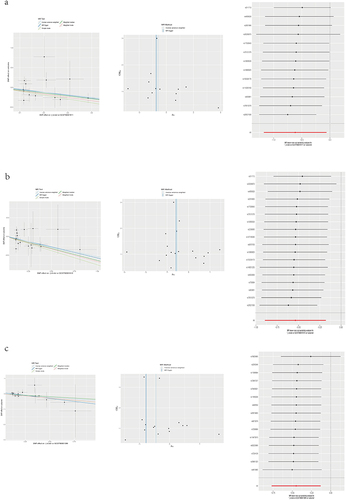
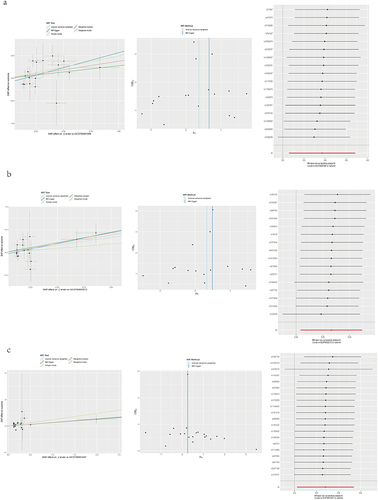
The MR Results are presented in and with a threshold of p<0.05, defining a causal relationship. When the IVW method yields significance (p < 0.05), even if the other methods do not, a positive result can be considered provided that the beta values of the other methods align in the same direction.Citation20 Based on these results, 31 immunocyte phenotypes associated with NSHL were identified. Among these, 19 immunocyte phenotypes were negatively associated with NSHL (), indicating their protective effects against NSHL. In contrast, the remaining 12 immunocyte phenotypes were positively associated with NSHL (). Details regarding each SNP are shown in Supplementary Table S1.
Table 1 Results of Mendelian Randomization of 19 Immune Cell Phenotypes Negatively Associated with NSHL
Table 2 Results of Mendelian Randomization of 12 Immune Cell Phenotypes Positive Associated with NSHL
Sensitivity Analysis
The results of the heterogeneity tests indicated no heterogeneity among the SNPs in all MR Analyses (Q_p>0.05) ( and ). In this study, we conducted all MR analyses using a “leave-one-out” approach. After sequential removal of each SNP, the direction of the beta values calculated using the IVW method remained consistent across all analyses (). This indicates the stability of all MR findings.Citation21 Furthermore, we subjected all intercept terms (egger_intercept) of the MR-Egger method to statistical testing, and the resulting p-values were >0.05. Therefore, we concluded that there was no evidence of horizontal pleiotropy ( and ).Citation22
Discussion
Immunotherapy has been utilized to treat numerous diseases, especially with the advent of Chimeric antigen receptor (CAR)-T-cell therapy,Citation23 which has brought hope to patients with malignant tumors owing to its exceptional therapeutic efficacy. Furthermore, its success has provided insights into the application of immunotherapy in the treatment of other diseases. Currently, there is limited research focusing on immunotherapy for the treatment of NSHL,Citation24 which raises questions regarding the specific immune cells to be targeted for investigation, potential immune cells that may confer a protective effect against NSHL, and immune cells that might be implicated in the exacerbation of NSHL. Further investigations are required to address these questions.
We sorted all immune cell phenotypes based on their OR values according to IVW; the top three phenotypes that were most negatively correlated with NSHL were CD4+ CD8dim T cell % leukocytes, CD4+ CD8dim T cell % lymphocytes, and CD86+ plasmacytoid dendritic Cell (). Thus, it can be inferred that CD4+ CD8dim T cells play a significant role in the protection against NSHL. CD4+ CD8dim T cells are a special type of immune cells belonging to the T cell family. In the immune system, leukocytes are an important type of cell that play a crucial role in protecting the body from infections.Citation25 “% leukocyte” represents the percentage of this cell type within the leukocyte population. The percentage of leukocytes can be used to measure the relative abundances of different cell types in the immune system. CD4+ CD8dim T cell leukocytes indicates the proportion of this specific type of T-cell within the leukocyte population. Typically, changes in this proportion may be associated with immune system function and disease status. CD86+ plasmacytoid dendritic cells are important components of the immune system that contribute to the regulation and coordination of immune responses, particularly in the context of viral infections.Citation26 It can be inferred that in-depth investigations of the immune cells closely associated with NSHL are crucial for advancing research on immunotherapy for this condition. In the context of NSHL immunotherapy, targeting and inhibiting immunosuppressive immune cells are considered a critical step towards augmenting the immune response against baldness and improving treatment outcomes. Therefore, a comprehensive exploration of the immune cells closely linked to the immune response in NSHL is pivotal in determining the potential of immunotherapy and developing efficacious treatment modalities.
In contrast, the three immune cell phenotypes that exert the strongest promoting effects on NSHL are phenotypes PDL-1 on CD14- CD16+ monocyte, CX3CR1 on CD14- CD16+ monocyte, and CD25 on resting CD4 regulatory T cell. The functional mechanisms of these immune cells are currently unclear, and studying these immune cells will help understand the pathogenic mechanisms of NSHL.
Although this study yielded rich results, it has certain limitations. Our data came exclusively from Europeans, and the causes of hair loss may vary among ethnic groups.Citation27 Therefore, larger, more globally representative datasets are required to support our findings.
Conclusion
This study revealed a correlation between NSHL and the immune system by utilizing MR analysis, identifying 31 immune cell phenotypes associated with NSHL. Our findings may provide a research direction for immune-based therapies for NSHL and establish a foundation for understanding the pathogenesis of NSHL.
Ethical Approval and Consent
This study has been approved by the Ethics Committee of the Second Affiliated Hospital of Guangxi Medical University. Approval Number:2024-KY(0500).
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgment
We would like to thank Editage (www.editage.cn) for English language editing.
References
- Gan DC, Sinclair RD. Prevalence of male and female pattern hair loss in Maryborough. J Investig Dermatol Symp Proc. 2005;10(3):184–189. doi:10.1111/j.1087-0024.2005.10102.x
- Cash TF. The psychosocial consequences of androgenetic alopecia: a review of the research literature. Br J Dermatol. 1999;141(3):398–405. doi:10.1046/j.1365-2133.1999.03030.x
- Randolph M, Tosti A. Oral minoxidil treatment for hair loss: a review of efficacy and safety. J Am Acad Dermatol. 2021;84(3):737–746. doi:10.1016/j.jaad.2020.06.1009
- York K, Meah N, Bhoyrul B, Sinclair R. A review of the treatment of male pattern hair loss. Expert Opin Pharmacother. 2020;21(5):603–612. doi:10.1080/14656566.2020.1721463
- Gokce N, Basgoz N, Kenanoglu S, et al. An overview of the genetic aspects of hair loss and its connection with nutrition. J Prev Med Hyg. 2022;63(2 Suppl 3).
- Phillips TG, Slomiany WP, Allison R. Hair Loss: common Causes and Treatment. Am Fam Physician. 2017;96(6):371–378.
- Snyder BK, Roghmann KJ, Sigal LH. Stress and psychosocial factors: effects on primary cellular immune response. J Behav Med. 1993;16(2):143–161. doi:10.1007/BF00844890
- Friedman SM. Lifestyle (Medicine) and Healthy Aging. Clin Geriatr Med. 2020;36(4):645–653. doi:10.1016/j.cger.2020.06.007
- Sterkens A, Lambert J, Bervoets A. Alopecia areata: a review on diagnosis, immunological etiopathogenesis and treatment options. Clin Exp Med. 2021;21(2):215–230. doi:10.1007/s10238-020-00673-w
- Watson VE, Faniel ML, Kamili NA, Krueger LD, Zhu C. Immune-mediated alopecias and their mechanobiological aspects. Cells Dev. 2022;170:203793. doi:10.1016/j.cdev.2022.203793
- Sekula P, Del Greco MF, Pattaro C, Köttgen A. Mendelian Randomization as an Approach to Assess Causality Using Observational Data. J Am Soc Nephrol. 2016;27(11):3253–3265. doi:10.1681/ASN.2016010098
- Elsworth B, Lyon M, Alexander T, et al. The MRC IEU OpenGWAS data infrastructure. bioRxiv. 2020.
- Orrù V, Steri M, Sidore C, et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat Genet. 2020;52(10):1036–1045. doi:10.1038/s41588-020-0684-4
- Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–525. doi:10.1093/ije/dyv080
- Yang S. The Weighted Kernel Estimators of Nonparametric Regression Function with Censored Data. Acta Mathematica Sinica. 1999.
- Jack B, George DS, Stephen B. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2).
- Auton A, Brooks LD, Durbin RM, et al. A global reference for human genetic variation. Nature. 2015;526:7571.
- Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997;315(7121):1533–1537. doi:10.1136/bmj.315.7121.1533
- Dessau RB, Pipper CB. “R”--project for statistical computing. Ugeskr Laeger. 2008;170(5):328–330.
- Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377–389. doi:10.1007/s10654-017-0255-x
- Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity Analyses for Robust Causal Inference from Mendelian Randomization Analyses with Multiple Genetic Variants. Epidemiology. 2017;28(1):30–42. doi:10.1097/EDE.0000000000000559
- Hemani G, Bowden J, Davey Smith G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet. 2018;27(R2):R195–R208. doi:10.1093/hmg/ddy163
- Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69. doi:10.1038/s41408-021-00459-7
- Okada M, Tashiro-Yamaji J, Takahashi T, et al. Regulation of hair regrowth in alopecic site of IFN-gamma-/- mice by macrophages infiltrating into allograft in IFN-gamma+/+ mice. J Interferon Cytokine Res. 2005;25(9):564–574. doi:10.1089/jir.2005.25.564
- Lambert C, Ibrahim M, Iobagiu C, Genin C. Significance of unconventional peripheral CD4+CD8dim T cell subsets. J Clin Immunol. 2005;25(5):418–427. doi:10.1007/s10875-005-5257-x
- Suzuki M, Yokota M, Matsumoto T, Ozaki S. Synergic Effects of CD40 and CD86 Silencing in Dendritic Cells on the Control of Allergic Diseases. Int Arch Allergy Immunol. 2018;177(2):87–96. doi:10.1159/000489862
- Lee WS, Lee HJ. Characteristics of androgenetic alopecia in asian. Ann Dermatol. 2012;24(3):243–252. doi:10.5021/ad.2012.24.3.243