Abstract
This case study outlines the management of a 24-year-old male with a history of juvenile nephronophthisis who underwent renal transplantation at age 12 and later required dialysis at 18 due to chronic rejection and hypertension. Subsequently, the patient developed severe Hidradenitis Suppurativa (HS) affecting the axillary, groin, and gluteal regions. Despite undergoing various systemic and intravenous antibiotic therapies, as well as Adalimumab treatment, the HS remained refractory. Adalimumab was discontinued due to a detected ejection fraction of 45% during cardiologic follow-up, likely due to COVID-19 related myocarditis. Following this, the patient was initiated on secukinumab therapy, initially undergoing an induction phase followed by maintenance dosing. Significant improvements were observed in quality of life, pain scores, and HS activity after 5 weeks of secukinumab therapy, with sustained benefits at the 6-month follow-up. Secukinumab was well tolerated, with no reported adverse events. This case underscores the effectiveness and safety of secukinumab as a therapeutic option for refractory HS, particularly in patients with comorbidities such as renal transplant recipients.
Introduction
Secukinumab, a monoclonal antibody of the IgG1 class, inhibits the activity of IL-17A and has demonstrated efficacy in treating plaque psoriasis and ankylosing spondylitis. As of October 31, 2023, secukinumab has also been approved for the treatment of moderate to severe forms of hidradenitis suppurativa.Citation1–3
Large molecules like immunoglobulins undergo minimal filtration by the kidneys. Similar biological agents are administered to patients with renal dysfunction without dose adjustment, suggesting that secukinumab can be administered at the standard dosage to such patients. However, there is currently insufficient real-world evidence regarding the safety and effectiveness of secukinumab in patients undergoing hemodialysis.
In the literature, there’s discussion about the affirmative impact of IL-17A inhibitors on kidney ailments. The Th17/IL-17 pathway fosters scarring, organ structure and function deterioration, resulting in podocyte harm. Hence, an IL-17A inhibitor can impede damage progression. HS is typified by persistent inflammation with potential ramifications on various bodily organs. Persistent buildup of inflammatory agents and cytokines may impede renal function, thus alleviating inflammation could prove beneficial for patients.
Case Report
Here we report the case of a 24-year-old boy suffering from juvenile nephronophthisis for which he performed renal transplantation at the age of 12 years; unfortunately, at age of 18 years he started dialysis treatment due to chronic rejection and concomitant hypertension.
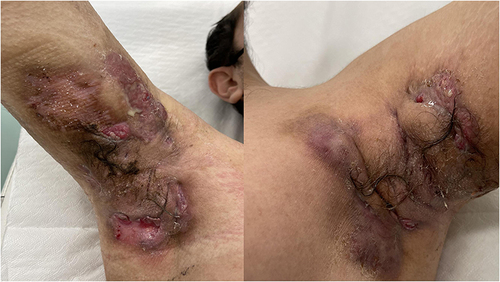
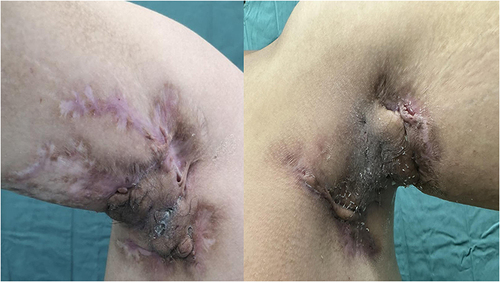
Three years later, he experienced the onset of severe HS localized to the axillary region bilaterally, groin and posterior gluteal region for which he practiced several systemic (rifampicin + clindamycin; doxycycline) and intravenous antibiotic therapies (ertapenem) with mild results. Then Adalimumab therapy for 8 months with fair results was performed; however, during the cardiologic follow-up visits that the patient regularly performed for hypertension, an ejection fraction of 45% (probably due to COVID-19 related myocarditis) was found. Hence, Adalimumab was discontinued. We decided to start secukinumab 300mg at Week 0, 1, 2, 3 and 4 (induction phase) and then 300 mg continued every 2 weeks. IHS4 at baseline was 51, DLQI 29 and severe pain (NRS, 10) ()
After 5 weeks, there was an improvement in quality of life (DLQI from 29 to 8) and a reduction in pain (NRS from 10 to 3). There was also a significant reduction in HS activity (IHS4 from 51 to 19).
Results have been confirmed and improved after 6 months of therapy (DLQI 4, NRS 1 and IHS4 15) (). Secukinumab was well tolerated by the patient and no adverse events occurred.
Discussion
Treating Hidradenitis Suppurativa (HS) has long posed a significant challenge for dermatologists. Up until recently, the first FDA-approved biologic drug for HS was adalimumab, an anti-tumor necrosis factor (TNF)-α medication, which gained approval in 2015. Real-world studies have indicated an average clinical success rate of approximately 60%. However, instances of primary or secondary lack of efficacy have been documented. Secukinumab, an anti-interleukin 17A drug initially approved for moderate to severe psoriasis, psoriatic arthritis, and ankylosing spondylitis, has shown promising results in clinical trials by improving signs and symptoms of HS, while maintaining a favorable safety profile and sustained response for up to 52 weeks. Initial real-world data are in line with these studies. Recently, the European Commission (EC) approved secukinumab for use in adults with active moderate to severe HS who have not responded adequately to conventional systemic HS therapy, while FDA approval was granted more recently on October 31, 2023.Citation4
To our knowledge, this is the first report evaluating the efficacy of secukinumab in a young patient with severe HS during haemodialysis, confirm the excellent efficacy and safety data reported by Rached at al. In addition, we reported a longer follow-up (6 months vs 3 months), and efficacy and safety even with secukinumab 300mg every 2 weeks respect to every 4 weeks.
Recently Rached et alCitation5 reported the case of a 57-year-old female on haemodialysis for end-stage renal disease treated with secukinumab for severe hidradenitis suppurativa (HS). The patient had severe, active HS (IHS4 score 63, mHSS score 120, Hurley III and SAHS score 13), which failed systemic antibiotics and adalimumab. Of note, the authors did not report details of systemic antibiotic treatment nor the duration of Adalimumab. After 4 weeks of secukinumab, there was an improvement in quality of life (DLQI from 29 to 4) and a reduction in pain (NRS from 10 to 1). There was also a significant reduction in HS activity (IHS4 from 63 to 28). Results have been confirmed after 3 months, but no values are reported by the authors. Literature supports the positive impact of IL-17A inhibitors on kidney disease.
To date, only limited reports and small-scale retrospective studies have been conducted to assess the efficacy and safety of secukinumab in patients suffering from both chronic kidney disease and psoriasis.Citation6–8
Consequently, inhibition of IL-17A holds promise for halting disease progression. Inflammatory mediators and cytokines may also affect renal function, suggesting that reduction of inflammation may offer benefits to patients.Citation6,Citation9,Citation10
Conclusion
Certainly, with the introduction and approval of secukinumab by the European Medical Agency (EMA) has allowed us to expand the available therapies for HS that are not always able to meet the needs of patients.Citation4,Citation6,Citation10–19 Apart from evidence deriving from case reports and series on psoriasis, literature should expand on the efficacy and safety of secukinumab also in HS patients, especially in complicated ones such as those on hemodialysis.Citation6,Citation9,Citation10 Initial data are reassuring, as highlighted by our case with the dosage of 300 mg every 2 weeks, but further studies are required.
Ethical Approval
Institutional approval is not required for a case report.
Informed Consent
Written informed consent for publication of their details was obtained from the patient, including images. The patient's earlier kidney transplant was donated voluntarily with written informed consent, and this was conducted in accordance with the Declaration of Istanbul.
Institutional approval is not required.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author.
Additional information
Funding
References
- Kimball AB, Jemec GBE, Alavi A, et al. Secukinumab in moderate-to-severe hidradenitis suppurativa (SUNSHINE and SUNRISE): week 16 and week 52 results of two identical, multicentre, randomised, placebo-controlled, double-blind Phase 3 trials [published correction appears in Lancet. Lancet. 2023;401(10378):747–761. doi:10.1016/S0140-6736(23)00022-3
- Martora F, Megna M, Battista T, et al. Adalimumab, Ustekinumab, and Secukinumab in the management of hidradenitis suppurativa: A review of the real-life experience. Clin Cosmet Invest Dermatol. 2023;16:135–148. doi:10.2147/CCID.S391356
- Ring HC, Maul JT, Yao Y, et al. Drug survival of biologics in patients with Hidradenitis Suppurativa. JAMA Dermatol. 2022;158(2):184–188. doi:10.1001/jamadermatol.2021.4805
- Martora F, Marasca C, Cacciapuoti S, et al. Secukinumab in Hidradenitis Suppurativa patients who failed Adalimumab: A 52-week real-life study. Clin Cosmet Invest Dermatol. 2024;17:159–166. doi:10.2147/CCID.S449367
- Abu Rached N, Ocker L, Stockfleth E, Bechara FG. Secukinumab is effective in a patient on haemodialysis for end-stage renal disease and severe hidradenitis suppurativa. J Eur Acad Dermatol Ven. doi:10.1111/jdv.19868
- Mukai M, Kurihara Y, Ito Y, et al. Successful treatment with secukinumab of three psoriatic patients un- dergoing dialysis. J Dermatol. 2020;47:e26–e28. doi:10.1111/1346-8138.15132
- Ikuma D, Oguro M, Hoshino J, et al. Efficacy of Secukinumab for Plaque Psoriasis in a Patient on Hemodialysis. CEN Case Rep. 2020;9(1):55–58. doi:10.1007/s13730-019-00426-z
- Pizzatti L, Mugheddu C, Sanna S, Atzori L, Rongioletti F. Erythrodermic psoriasis in a dialyzed patient successfully treated with Secukinumab. Dermatol Ther. 2020;33(3):e13348. doi:10.1111/dth.13348
- Paquissi FC, Abensur H. The Th17/IL-17 Axis and kidney diseases, with focus on lupus nephritis. Front Med Lausanne. 2021;8:654912. doi:10.3389/fmed.2021.654912
- Panickar KS, Jewell DE. The benefit of anti-inflammatory and renal- protective dietary ingredients on the biological processes of aging in the kidney. Biology. 2018;7:45. doi:10.3390/biology7040045
- Martora F, Scalvenzi M, Ruggiero A, Potestio L, Battista T, Megna M. Hidradenitis Suppurativa and JAK Inhibitors: A Review of the published literature. Medicina. 2023;59(4):801. doi:10.3390/medicina59040801
- Martora F, Martora L, Fabbrocini G, Marasca C. A case of pemphigus vulgaris and hidradenitis suppurativa: May systemic steroids be considered in the standard management of Hidradenitis Suppurativa? Skin Append Disord. 2022;8(3):265–268. doi:10.1159/000521712
- Maghfour J, Elliott E, Gill F, Stumpf B, Murina A. Effect of biologic drugs on renal function in psoriasis patients with chronic kidney dis- ease. J Am Acad Dermatol. 2020;82:1249–1251. doi:10.1016/j.jaad.2019.12.043
- Martora F, Scalvenzi M, Battista T, et al. Guselkumab, Risankizumab, and Tildrakizumab in the management of hidradenitis suppurativa: A review of existing trials and real-life data. Clin Cosmet Invest Dermatol. 2023;16:2525–2536. doi:10.2147/CCID.S418748
- Zouboulis CC, Frew JW, Giamarellos-Bourboulis EJ, et al. Target molecules for future hidradenitis suppurativa treatment. Exp Dermatol. 2021;30(Suppl):8–17. doi:10.1111/exd.14338
- Aarts P, Dudink K, Vossen ARJV, et al. Clinical implementation of biologics and small molecules in the treatment of Hidradenitis Suppurativa. Drugs. 2021;81(12):1397–1410. doi:10.1007/s40265-021-01566-2
- Čagalj A M, Marinović B, Bukvić Mokos Z. New and emerging targeted therapies for Hidradenitis Suppurativa. Int J Mol Sci. 2022;23(7):3753. doi:10.3390/ijms23073753
- Martora F, Marasca C, Battista T, Fabbrocini G, Ruggiero A. Management of patients with hidradenitis suppurativa during COVID-19 vaccination: an experience from southern Italy. Comment on: ‘Evaluating the safety and efficacy of COVID-19 vaccination in patients with hidradenitis suppurativa’. Clin Exp Dermatol. 2022;47(11):2026–2028. doi:10.1111/ced.15306
- Martora F, Picone V, Fabbrocini G, Marasca C. Hidradenitis suppurativa flares following COVID-19 vaccination: a case series. JAAD case reports. 2022;23:42–45. doi:10.1016/j.jdcr.2022.03.008