Abstract
Vitiligo has been reported to occur in association with lupus erythematosus (LE) and other autoimmune diseases. However, it remains unclear whether this association occurs because of shared immunopathogenesis. We hereby describe a case of discoid lupus erythematosus (DLE) in a 51-year-old man with a 3 years history of skin lesions on his face, arms, and the V zone of the neck, and with the coexistence of vitiligo for 12 years, who developed from DLE to hypertrophic discoid lupus erythematosus (HDLE) after 10 months. We reviewed the previously reported cases to summarize the clinical characteristics of these patients and hope it may provide a reference for dermatologists.
Introduction
Cutaneous lupus erythematosus (CLE) is an autoimmune skin disease that has a broad range of dermatologic manifestations. Discoid lupus erythematosus (DLE) is a variant of chronic CLE that is characterized by erythematous patches and plaques with adherent scale, HDLE is a very rare subgroup of chronic CLE, estimated to represent 2% of all chronic cutaneous lesions of lupus erythematosus (LE).Citation1 The coexistence of discoid lupus erythematosus and vitiligo has been infrequently reported. However, cases of vitiligo coexisting with HDLE have been much rarer. Herein, we present a case of a male patient with vitiligo who developed from DLE to disseminated HDLE. We also performed a systematic review of similar literature and compiled nine cases to discuss the relationship between cutaneous lupus erythematosus and vitiligo. We searched PubMed for original articles on LE, published between 1984 and 2023. On PubMed, we performed the following literature search: (“discoid lupus erythematosus” OR “DLE” OR “cutaneous lupus erythematosus” OR “CLE”) AND (“vitiligo”) to identify and assess all cases of CLE with vitiligo had been reported in English language publications. In total, 9 cases were documented and analyzed in this present study.
Case Presentation
A 51-year-old man with no family history of autoimmune disease was consulted by our department in 2022 for the appearance of erythematous papules on the face, arms, and the V zone of the neck, with pruritus for 3 years (). He had a medical history of vitiligo on his face, mouth mucosa, arms, and trunk for 12 years. The patient had photosensitivity and denied joint pain or swelling, oral ulcers, or nasal ulcers, and fever. The pathological biopsy was consistent with discoid lupus erythematosus (DLE). Immunoserological tests for anti-nuclear antibodies (ANA), anti-dsDNA antibodies, anti-SM antibodies, anti-SSA/Ro antibodies, anti-SSB /La antibodies, and anti-cardiolipin antibodies were all negative. Oral hydroxychloroquine sulfate tablets 200 mg twice a day, topical corticosteroid therapy with compound flumethasone ointment, and sun protection measures were prescribed, and the patient’s lesions improved. After that, the patient was lost to follow-up and stopped the drugs by himself. However, 10 months ago, the patient suffered from sun exposure, and lesions on his face, fore chest, both forearms and hands reoccurred and mainly localized to areas of pre-existing vitiligo patches, meanwhile, the surface hypertrophy was obvious (). The patient lacked systemic complaints. Subsequently, he visited our department again.
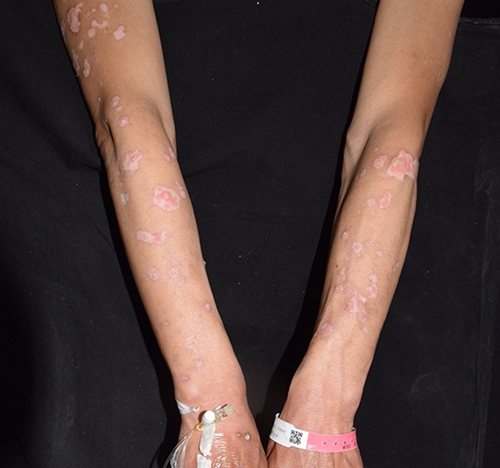
Figure 1 The initial lesions on the right arm, showing well-demarcated erythematous plaques and white macules at the first visit in 2022.
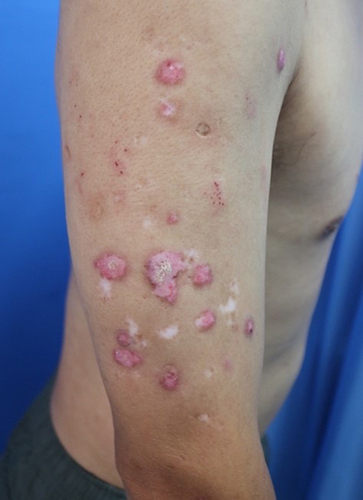
Figure 2 The arms showed generalized with varying sizes and irregularly shaped red maculopapules, plaques, warty hyperplasia, and local surface covered with white adhesive scales, partially accompanied by depigmentation at this visit.
Dermatologic examination revealed hypertrophic thickening erythema with clear boundaries on the face, arms, and the V zone of the neck and covered with a thick white crust. The scattered depigmentation spots on his face, mouth mucosa, arms, and trunk, part of the patches are distributed around the erythema.
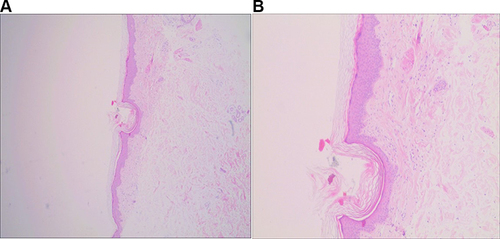
Imageological examination, thyroid function, and autoimmunity tests showed no abnormality. Biopsy specimens were taken from the right upper arm, right forearms, and right hand. The histopathologic findings were similar and demonstrated hyperkeratosis of the epidermis, hair follicle horn supposition, irregular hyperplasia of the spinous layer, spongy edema, intact basal cells, and no obvious liquefied degeneration. There is a focal infiltration of predominantly lymphocytes around the dermal vessels and skin appendages ().
Figure 3 Histologic section showing dermal vessels and periadnexal lymphocytic focal infiltrate (A. H&E, ×40; B. H&E, ×100).
Our patient was diagnosed with disseminated hypertrophic discoid lupus erythematosus (HDLE) and vitiligo, according to the clinical manifestations and pathological biopsy. Then he was advised strict photoprotection and started on oral hydroxychloroquine 200 mg twice a day dosage and topical halometasone cream twice daily. In the subsequent week, he reported a reduction in pruritus, erythema, and scale. He is still being followed up.
Discussion
HDLE is characterized by prominent warty hyperplasia, papules, and nodules those are typically present on sun-exposed skin. In a study of 14 cases of HDLE, all hypertrophic lesions occurred on sun-exposed sites and all patients demonstrated classic discoid lesions as well.Citation2 Valandro’s study showed that in HDLE, the immunoexpression of p53 was significantly higher than in DLE. P53 protein suppresses genomic instability that triggers apoptosis. In the skin, p53 expression is increased after DNA damage caused by UV irradiation. In turn, prolonged or repeated exposure to UV-B leads to p53 mutations.Citation3 The histopathology of HDLE often includes spinous layer hypertrophy, spongy edema, poor keratosis, basal layer destruction and liquefaction degeneration, and lymphocyte infiltration around perivascular and peri adnexal.
HDLE poses a challenge in diagnosis, as it can resemble hypertrophic lichen planus or hypertrophic actinic keratosis clinically and histopathologically. Hypertrophic lichen planus demonstrates hyperkeratosis with parakeratosis, hypergranulosis, pseudoepitheliomatous hyperplasia, cytoid bodies, and sawtooth rete ridges. Hypertrophic actinic keratosis demonstrates hyperkeratosis, hypergranulosis, acanthosis, and dysplasia of the basal layer and deepest cells of the stratum spinosum. However, the presence of photosensitivity, the absence of clinically lichenoid lesions, and peri adnexal infiltrates supported the diagnosis of DLE and ruled-out lichen planus and actinic keratosis.
As we mentioned that HDLE or DLE is a subgroup of chronic CLE, so we summarized the clinical characteristics of patients with CLE with vitiligo ().
Table 1 Case Reports of Discoid Lupus Erythematosus with Vitiligo and Other Diseases
Demographics
There were three males and five females, and one patient whose gender was not mentioned of documented patients. The ratio of male to female was 4:5. The mean age at diagnosis was 49.2 (range 36–68) years old except for one patient whose age was unknown. The median duration of symptoms prior to diagnosis with LE was 48 (range 0.25–96) months. Our document showed that 5/10 (50.0%) of patients had vitiligo lesions followed by lupus erythematosus lesions, while the rest showed the opposite. Almost all patients have no family history of lupus erythematosus or vitiligo.
Inducing Factor
No obvious factors were mentioned in the majority of the cases except for some patients who complained of sun exposure (3/10 cases, 30.0%), PUVA-radiation (1/10 cases, 10.0%), and hydroxychloroquine treatment (1/10 case, 10.0%). Lupus erythematosus is considered to be related to genetics, viral infections, drugs, and environmental and hormonal factors. Common internal and external triggers of vitiligo include genetic predisposition, viral infections, oxidative stress, and drugs.Citation13 Our document indicated two factors, ultraviolet light and drug.
Localization and Manifestations
Our data have shown that almost all of the cases (9/10, 90.0%) of LE involve face and neck. There were 8/10 (80.0%) cases in which the arms or hands were affected. Five of 10 (50.0%) cases in which the trunk was affected, including shoulder, back, or chest. Besides, 1/10 (10.0%) cases involved feet. Thus, it can be seen that the involvement of LE lesions concentrated on the sun-exposed area.
2/10 (20.0%) vitiliginous lesions had appeared in places other than the area of LE lesions and skin lesions typical of LE outside the vitiliginous areas in 1/10 (10.0%) cases. In the other 7/10 (70.0%) cases, either the LE lesions localized only to preexisting depigmented patches or in the opposite. We already know that DLE can develop on the normal skin of individuals with vitiligo or on the vitiliginous skin.
Complications
In all, 1/10 (10.0%) patients who had vitiligo coexisting with CLE have a history of 2 comorbid diseases; 6/10 (60.0%) patients had only 1 comorbid disease, including 3/10 (30.0%) cases involving thyroid disease, 3/10 (30%) patients had no comorbid disease recorded. The patient who was afflicted with 2 comorbid diseases had malignant melanoma and urticaria; the remaining patient with 1 comorbid disease had dermatophyte infection, diabetes mellitus, or achromotrichia. So, cooperation between dermatologists and physicians is important to treat and prevent further damage.
Laboratory Examinations
It has previously been reported that approximately 20% of CLE patients have a positive ANA titer. Antibody analysis was detected in 8/10 (80.0%) cases, and 3/8 (37.5%) cases were ANA positive, (SSA/SSB)-antibodies of 3/8 (37.5%) cases were detected positive. We did not find positive anti-dsDNA antibodies in all cases. ESR and serum complement levels can be abnormal in a few patients. The patients complicated with thyroid disease had abnormal thyroid function.
Treatment
All patients with a diagnosis of CLE should take photoprotection. 2/10 (20.0%) patients’ treatment is unknown. Systemic hydroxychloroquine and topical corticosteroids were used for the treatment of 8/10 (80.0%) cases.
HDLE is a chronic disease course, the lesions are difficult to subside, and there is no clear treatment method. Treatments reported to be helpful for HDLE include intralesional triamcinolone acetonide, oral hydroxychloroquine, acitretin, thalidomide, and isotretinoin.
Vitiligo is an acquired chronic depigmented skin disorder characterized by white macules’ development, features selective loss of functional epidermal melanocytes, and patchy decolorization of the skin and mucous membranes. Vitiligo is also characterized by the destruction of T-cell-driven melanocytes. Although the etiology and pathogenesis are unclear, most think it is an autoimmune disease, the immune cells with are important in the pathogenesis, are widespread resistance to melanin cells in serum antibodies. The autoimmune theory is the predominant hypothesis for non-segmental vitiligo development,Citation14 it is suggested that melanocytes are destroyed by autoimmune mechanisms. The relationship between autoimmunity and vitiligo has been paid more and more attention, and 20 ~ 30% of vitiligo patients have been reported to be accompanied by various comorbidities autoimmune diseases, systemic diseases, and skin disorders,Citation15 including Hashimoto thyroiditis, alopecia areata,Citation16 Addison disease,Citation17 pernicious anemia,Citation18 inflammatory bowel disease, rheumatoid arthritis, psoriasis, systemic lupus erythematosus (SLE).Citation19
The coexistence of autoimmune diseases and vitiligo supports the autoimmune theory of vitiligo, and recent genome-wide association studies have shown that they share an underlying genetic susceptibility site.Citation20 According to Jin’s research, NALP1 polymorphisms increase a person’s risk of developing vitiligo-related autoimmune and autoinflammatory illnesses.Citation21 A genetic explanation for the association between lupus erythematosus and vitiligo has recently been attempted, there are also studies that have provided evidence for the association between vitiligo and DLE,Citation22 but reports of the coexistence of vitiligo complicated with disseminated HDLE are rare. Literatures reported that the majority of patients with concurrent CLE and vitiligo resided in regions with potential chronic sun exposure.Citation23 Our patient is also due to prolonged sun exposure resulting in aggravated skin lesions. We already know that ultraviolet B induced apoptotic and necrotic keratinocytes are putative targets for autoantibodies and mediators of inflammatory cascades for lupus erythematosus.Citation8 Thus, photosensitivity and chronic sun exposure may serve as triggering factors.
The main limitation of this study is the small number of cases. Despite multiple attempts, we were unable to obtain the original full text for some of the qualified articles.
Conclusion
To conclude, we report an exceptional vitiligo patient who developed from DLE to HDLE, which represented a diagnostic challenge. We speculate that this patient’s disease progression may be strongly related to sun exposure. However, HDLE combined with vitiligo is relatively rare, further mechanistic studies are needed to elucidate the factors associated with CLE and vitiligo.
Ethics and Consent Statements
Our institution does not require ethical approval for reporting individual cases or case series. We have obtained written informed consent from the patient to include his anonymized information, including images, in this publication.
Disclosure
Qing Zhu and Yijia He are co-first authors for this study. The authors have no conflicts of interest to declare in this work.
References
- Walsh NM, Lai J, Hanly JG, et al. Plasmacytoid dendritic cells in hypertrophic discoid lupus erythematosus: an objective evaluation of their diagnostic value. J Cutan Pathol. 2015;42(1):32–38. doi:10.1111/cup.12416
- Daldon PE, Macedo de Souza E, Cintra ML. Hypertrophic lupus erythematosus: a clinicopathological study of 14 cases. J Cutan Pathol. 2003;30(7):443–448. doi:10.1034/j.1600-0560.2003.00082.x
- Valandro LDS, Beleli M, Fogagnolo L, et al. P53 and Granzyme B may have a role in progression to malignancy in hypertrophic discoid lupus erythematosus. JAAD Int. 2022;8:111–113. doi:10.1016/j.jdin.2022.06.008
- Callen JP. Discoid lupus erythematosus in a patient with vitiligo and autoimmune thyroiditis. Int J Dermatol. 1984;23(3):203–204. doi:10.1111/j.1365-4362.1984.tb04512.x
- Khosravi AR, Mansouri P, Moazzeni M. Case report: chronic dermatophyte infection in a patient with vitiligo and discoid lupus erythematosus. Mycoses. 2000;43(7–8):317–319. doi:10.1046/j.1439-0507.2000.00573.x
- Kürkçüoğlu N, Sahin S. PUVA-induced discoid lupus erythematosus in a patient with vitiligo. J Am Acad Dermatol. 1991;24(3):515. doi:10.1016/s0190-9622(08)80093-x
- Gül U, Kiliç A, Tulunay O, Kaygusuz G. Vitiligo associated with malignant melanoma and lupus erythematosus. J Dermatol. 2007;34:142–145.
- Johnson H, Bossenbroek NM, Rosenman K, Meehan SA, Robles M, Pomeranz MK. Chronic cutaneous lupus erythematosus in vitiligo. Dermatol Online J. 2008;14:10.
- Monsálvez V, García-Cano I, Fuertes L, Llamas R, Vanaclocha F. Lupus eritematoso cutáneo y vitíligo [Cutaneous lupus erythematosus and vitiligo]. Actas Dermosifiliogr. 2010;101(4):375–377. Spanish.
- Sharma S, Sarkar R, Garg VK, Bansal S. Coexistence of lip-tip vitiligo and disseminated discoid lupus erythematosus with hypothyroidism: need for careful therapeutic approach. Indian Dermatol Online J. 2013;4(2):112–114. doi:10.4103/2229-5178.110636
- Mayou S, Munro D. Discoid lupus erythematosus in vitiligo. Br J Dermatol. 1991;125(38):67.
- Niebel D, Braegelmann C, Bieber T, Wenzel J. Vitiligo-like depigmentation subsequent to subacute cutaneous lupus erythematosus and hydroxychloroquine treatment. J Dtsch Dermatol Ges. 2020;18(12):1470–1473. doi:10.1111/ddg.14221
- Yaghoobi R, Omidian M, Bagherani N. Vitiligo: a review of the published work. J Dermatol. 2011;38(5):419–431. PMID: 21667529. doi:10.1111/j.1346-8138.2010.01139.x
- Malhotra N, Dytoc M. The pathogenesis of vitiligo. J Cutan Med Surg. 2013;17(3):153–172. doi:10.2310/7750.2012.12005
- Kroon MW, Vrijman C, Chandeck C, et al. High prevalence of autoimmune thyroiditis in children and adolescents with vitiligo. Horm Res Paediatr. 2013;79:137–144. doi:10.1159/000348388
- Huang KP, Mullangi S, Guo Y, Qureshi AA. Autoimmune, atopic, and mental health comorbid conditions associated with alopecia areata in the United States. JAMA Dermatol. 2013;149(7):789–794. Erratum in: JAMA Dermatol. 2014 Jun;150(6):674. doi:10.1001/jamadermatol.2013.3049
- Amerio P, Di Rollo D, Carbone A, et al. Polyglandular autoimmune diseases in a dermatological clinical setting: vitiligo-associated autoimmune diseases. Eur J Dermatol. 2010;20(3):354–358. doi:10.1684/ejd.2009.0939
- Laberge G, Mailloux CM, Gowan K, et al. Early disease onset and increased risk of other autoimmune diseases in familial generalized vitiligo. Pigment Cell Res. 2005;18(4):300–305. doi:10.1111/j.1600-0749.2005.00242.x
- Sheth VM, Guo Y, Qureshi AA. Comorbidities associated with vitiligo: a ten-year retrospective study. Dermatology. 2013;227(4):311–315. doi:10.1159/000354607
- Roberts GHL, Santorico SA, Spritz RA. The genetic architecture of vitiligo. Pigm Cell Melanoma Res. 2020;33(1):8–15. doi:10.1111/pcmr.12848
- Jin Y, Mailloux CM, Gowan K, et al. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med. 2007;356(12):1216–1225. doi:10.1056/NEJMoa061592
- Gill L, Zarbo A, Isedeh P, Jacobsen G, Lim HW, Hamzavi I. Comorbid autoimmune diseases in patients with vitiligo: a cross-sectional study. J Am Acad Dermatol. 2016;74(2):295–302. doi:10.1016/j.jaad.2015.08.063
- Park HS, Lee YS, Chun DK. Squamous cell carcinoma in vitiligo lesion after long-term PUVA therapy. J Eur Acad Dermatol Venereol. 2003;17(5):578–580. doi:10.1046/j.1468-3083.2003.00815.x
