Abstract
(Myo)fibroblasts are key players for maintaining skin homeostasis and for orchestrating physiological tissue repair. (Myo)fibroblasts are embedded in a sophisticated extracellular matrix (ECM) that they secrete, and a complex and interactive dialogue exists between (myo)fibroblasts and their microenvironment. In addition to the secretion of the ECM, (myo)fibroblasts, by secreting matrix metalloproteinases and tissue inhibitors of metalloproteinases, are able to remodel this ECM. (Myo)fibroblasts and their microenvironment form an evolving network during tissue repair, with reciprocal actions leading to cell differentiation, proliferation, quiescence, or apoptosis, and actions on growth factor bioavailability by binding, sequestration, and activation. In addition, the (myo)fibroblast phenotype is regulated by mechanical stresses to which they are subjected and thus by mechanical signaling. In pathological situations (excessive scarring or fibrosis), or during aging, this dialogue between the (myo)fibroblasts and their microenvironment may be altered or disrupted, leading to repair defects or to injuries with damaged and/or cosmetic skin alterations such as wrinkle development. The intimate dialogue between the (myo)fibroblasts and their microenvironment therefore represents a fascinating domain that must be better understood in order not only to characterize new therapeutic targets and drugs able to prevent or treat pathological developments but also to interfere with skin alterations observed during normal aging or premature aging induced by a deleterious environment.
Introduction to the myofibroblastic phenotype
Myofibroblasts were first described in healing skin wounds, where it was hypothesized that they were responsible for the phenomenon of wound contraction.Citation1 Since then, cells morphologically similar to myofibroblasts have been described in many tissues, predominantly in pathological states where their sustained presence is generally a marker of fibrosis and scarring.Citation2
Early studies identified myofibroblasts on the basis of their ultrastructural morphology, with prominent microfilament bundles in their cytoplasm distinguishing them from “normal” quiescent tissue fibroblasts. Myofibroblasts also possessed fibronexus junctions between cells and the surrounding extracellular matrix (ECM), thus in some ways appearing to share some of the morphological characteristics of smooth muscle (SM) cells.Citation3
Many tissues and pathologies have been described in which myofibroblasts have been identified, including hypertrophic and keloid scars in the skin, fibrotic liver as seen in liver cirrhosis and other liver pathologies, renal fibrosis, and idiopathic pulmonary fibrosis.Citation4 More recently, cells with phenotypic features of myofibroblasts have also been found in and around a number of epithelial tumors, where they have been termed cancer-associated fibroblasts or stromal myofibroblasts.Citation5–Citation7 The role of myofibroblasts in driving fibrotic diseases, and the recent finding that cancer-associated myofibroblasts likely influence tumor growth and correlate with poor clinical prognosis, has increased our interest in their cellular origins, their regulation, and their role in repair and disease.Citation8
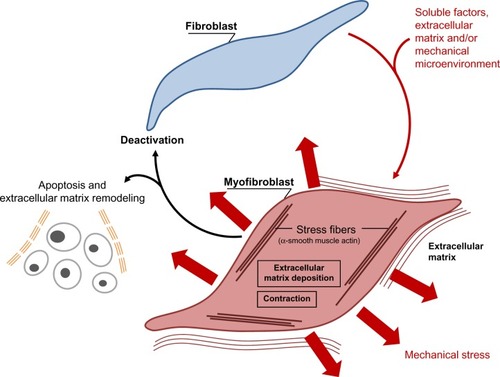
After early studies that defined myofibroblasts on the basis of their ultrastructural morphology, later research using antibodies and immunohistochemistry resulted in myofibroblasts and their microenvironment being more clearly defined.Citation9 It is now accepted that myofibroblasts go through a precursor stage of expressing large stress fibers that are not seen in quiescent fibroblasts, prominent bundles of microfilaments that permit some contraction and pre-stressing and remodeling of the surrounding ECM.Citation10 Later, fully differentiated myofibroblasts show expression of the usually SM-specific cytoskeletal protein, α-SM actin, which is now often used to define the myofibroblast phenotype.Citation9,Citation11
The presence of a splice variant form of fibronectin (ED-A fibronectin) in the microenvironment adjacent to the myofibroblast is also a defining feature and appears to be required for their differentiation.Citation12 De novo expression of osteoblast (OB) cadherin has also been reported to be found on the surface of differentiated myofibroblasts, and is not seen on α-SM actin-negative fibroblasts.Citation13
The other defining feature of myofibroblasts is that they fail to express the full repertoire of SM cell markers, allowing myofibroblasts to be distinguished from SM cells. Specifically, myofibroblasts in most cases are negative for SM cell markers such as SM myosin heavy chain,Citation14 n-caldesmon,Citation15 and smoothelin.Citation16 Desmin has also been used as a negative marker of myofibroblasts. Generally, SM cells express desmin and vimentin as well as SM myosin, while myofibroblasts express only vimentin. However, some situations have been reported in the literature where myofibroblasts in some pathologies have been found to be desmin positive.Citation17 Distinguishing myofibroblasts from pericytes is perhaps more problematic since pericytes can closely resemble myofibroblasts in being α-SM actin positive, vimentin and desmin positive, but SM myosin negative.Citation15,Citation18 Indeed, pericytes may in some cases be a source of myofibroblasts in some conditions, including wound repair, where myofibroblasts may represent a pericyte that has lost some phenotypic features such as desmin expression.Citation19 Similarly, SM cells from the media of an injured blood vessel may lose late differentiation markers such as desmin, smoothelin, and SM myosin and acquire a myofibroblast phenotype.Citation20
Lastly, myofibroblasts show both fibronexus junctions with other cells and specialized junctional complexes with the ECM; these large mature focal adhesions allow them to make strong attachments, contract and remodel the ECM, and provide a means of transducing mechanical force in the tissue.Citation21,Citation22 The contractile nature of myofibroblasts has some similarities to SM cells, despite the differences in expression of cytoskeletal features. For example, the calcium signaling in myofibroblasts appears to be similar to that in SM cells, and the arrangement of cells into something resembling that of SM cells in tissues with single-unit SM is also similar, that is, with cells having junctional complexes and connections (including gap junctions) that allow the spread of contraction signals through the tissue. Contraction of myofibroblasts seems to be possible through Ca2+-dependent mechanisms that are similar to those present in SM cells, with increased free Ca2+ regulating phosphorylation of myosin light chain. This may explain the rapid contractile responses of myofibroblasts in vitro to agonists such as angiotensin II or endothelin. Slower and more sustained contraction, which is perhaps more typical of what occurs during slow retractile contraction of connective tissue in granulation tissue, involves activity of the guanosine triphosphate (GTP)-ase RhoA and activation of its downstream target Rho-associated kinase (ROCK). This results in more continued phosphorylation of myosin light chain and thus sustained contraction.Citation23
Role in wound healing
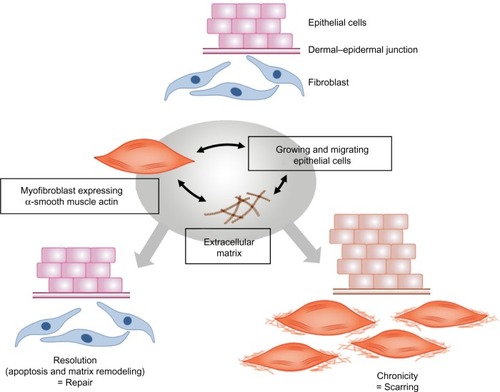
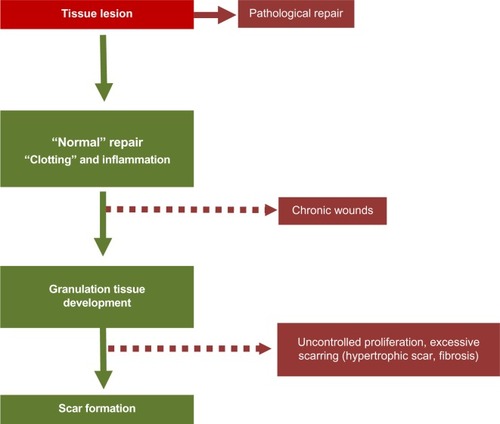
Immediately after wounding, the healing process commences, leading to (partial) restoration of injured tissue. Wound healing proceeds in three interrelated dynamic phases that temporally overlap (). Based on morphological changes over the course of the healing process, these phases are defined as the inflammatory phase, the proliferative phase (the development of granulation tissue), and the regeneration phase, including maturation, scar formation, and re-epithelialization.Citation24 The inflammatory phase begins with damage of capillaries, triggering the formation of a blood clot consisting of fibrin and fibronectin. This provisional matrix fills in the lesion and allows a variety of recruited cells to migrate into the lesion. Platelets present in the blood clot release multiple chemokines, which participate in the recruitment of inflammatory cells, neutrophils, and macrophages, but also in chemotaxis and recruitment of fibroblasts and endothelial cells. The second stage of wound healing is the proliferative phase. Angiogenesis, which is critical for the wound healing process, allows new capillaries to deliver nutrients to the wound, and contributes to the proliferation of fibroblasts. Initially the wound is hypoxic due to the loss of vascular perfusion, but with the development of a new capillary network, vascular perfusion is restored. Regulating wound angiogenesis in itself may represent a means for improving healing in some cases, particularly where delayed or defective angiogenesis is implicated in healing impairment.Citation25 In the granulation tissue, fibroblasts are activated and acquire α-SM actin expression and become myofibroblasts. These myofibroblastic cells synthesize and deposit the ECM components that eventually replace the provisional matrix (). These cells exhibit contractile properties, due to the expression of α-SM actin in microfilament bundles or stress fibers, playing a major role in the contraction and maturation of the granulation tissue.Citation26 Presently, it is accepted that the myofibroblastic modulation of fibroblastic cells begins with the appearance of the protomyofibroblast, whose stress fibers contain only β- and γ-cytoplasmic actins. Protomyofibroblasts generally evolve into differentiated myofibroblasts, the most common variant of this cell, with stress fibers containing α-SM actin (for review, see Tomasek et alCitation27). Myofibroblasts can, depending on the experimental or clinical situation, express other SM-related contractile proteins, such as SM myosin heavy chains or desmin; however, the presence of α-SM actin represents the most reliable marker of the myofibroblastic phenotype.Citation27 The third phase of healing, scar formation, involves a progressive remodeling of the granulation tissue. During this remodeling process, proteolytic enzymes, essentially matrix metalloproteinases (MMPs) and their inhibitors (tissue inhibitor of metalloproteinases [TIMPs]) play a major role.Citation28 The synthesis of ECM is not totally stopped, but considerably reduced, and the synthesized components are modified as the matrix is remodeled. Progressively, collagen type III, the major component of the granulation tissue, is replaced by collagen type I, which is the main structural component of the dermis. Lastly, elastin, which contributes to skin elasticity and is absent in the granulation tissue, also reappears. In the resolution phase of healing, the cell number is dramatically reduced by apoptosis of both vascular cells and myofibroblasts.Citation29 To date, it is not known whether myofibroblasts can reacquire a quiescent phenotype, that is, return to a normal dermal fibroblast phenotype with no expression of α-SM actin ().
Figure 1 The various phases of the healing process.
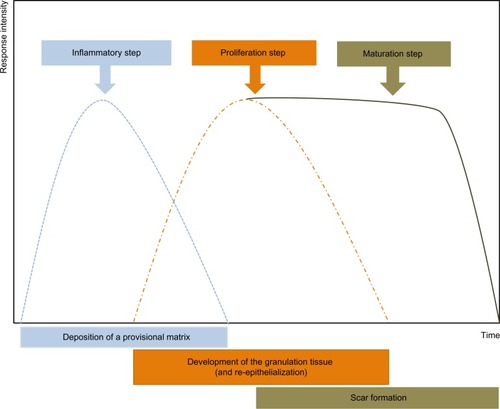
Figure 2 Schematic illustration showing the evolution of the (myo)fibroblast phenotype.
Origin of wound myofibroblasts
It is generally accepted that the major source of myofibroblasts are local connective tissue fibroblasts that are recruited into the wound.Citation30 Dermal fibroblasts located at the edges of the wound can acquire a myofibroblastic phenotype and participate in tissue repair.Citation31 However, important heterogeneity in fibroblastic cell subpopulations has also been observed. These subpopulations reside in different locations within the skin and have specific activation and deactivation properties. At least three subpopulations have been identified in the dermis: superficial (or papillary) fibroblasts (papillary dermis is around 300–400 μm deep and is arranged as a ridge-like structure), reticular fibroblasts, which reside in the deep dermis (made of thick collagen and elastin fibers arranged parallel to the surface of the skin), and fibroblasts associated with hair follicles. These cell subpopulations can be isolated and exhibit, depending of the nature and age of the original skin, distinct phenotypic differences when cultured separately.Citation32,Citation33
Recently, the involvement in tissue repair of local mesenchymal stem cells has been increasingly raised. These progenitor cells have been described in the dermal sheath that surrounds the outside of the hair follicle facing the epithelial stem cells. They are involved in the regeneration of the dermal papilla and can also became wound healing (myo)fibroblasts after a lesion or injury.Citation34,Citation35
Recent data have also implicated circulating cells, dubbed fibrocytes, in the tissue repair process. Fibrocytes enter injured skin together with inflammatory cells and may then acquire a myofibroblastic phenotype.Citation36 In postburn scars, fibrocytes are recruited to the site of the lesion where they stimulate the local inflammatory response and produce ECM proteins, thus contributing to hypertrophic scar formation.Citation37
Another type of circulating cell originating from bone marrow has also been suggested to play a role in tissue repair. Mesenchymal stem cells are bone marrow-derived non-hematopoietic precursor cellsCitation38 that contribute to the maintenance and regeneration of connective tissues through engraftment.Citation39 Indeed, they have the capacity to seed into several organs and to differentiate into wound-healing myofibroblasts. This engraftment in injured organs is regulated by the severity of the damage.Citation40
Finally, differentiated (or malignant) epithelial and endothelial cells can undergo a phenotypic conversion that gives rise to matrix-producing fibroblasts and myofibroblasts (through epithelial- and endothelial-to-mesenchymal transition processes).Citation41 This mechanism is increasingly recognized as an integral part of tissue fibrogenesis after injury, but seems to play a limited role during normal tissue repair.
Overall, mesenchymal stem cells, fibrocytes, bone marrow-derived cells, and cells derived from epithelial-and endothelial-to-mesenchymal transition processes may represent alternative sources of myofibroblasts when local fibroblasts are not sufficient to carry out tissue repair and remodeling.
Role of myofibroblasts in diseases (excessive scarring/fibrosis)
Myofibroblasts are implicated in many fibrotic and scarring diseases, where they carry out the important process after initial injury of providing mechanical support and integrity to the tissue. In normal physiological conditions, they are then lost via apoptosis, generally when the tissue integrity has been sufficiently restored to be mechanically coherent.Citation9,Citation29 Thus, in normal physiological situations like skin wound healing, myofibroblasts disappear in a prominent wave of apoptosis, leaving a markedly less cellular scar. However, it is now assumed that, in many fibrotic and scarring conditions, as well as in the stromal response to tumors, myofibroblasts fail to undergo cell death, persist, and thus in turn lead to ongoing pathology and scarring (). An example of reduced or inhibited apoptosis leading to scarring is in a model of hypertrophic scarring, where mechanical loading increases survival of myofibroblasts and was found to lead to greater scar formation.Citation42,Citation43 A better understanding of the control and signaling that governs apoptosis (or autophagy) in myofibroblasts may lead to more targeted approaches to combatting fibrosis and scarring. It has been demonstrated that elevated nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 4-derived hydrogen peroxide, supported by concomitant decreases in nitric oxide signaling and reactive oxygen species scavengers, are central factors in the molecular pathogenesis of fibrosis.Citation44 Redox signaling could therefore represent an interesting target to restore the physiological fibroblast–myofibroblast ratio. Apoptosis in myofibroblasts is thought to be regulated by a reduction in the local growth factors that drive and sustain myofibroblast differentiation. In particular, local concentrations of transforming growth factor (TGF)-β1 and endothelin-1 play a role in stimulating myofibroblast survival via protein kinase B (AKT) activation.Citation45 However, changes in mechanical signaling such as unloading of mechanical force likely also plays a role (discussed below).
Figure 3 Pathological situations.
The importance of myofibroblasts in causing fibrosis in internal organs and the skin (hypertrophic scars), and the role that persistence of stromal myofibroblasts appear to play in tumor growth and spread, makes the (down)regulation of myofibroblasts and the potential regulation of myofibroblast disappearance through apoptosis of increasing interest () (for review, see Hinz and GabbianiCitation46).
Regulation of myofibroblasts by mechanical forces
Mechanical signals have been shown to play a role in myofibroblast differentiation as the ECM that surrounds the fibroblasts in damaged tissue changes its composition and its stiffness as tissue repair proceeds.Citation47 The early ECM present in damaged tissue, or provisional matrix, is rich in fibrin and has been estimated to be very compliant. Fibroblasts cultured in compliant ECM such as soft three-dimensional (3D) collagen gels, show little development of stress fibers. These fibroblasts then form only small adhesions with the ECM.Citation48,Citation49 Fibroblasts grown in stiffer collagen matrices have been shown to form stress fibers and mature focal adhesions, though they are still negative for the myofibroblast marker α-SM actin. Lastly, the stiff matrix found either in 3D cultures using stiff (higher concentration) collagen matrix or in vivo in granulation tissue and fibrotic tissues is able to induce full myofibroblast differentiation in concert with growth factor stimulation from TGF-β1.Citation22 The contractile nature of myofibroblasts itself leads to an increase in stiffness and mechanical stress of the ECM as healing progresses, leading to a positive-feedback loop where increased stress signals myofibroblast differentiation and also increases myofibroblast survival.Citation42 For this reason, mechanical feedback is considered to be important in driving pathological conditions such as contractures post-injury. The role of mechanical stress in stimulating myofibroblast activity has also been shown in experiments where dermal wounds in mice are mechanically stressed by stretching or splinting the wound, where increased myofibroblast activity results in increased scar formation, to some extent mimicking hypertrophic scarring that is seen in humans.Citation42
In cancer biology, matrix stiffness can be used as a diagnostic indicator of the risk of malignancy and appears to correlate with increased invasiveness of tumors, for example in breast cancer where density of tissue on mammography correlates strongly with the risk of cancer formation. Recent studies have suggested this may be due to increased cell proliferation of epithelial and mesenchymal cells on stiffer matrices.
Conversely, releasing mechanical stress or reducing stiffness has been shown to induce both apoptosis and a reduction in α-SM actin expression and contractility in myofibroblasts.Citation50,Citation51
Mechanical signaling and stress modulate myofibroblast differentiation via a number of pathways and mechanisms. Stress may directly activate transcription of the α-SM actin gene, since application of force across integrin binding sites has been shown to up-regulate α-SM actin promoter activity.Citation52 As mentioned above, mechanical force alone is not generally sufficient to induce myofibroblast differentiation and other factors are needed to act in concert, specifically TGF-β1. Mechanical signaling and TGF-β1 stimulation also increase collagen gene expression by fibroblasts, emphasizing the role these factors play in stimulating a pro-fibrotic phenotype as is shown by activated myofibroblasts. TGF-β1 also favors deposition of ECM proteins over degradation by up-regulating TIMPs while decreasing expression of the MMPs themselves.Citation53
Stimulation of myofibroblasts by TGF-β1 itself is affected by mechanical forces within the damaged or fibrotic tissue. TGF-β1 released from a variety of inflammatory cells and platelets in the microenvironment of damaged or fibrotic tissue is in a latent form. Indeed, myofibroblasts themselves release latent TGF-β1 complexed with latency-associated peptide (LAP). Together with a binding protein, TGF-β1 is bound to ECM proteins, providing a reservoir of latent TGF-β1 that can be activated as healing and scar formation progress.Citation54,Citation55 Myofibroblasts express integrins that can bind to the LAP, and mechanical stress applied to the integrins, either by mechanical stress on the matrix and/or via myofibroblast contraction, can effectively activate TGF-β1 without cleaving the LAP and allow its binding to cell membrane receptors.Citation51 Thus, both increased mechanical stress and contraction can further increase myofibroblast contractile and matrix synthetic activity. This mode of activation provides another possible pathway for regulating myofibroblast activity by blocking integrin binding to latent TGF-β1, for example by blocking the integrin involved in latent TGF-β1 activation, αvβ5.Citation56 Inhibition of other integrin-binding sites has also been shown to inhibit myofibroblast development, including blocking of integrins α3β1,Citation57 α11β1,Citation58 αvβ5,Citation59 or β1.Citation60
Hypoxia
Tissue oxygenation or hypoxia may play a role in both normal healing and pathological situations. In normal wound healing, the wound is transiently hypoxic as vascular perfusion is disrupted by the initial injury. Staining for the hypoxia-induced transcription factor, hypoxia inducible factor (HIF)-1α shows both areas of the early granulation tissue and the overlying migrating keratinocytes to be hypoxic. During normal healing, this hypoxia is resolved within a few days of injury and expression of HIF-1α declines. Hypoxia signaling can induce a number of growth factors that are beneficial to the healing process, prominent amongst them being vascular endothelial growth factor (VEGF) and, thus, acute hypoxia likely plays a beneficial role in healing. However, the same may not be true for chronic hypoxia and chronic hypoxia signaling. Hypoxia has been reported to reduce myofibroblast activation and reduce collagen synthesis and α-SM actin expression.Citation61 In vivo studies using HIF-1-deficient mice showed that reducing HIF-1 availability during healing resulted in reduced collagen synthesis and delayed myofibroblast differentiation, suggesting that, overall, in vivo acute hypoxia during healing was normally compensated by induction of genes that allow tissue to adapt to transient hypoxia, such as VEGF.Citation62 In fact, fibroblasts that show reduced HIF-1α expression during hypoxia show inhibited migration and collagen synthesis in vitro. It is possible that, in some organs where there is pathological fibrosis and scarring, hypoxia and HIF signaling, possibly during more chronic hypoxic states, actually drives fibrosis, which has been suggested in the case of renal fibrosis. In some cells at least, there is cross talk between hypoxia signaling and TGF-β signaling that may exacerbate matrix synthesis and thus fibrosis.Citation63,Citation64
Therapies
Anti-fibrotic and anti-scarring therapies have proven to be a difficult and elusive area for research, with relatively few advances until quite recently. As the growth factor TGF-β is central to many of the mechanisms of pathological scarring and fibrosis, it has been the target of some therapeutic strategies. Some positive results have been reported with the drug pirfenidone, particularly in lung fibrosis, where the drug lowered TGF-β expression and both tissue and lavage fluid levels of the protein.Citation65 Interfering with activation of latent TGF-β is another potential target for anti-scarring therapies, and the role of integrin binding in TGF-β activation makes integrin-blocking antibodies a potential therapy for lowering TGF-β activation and thus downstream signaling.Citation66,Citation67 Specific inhibitors of TGF-β signaling have also been suggested as possible treatments for scarring and fibrosis. TGF-β exerts its pro-fibrotic effects through transcription factor signaling Smad3, and selective inhibition of Smad3 phosphorylation and inhibition of Smad3 interaction with Smad4 has been shown to reduce fibroblast activation to the myofibroblast phenotype and also reduce ECM synthetic activity of the cells.Citation68 Other molecular targets include tyrosine kinases, and the drug imatinib mesylate has been reported to be anti-fibrotic through inhibiting downstream molecules that are required for the TGF-β response while having an additional anti-fibrotic role by also inhibiting platelet-derived growth factor signaling.Citation69 Interestingly, HMG-coA reductase inhibitors such as statins have been shown to have anti-fibrotic effects, likely through inhibition of ROCK.Citation70 The widespread use of, and low rate of side effects associated with, these drugs may make them promising as anti-fibrotic therapies in the future.
Conclusion
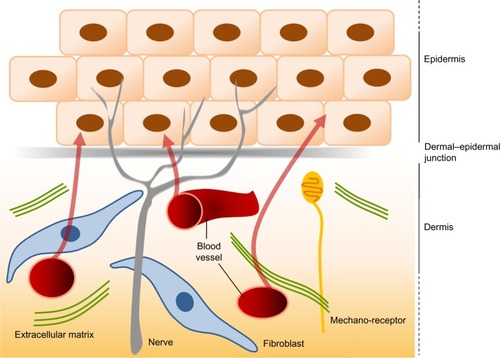
Since their first description in the early 1970s, our knowledge about myofibroblast biology has increased greatly, and our interest in the biology of myofibroblasts has also increased, as this cell has been implicated in many pathological situations in addition to their role in normal wound repair. Despite major advances in our understanding of the origins of myofibroblasts and the factors that regulate their differentiation and activity, it remains a challenge and a major goal of researchers to find ways in which to regulate their activity to improve healing and scarring in the clinic. It is interesting that the skin is a highly sensitive organ. It is densely innervated by different sensory nerve fiber subtypes that react to tissue injury, temperature variation, and tactile stimuli (). Cutaneous sensory nerve fibers are endings of dorsal root ganglia (or spinal ganglia) neurons that carry signals from sensory organs toward the appropriate integration center of the brain or of the spinal cord. Several clinical observations indicate that damage to the peripheral nervous system influences wound healing, sometimes resulting in chronic wounds within the affected area. Patients with cutaneous sensory defects due to spinal cord injury or diabetic neuropathy have an increased risk of developing ulcers that fail to heal. In addition, in aged patients, cutaneous repair processes are less efficient and this could be partly due to a deterioration of the peripheral nervous system at the skin level. Interestingly, factors that are required for sustaining peripheral nerves, the neurotrophin network, have also been shown to have direct effects on dermal fibroblasts in regulating ECM secretion, fibroblast differentiation, and tensile strength via effects on myofibroblasts.Citation71 Understanding the role of innervation during wound healing and myofibroblastic differentiation therefore represents an interesting domain. In addition, cutaneous innervation is certainly necessary to provide good hemostasis and to maintain the mechanical and cosmetic properties of the skin.Citation72 In conclusion, the recent advances made in understanding control of differentiation, proliferation, and survival of myofibroblasts will hopefully lead to new therapeutic approaches to limit scarring and improve healing in the not-too-distant future.Citation73
Figure 5 The interactions between the dermis and the epidermis.
Acknowledgments
This work was supported in part by the French Armaments Procurement Agency (DGA, No 2013 94 0903). Betty Laverdet was recipient of a fellowship from the French Armaments Procurement Agency. The authors thank Régine Baudet (LVMH Recherche) for the production of the figures.
Disclosure
The authors report no conflicts of interest directly relevant to the content of this paper.
References
- GabbianiGRyanGBMajnoGPresence of modified fibroblasts in granulation tissue and their possible role in wound contractionExperientia19712755495505132594
- DesmoulièreADarbyIAGabbianiGNormal and pathologic soft tissue remodeling: role of the myofibroblast, with special emphasis on liver and kidney fibrosisLab Invest200383121689170714691287
- EydenBThe myofibroblast: a study of normal, reactive and neoplastic tissues, with an emphasis on ultrastructure. part 2 – tumours and tumour-like lesionsJ Submicrosc Cytol Pathol2005373–423129616612972
- DarbyIAHewitsonTDFibroblast differentiation in wound healing and fibrosisInt Rev Cytol200725714317917280897
- DesmoulièreAGuyotCGabbianiGThe stroma reaction myofibroblast: a key player in the control of tumor cell behaviorInt J Dev Biol2004485–650951715349825
- KalluriRZeisbergMFibroblasts in cancerNat Rev Cancer20066539240116572188
- De WeverODemetterPMareelMBrackeMStromal myofibroblasts are drivers of invasive cancer growthInt J Cancer2008123102229223818777559
- TsujinoTSeshimoIYamamotoHStromal myofibroblasts predict disease recurrence for colorectal cancerClin Cancer Res20071372082209017404090
- DarbyISkalliOGabbianiGAlpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healingLab Invest199063121292197503
- HinzBFormation and function of the myofibroblast during tissue repairJ Invest Dermatol2007127352653717299435
- SandboNDulinNActin cytoskeleton in myofibroblast differentiation: ultrastructure defining form and driving functionTransl Res2011158418119621925115
- SeriniGBochaton-PiallatMLRoprazPThe fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1J Cell Biol199814238738819700173
- HinzBPittetPSmith-ClercJChaponnierCMeisterJJMyofibroblast development is characterized by specific cell-cell adherens junctionsMol Biol Cell20041594310432015240821
- BenzonanaGSkalliOGabbianiGCorrelation between the distribution of smooth muscle or non muscle myosins and alpha-smooth muscle actin in normal and pathological soft tissuesCell Motil Cytoskeleton19881142602743064925
- EydenBThe myofibroblast: a study of normal, reactive and neoplastic tissues, with an emphasis on ultrastructureJ Submicrosc Cytol Pathol2007716618277533
- van der LoopFTSchaartGTimmerEDRamaekersFCvan EysGJSmoothelin, a novel cytoskeletal protein specific for smooth muscle cellsJ Cell Biol199613424014118707825
- HinzBMastrangeloDIselinCEChaponnierCGabbianiGMechanical tension controls granulation tissue contractile activity and myofibroblast differentiationAm J Pathol200115931009102011549593
- ArmulikAAbramssonABetsholtzCEndothelial/pericyte interactionsCirc Res200597651252316166562
- RajkumarVSShiwenXBostromMPlatelet-derived growth factor-beta receptor activation is essential for fibroblast and pericyte recruitment during cutaneous wound healingAm J Pathol200616962254226517148686
- HaoHGabbianiGCamenzindEBacchettaMVirmaniRBochaton-PiallatMLPhenotypic modulation of intima and media smooth muscle cells in fatal cases of coronary artery lesionArterioscler Thromb Vasc Biol200626232633216339500
- DuginaVFontaoLChaponnierCVasilievJGabbianiGFocal adhesion features during myofibroblastic differentiation are controlled by intracellular and extracellular factorsJ Cell Sci2001114Pt 183285329611591817
- GoffinJMPittetPCsucsGLussiJWMeisterJJHinzBFocal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibersJ Cell Biol2006172225926816401722
- Follonier CastellaLGabbianiGMcCullochCAHinzBRegulation of myofibroblast activities: calcium pulls some strings behind the sceneExp Cell Res2010316152390240120451515
- SingerAJClarkRACutaneous wound healingN Engl J Med19993411073874610471461
- WietechaMSDipietroLATherapeutic approaches to the regulation of wound angiogenesisAdv Wound Care (New Rochelle)201323818624527330
- HinzBGabbianiGCell-matrix and cell-cell contacts of myofibroblasts: role in connective tissue remodelingThromb Haemost2003906993100214652629
- TomasekJJGabbianiGHinzBChaponnierCBrownRAMyofibroblasts and mechano-regulation of connective tissue remodellingNat Rev Mol Cell Biol20023534936311988769
- VisseRNagaseHMatrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistryCirc Res200392882783912730128
- DesmoulièreARedardMDarbyIGabbianiGApoptosis mediates the decrease in cellularity during the transition between granulation tissue and scarAm J Pathol1995146156667856739
- HigashiyamaRNakaoSShibusawaYDifferential contribution of dermal resident and bone marrow-derived cells to collagen production during wound healing and fibrogenesis in miceJ Invest Dermatol2011131252953620962852
- DriskellRRLichtenbergerBMHosteEDistinct fibroblast lineages determine dermal architecture in skin development and repairNature2013504747927728124336287
- SorrellJMCaplanAIFibroblast heterogeneity: more than skin deepJ Cell Sci2004117Pt 566767514754903
- MineSFortunelNOPageonHAsselineauDAging alters functionally human dermal papillary fibroblasts but not reticular fibroblasts: a new view of skin morphogenesis and agingPLoS One2008312e406619115004
- JahodaCAReynoldsAJHair follicle dermal sheath cells: unsung participants in wound healingLancet200135892911445144811705511
- JahodaCAWhitehouseJReynoldsAJHoleNHair follicle dermal cells differentiate into adipogenic and osteogenic lineagesExp Dermatol200312684985914714566
- AbeRDonnellySCPengTBucalaRMetzCNPeripheral blood fibrocytes: differentiation pathway and migration to wound sitesJ Immunol2001166127556756211390511
- YangLScottPGDoddCIdentification of fibrocytes in postburn hypertrophic scarWound Repair Regen200513439840416008729
- PittengerMFMackayAMBeckSCMultilineage potential of adult human mesenchymal stem cellsScience1999284541114314710102814
- OpalenikSRDavidsonJMFibroblast differentiation of bone marrow-derived cells during wound repairFASEB J200519111561156316014399
- DirekzeNCForbesSJBrittanMMultiple organ engraftment by bone-marrow-derived myofibroblasts and fibroblasts in bone-marrow-transplanted miceStem Cells200321551452012968105
- NakamuraMTokuraYEpithelial-mesenchymal transition in the skinJ Dermatol Sci201161171321167690
- AarabiSBhattKAShiYMechanical load initiates hypertrophic scar formation through decreased cellular apoptosisFASEB J200721123250326117504973
- van der VeerWMBloemenMCUlrichMMPotential cellular and molecular causes of hypertrophic scar formationBurns2009351152918952381
- SampsonNBergerPZenzmaierCRedox signaling as a therapeutic target to inhibit myofibroblast activation in degenerative fibrotic diseaseBiomed Res Int2014201413173724701562
- KulasekaranPScavoneCARogersDSArenbergDAThannickalVJHorowitzJCEndothelin-1 and transforming growth factor-beta1 independently induce fibroblast resistance to apoptosis via AKT activationAm J Respir Cell Mol Biol200941448449319188658
- HinzBGabbianiGFibrosis: recent advances in myofibroblast biology and new therapeutic perspectivesF1000 Biol Rep201027821170369
- HinzBThe myofibroblast: paradigm for a mechanically active cellJ Biomech201043114615519800625
- TamarizEGrinnellFModulation of fibroblast morphology and adhesion during collagen matrix remodelingMol Biol Cell200213113915392912429835
- YeungTGeorgesPCFlanaganLAEffects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesionCell Motil Cytoskeleton2005601243415573414
- GrinnellFHoCHLinYCSkutaGDifferences in the regulation of fibroblast contraction of floating versus stressed collagen matricesJ Biol Chem199927429189239873032
- WipffPJRifkinDBMeisterJJHinzBMyofibroblast contraction activates latent TGF-beta1 from the extracellular matrixJ Cell Biol200717961311132318086923
- WangJChenHSethAMcCullochCAMechanical force regulation of myofibroblast differentiation in cardiac fibroblastsAm J Physiol Heart Circ Physiol20032855H1871H188112842814
- LeaskAAbrahamDJTGF-beta signaling and the fibrotic responseFASEB J200418781682715117886
- AnnesJPChenYMungerJSRifkinDBIntegrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1J Cell Biol2004165572373415184403
- BuscemiLRamonetDKlingbergFThe single-molecule mechanics of the latent TGF-β1 complexCurr Biol201121242046205422169532
- ZhouYHagoodJSLuBMerrymanWDMurphy-UllrichJEThy-1-integrin alphav beta5 interactions inhibit lung fibroblast contraction-induced latent transforming growth factor-beta1 activation and myofibroblast differentiationJ Biol Chem201028529223822239320463011
- KimKKWeiYSzekeresCEpithelial cell alpha3beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosisJ Clin Invest2009119121322419104148
- CarracedoSLuNPopovaSNJonssonREckesBGullbergDThe fibroblast integrin alpha11beta1 is induced in a mechanosensitive manner involving activin A and regulates myofibroblast differentiationJ Biol Chem201028514104341044520129924
- AsanoYIhnHYamaneKJinninMTamakiKIncreased expression of integrin alphavbeta5 induces the myofibroblastic differentiation of dermal fibroblastsAm J Pathol2006168249951016436664
- ChanMWChaudaryFLeeWCopelandJWMcCullochCAForce-induced myofibroblast differentiation through collagen receptors is dependent on mammalian diaphanous (mDia)J Biol Chem2010285129273928120071339
- ModarressiAPietramaggioriGGodboutCVigatoEPittetBHinzBHypoxia impairs skin myofibroblast differentiation and functionJ Invest Dermatol2010130122818282720686497
- MusyokaJNLiuMCPouniotisDSSiah2-deficient mice show impaired skin wound repairWound Repair Regen201321343744723627548
- HigginsDFKimuraKBernhardtWMHypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transitionJ Clin Invest2007117123810382018037992
- BasuRKHubchakSHayashidaTRunyanCESchumackerPTSchnaperHWInterdependence of HIF-1α and TGF-β/Smad3 signaling in normoxic and hypoxic renal epithelial cell collagen expressionAm J Physiol Renal Physiol20113004F898F90521209004
- IyerSNGurujeyalakshmiGGiriSNEffects of pirfenidone on transforming growth factor-beta gene expression at the transcriptional level in bleomycin hamster model of lung fibrosisJ Pharmacol Exp Ther1999291136737310490926
- WipffPJHinzBIntegrins and the activation of latent transforming growth factor beta1 – an intimate relationshipEur J Cell Biol2008878–960161518342983
- PuthawalaKHadjiangelisNJacobySCInhibition of integrin alpha(v)beta6, an activator of latent transforming growth factor-beta, prevents radiation-induced lung fibrosisAm J Respir Crit Care Med20081771829017916808
- JinninMIhnHTamakiKCharacterization of SIS3, a novel specific inhibitor of Smad3, and its effect on transforming growth factor-beta1-induced extracellular matrix expressionMol Pharmacol200669259760716288083
- DistlerJHJüngelAHuberLCImatinib mesylate reduces production of extracellular matrix and prevents development of experimental dermal fibrosisArthritis Rheum200756131132217195235
- LounevaNHuamanGFertalaJJiménezSAInhibition of systemic sclerosis dermal fibroblast type I collagen production and gene expression by simvastatinArthritis Rheum20065441298130816575855
- PalazzoEMarconiATruzziFRole of neurotrophins on dermal fibroblast survival and differentiationJ Cell Physiol201222731017102521503896
- RoostermanDGoergeTSchneiderSWBunnettNWSteinhoffMNeuronal control of skin function: the skin as a neuroimmunoendocrine organPhysiol Rev20068641309137917015491
- HinzBPhanSHThannickalVJRecent developments in myofibroblast biology: paradigms for connective tissue remodelingAm J Pathol201218041340135522387320