Abstract
During aging, the reduction of elastic and collagen fibers in dermis can lead to skin atrophy, fragility, and aged appearance, such as increased facial wrinkling and sagging. Microfibril-associated glycoprotein-1 (MAGP-1) is an extracellular matrix protein critical for elastic fiber assembly. It integrates and stabilizes the microfibril and elastin matrix network that helps the skin to endure mechanical stretch and recoil. However, the observation of MAGP-1 during skin aging and its function in the dermis has not been established. To better understand age-related changes in the dermis, we investigated MAGP-1 during skin aging and photoaging, using a combination of in vitro and in vivo studies. Gene expression by microarray was performed using human skin biopsies from young and aged female donors. In addition, immunofluorescence analysis on the MAGP-1 protein was performed in dermal fibroblast cultures and in human skin biopsies. Specific antibodies against MAGP-1 and fibrillin-1 were used to examine protein expression and extracellular matrix structure in the dermis via biopsies from donors of multiple age groups. A reduction of the MAGP-1 gene and protein levels were observed in human skin with increasing age and photoexposure, indicating a loss of the functional MAGP-1 fiber network and a lack of structural support in the dermis. Loss of MAGP-1 around the hair follicle/pore areas was also observed, suggesting a possible correlation between MAGP-1 loss and enlarged pores in aged skin. Our findings demonstrate that a critical “pre-elasticity” component, MAGP-1, declines with aging and photoaging. Such changes may contribute to age-related loss of dermal integrity and perifollicular structural support, which may lead to skin fragility, sagging, and enlarged pores.
Introduction
Cutaneous aging manifests itself as a progressive reduction in both the function and reserve capacity of skin tissues, and the alterations in functional connective tissue, especially elastic fibers in the dermis, results in distensibility and loss of skin elasticity, which may lead to gradual deepening of facial rhytides and increased skin sagging and laxity.Citation1–Citation3 Microfibril-associated glycoprotein-1 (MAGP-1) is a key extracellular matrix protein. It plays essential roles during elastic fiber assembly by integrating elastin, microfibrils, and proteoglycans together, and helps organize them into a complex dermal structure.Citation4,Citation5 MAGP-1 has been shown to be a key regulator during bone development and remodeling.Citation6,Citation7 Its function in maintaining blood vessel integrity was also previously discussed;Citation8,Citation9 however, the role of MAGP-1 during skin aging has not been understood. As essential dermal matrix structures, elastic fibers and their associated proteins are critical to skin’s flexibility, resilience, and recoil. Functional elastic fibers decline with aging and sun exposure as a result of a combination of declined synthesis, faulty assembly, and increased degradation, leading to skin fragility, sagging, loss of elasticity, and wrinkles.Citation3,Citation10 Therefore, elastic fibers and their associated proteins are of interest for understanding cutaneous aging, and they serve as a targeted area for antiaging interventions.
MAGP-1 is an integral component of the extracellular matrix that plays a critical role in the organization of elastic fibers.Citation11 It has been proposed as a bridging molecule that binds tropoelastin onto the fibrillin-containing microfibrils during elastic fiber assembly.Citation12,Citation13 It mediates the binding and alignment of matrix components during elastogenesis, and facilitates the assembly of complex matrix structures ().Citation14,Citation15,Citation16 Together with other microfibril components, MAGP-1 forms a “pre-elastic fiber” module for proper elastin deposition and mature fiber formation. Among elastic fiber-associated proteins, elastin and fibrillin-1 have been widely studied;Citation2,Citation3 however, the function of MAGP-1 during skin aging was not investigated or understood previously.
Figure 1 Function of MAGP-1 in human skin.
Abbreviation: MAGP-1, microfibril-associated glycoprotein-1.
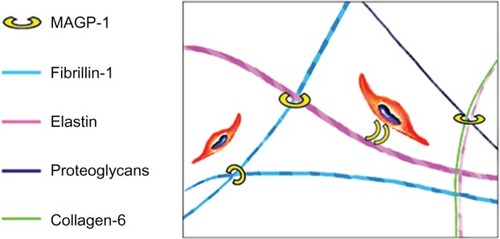
Given the role of MAGP-1 in elastic fiber formation, organization, and stabilization, we hypothesize that it serves a critical function in maintaining the foundation and architecture of the dermal matrix, especially the elastic matrix. In this study, we investigated the changes of the MAGP-1 gene and protein network during aging and photoaging through a combination of in vitro and in vivo studies.
Materials and methods
Gene microarray study
Gene array was performed using skin biopsies from healthy female donors in different age groups. Ribonucleic acid samples were prepared freshly and subjected to quality analysis. Affymetrix GeneChip® human whole genome U133 Plus 1.0 Array plates were used to perform the gene microarray (Expression Analysis, Inc., Durham, NC, USA). A comparison between the two age groups was performed to identify differentially expressed genes, and Student’s t-test was used to measure the statistic difference; a P-value smaller than 0.05 was considered to be statistically different.
UV exposure in vitro
Human dermal fibroblasts from healthy female donors aged 32 years, 38 years, and 39 years (PromoCell GmbH, Heidelberg, Germany; Cell Applications, Inc., San Diego, CA, USA) were grown on supplemented Dulbecco’s Modified Eagle’s Medium to subconfluence. Cells were subjected to three small doses of ultraviolet (UV) irradiation (UV dose: 10 mJ/cm2 UVB and 50 mJ/cm2 UVA) with a 24-hour recovery time between each dose, in order to mimic repetitive exposure to UV. Cells were fixed and subjected to immunocytochemistry studies. After blocking with 1% bovine albumin and 0.5% normal goat serum, goat antihuman MAGP-1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) or rabbit antihuman fibrillin-1 (Elastin Products Company, Inc., Owensville, MO, USA) were applied at 1:50 in Tris buffered saline with 1% bovine serum albumin. Experiments were repeated three times, and fluorescent images were captured using the Zeiss Axionskop2 Plus system (Carl Zeiss Meditec AG, Jena, Germany).
Western blot analysis
Cell supernatants were collected from control and UV-irradiated dermal fibroblasts, as described. A total of 10 μL of resuspended supernatant protein sample was loaded onto each lane of a 4%–20% gradient sodium dodecyl sulfate gel. Primary anti-MAGP-1 was obtained from Novus Biologicals, LLC (Littleton, CO, USA), and was used at 1:1000 dilution. Experiments were repeated three times. Protein band intensity was measured by ImageJ (National Institutes of Health, Bethesda, MD, USA), and the data was expressed as a percentage of control.
Immunohistochemistry
Skin biopsies were collected from the photoexposed areas (forearms) and photoprotected areas (upper underarms or upper thighs) of healthy Caucasian female donors in three different age groups (young, 18–21 years old, n=13; middle-aged, 34–42 years old, n=10; and aged, 57–79 years old, n=12). All protocols were approved by the Greater Delaware Valley Institutional Review Board (Conshohocken, PA, USA), and written informed consent was obtained from all subjects. Biopsies were fixed with neutral buffered formalin; they were then paraffin-embedded and sectioned for immunohistochemical staining. Primary goat antihuman MAGP-1 and antihuman fibrillin-1 (Santa Cruz Biotechnology, Inc.) were applied, followed by Alexa-488 or rhodamine-conjugated secondary antibodies (Vector Laboratories, Inc., Burlingame, CA, USA). Confocal images using 15-micron skin sections were captured by a Leica TCS SP2 system (Leica Microsystems, Wetzlar, Germany), and epifluorescence images were captured by a Zeiss Axionskop2 Plus system (Carl Zeiss Meditec AG).
Results
MAGP-1 is highly expressed in human skin dermis and colocalizes with fibrillin-1
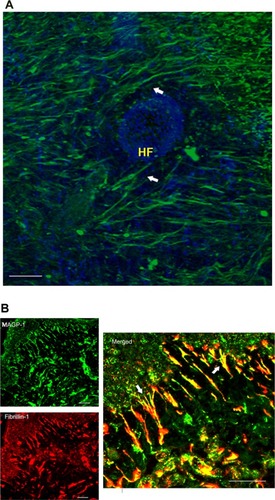
Confocal imaging of MAGP-1 in horizontal skin sections demonstrated that this protein was highly expressed in dermal tissue from young donors (; green fluorescence). This protein forms fibril-like structures with high density in the dermis, and wraps around hair follicles (; arrows). Similar to fibrillin-1 (a key microfibril component), MAGP-1 condensed at the papillary dermal region, connecting epidermis and papillary dermis (). Both proteins were visualized in human skin biopsies using immunofluorescence staining (green, MAGP-1; red, fibrillin-1). Colocalization of MAGP-1 with fibrillin-1 oxytalan fibers underneath the dermal–epidermal junction region was observed (, merged in yellow; the figures that are shown are representative images of the skin biopsy samples from multiple donors, n=13).
Figure 2 MAGP-1 protein localization in young skin.
Abbreviations: HF, hair follicle; MAGP-1, microfibril-associated glycoprotein-1; DAPI, 4′,6-diamidino-2-phenylindole.
MAGP-1 gene expression declines with age in human skin biopsies
Gene array from young and aged donors showed that the MAGP-1 gene declined significantly (over twofold) in aged skin, along with a panel of elastic fiber-related genes (). The details of each donor are listed in . Although the elastin gene only showed a trend of age-related decline (not significant), critical microfibril components (such as fibrillin-1 and MAGP-1), as well as “tethering” proteoglycans (such as biglycan and decorin), and elastic fiber and cell interface molecules (such as fibulin-5) had significantly lower levels of expression in aged skin. This change may contribute to atrophy, elasticity loss, and fragility in chronically aged skin.
Table 1 Age-related changes in key elastic fiber-associated genes from a human full-thickness skin biopsy microarray study
Table 2 Microfibril-associated glycoprotein-1 expression levels in all donors
UV irradiation disrupted the MAGP-1 protein network in vitro
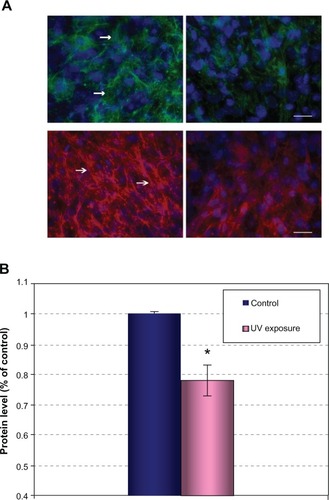
Unlike intrinsic aging that is preprogrammed genetically, extrinsic aging primarily results from exposure to UV light and other environmental insults. It has been previously reported that nearly 80% of facial aging can be attributed to UV exposure.Citation17 Changes in the functionality of the dermal extracellular components account for the major visible changes that are associated with UV-induced skin damage.Citation2,Citation3 Therefore, we evaluated the impact of UV irradiation on MAGP-1 protein in vitro and in vivo. In dermal fibroblast cultures, it was observed that MAGP-1 forms very fine and relatively short fibrils compared to fibrillin-1 (; left panels, arrows). After repetitive low-dose UV exposure, a significant reduction in the MAGP-1 level was observed (; upper right). This protein lost its regular fibril staining pattern and became sparse and disoriented, suggesting that UV inhibits the formation and/or accelerates the structural degradation of the MAGP-1 protein network. Similar changes were observed for fibrillin-1 (; lower right). The level of protein change with UV irradiation, as described, was quantified by Western blot analysis. Experiments were repeated three times. A significant reduction in the MAGP-1 protein level was observed, as quantified by ImageJ (P<0.05) ().
Figure 3 Reduction of MAGP-1 and FBN-1 levels after UV irradiation in dermal fibroblast culture.
Abbreviations: UV, ultraviolet; MAGP-1, microfibril-associated glycoprotein-1; FBN-1, fibrillin-1; DAPI, 4′,6-diamidino-2-phenylindole; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
MAGP-1 declines in dermis with chronological aging, and photoexposure accelerates this process
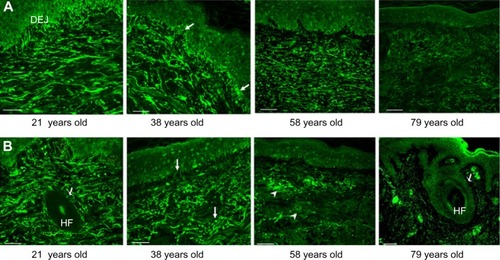
Next, we observed the in vivo change of MAGP-1 in human skin. Full thickness biopsies from photoprotected and photoexposed areas were examined to understand the in vivo distribution and expression pattern of this protein (). In photoprotected skin (similar to the gene array data), a significant decline in the MAGP-1 protein was observed in aged skin (from the 57–79-year-old group) compared to the young skin (from the 18–21-year-old group). Such change was not apparent in the middle-aged group (34–42 year-olds) (). However, in photoexposed skin, a change in the MAGP-1 staining pattern was observed early in life. Around the late 30s, the MAGP-1 fibers became more “wavy” and fragmented, and the candelabra-like structures in the papillary dermis were significantly reduced compared to the younger group (; arrows). In the aged group, a large amount of amorphous solar elastosis-like material accumulated in the dermis (; arrowheads). This phenomenon can be observed from donors in their late 50s. As age progresses, a near complete loss of MAGP-1 immunoreactivity was observed (; 79 year-olds). Similar age-related changes were observed in fibrillin-1 immunostaining, supporting the findings by Watson et al.Citation18
Figure 4 Age-related change of the MAGP-1 protein in human skin biopsies.
Abbreviations: MAGP-1, microfibril-associated glycoprotein-1; n, number; DEJ, dermal-epidermal junction; HF, hair follicle.
Significant changes in skin structure and elasticity profile are often reported in women of postmenopausal age.Citation1,Citation19 However, as UittoCitation3 pointed out, such changes can be accelerated greatly by sun exposure. Our study demonstrated that the disruption of the underlying microfibril foundation of the skin can happen as early as in the 30s in photoexposed skin. This suggests that possible damage of the MAGP-1 fibrils with chronic photoaging can happen relatively early in life.
Progression of MAGP-1 loss in reticular dermis and around hair follicles with photoaging
The reticular dermis, rich in mature elastic and collagen fibers, provides density and strength to the skin, and also anchors the hair follicles. A cumulative effect of photoexposure can lead to the degradation of elastin and fibrillin in the reticular dermis.Citation18,Citation20 In the reticular dermis of young skin, an abundant amount of MAGP-1 fibers, oriented parallel to the skin’s surface, was observed. These fibril structures were also found to be wrapping around the hair follicles (; arrowheads), suggesting that these fibrils may play a role in providing structural support to anchor the fibers and to regulate the elasticity of follicles and pores. In the aged group, a significant reduction in the MAGP-1 level in both the reticular dermis as well as in areas surrounding the hair follicles was observed (; arrowheads). This suggests that declines in MAGP-1 may contribute to the disruption in the structural integrity of follicular regions in aged skin.
Discussion
MAGP-1 has been reported as a critical component of elastic fiber, and it plays an important role in maintaining the structure of elastic tissue.Citation1–Citation3 However, its role during human skin aging was not previously investigated. This study demonstrated that MAGP-1 colocalized with key microfibril components in the papillary dermis and dermal vasculatures, and that UV irradiation can damage this protein network. Furthermore, a decline in MAGP-1 expression with age was observed both at the gene level and at the protein level. Such change was accelerated by photoexposure and can be observed as early as in one’s 30s, which represents a relatively young age group. Interestingly, age-related changes in other organs and systems can be observed at a similar age, as described by Ford,Citation21,Citation22 suggesting that systematic alterations in the human body’s functions begins to manifest in this age group. In the skin (and in addition to UV damage), cumulative impacts from combined internal and external aging factors such as gene modulation, hormone changes, oxidative stress, climate and dryness, lifestyle, and so on, could all play a part in the skin’s aging process.
Long-term photoaging appears to result in the progression of MAGP-1 loss in the follicular regions and in the reticular dermis, which may lead to fragility and instability of the dermal matrix, and contribute to the various signs of an aged appearance such as wrinkles, sagging, loss of elasticity and bounciness, and enlarged pores.
While collagen comprises a major part of the protein network that contributes to the firmness and tensile strength of skin, the healthy elastic fibers are important for the stretching and recoiling of the tissue, and they are also critical in tissue integrity as well. The microfibril components associated with elastin help regulate the formation, assembly, and stabilization of elastic fibers, and they are also critical for dermal integrity and elasticity. Such components should not be overlooked in antiaging investigations. An interesting observation in this study is that the dermal MAGP-1 level can significantly decline under the dermal–epidermal junction at a relatively young age, when the majority of collagen and elastic fibers have been reported to still remain intact.Citation23 Therefore, methods for the early prevention or correction of such changes should be adopted to avoid further diminishment and subsequent damage to the dermal foundation. Further functional assays pertaining to MAGP-1, such as gene knock-down studies in skin cells and tissues, should be carried out to better elucidate how this type of age-related change affects the total elastic network.
In summary, our studies helped to better understand the role of MAGP-1 during skin aging, and the findings indicated that the loss of MAGP-1 might contribute to or accelerate the visible signs of aging in the skin. The modulation of MAGP-1 or the prevention of protein loss may provide a new way to help reestablish elastic properties in the skin.
Acknowledgments
The authors would like to acknowledge Professor Adilson Costa for his critical review and support of the manuscript, and they would also like to acknowledge Katherine Cintron and Gerald Heenan for their technical support.
Disclosure
The authors report no conflicts of interest in this work.
References
- Calleja-AgiusJMuscat-BaronYBrincatMPSkin ageingMenopause Int2007132606417540135
- JenkinsGMolecular mechanisms of skin ageingMech Ageing Dev2002123780181011869737
- UittoJThe role of elastin and collagen in cutaneous aging: intrinsic aging versus photoexposureJ Drugs Dermatol20087Suppl 2s12s1618404866
- SegadeFFunctional evolution of the microfibril-associated glycoproteinsGene20094391–2435419332111
- TsurugaEYajimaTIrieKMicrofibril-associated glycoprotein-1 and fibrillin-2 are associated with tropoelastin deposition in vitroInt J Biochem Cell Biol200537112012915381155
- CraftCSBroekelmannTJZouWChappelJCTeitelbaumSLMechamRPOophorectomy-induced bone loss is attenuated in MAGP1-deficient miceJ Cell Biochem20121131939921898536
- HayesAJSmithSMGibsonMAMelroseJComparative immunolocalization of the elastin fiber-associated proteins fibril-lin-1, LTBP-2, and MAGP-1 with components of the collagenous and proteoglycan matrix of the fetal human intervertebral discSpine (Phila Pa 1976)20113621E1365E137221540769
- ChenELarsonJDEkkerSCFunctional analysis of zebrafish microfibril-associated glycoprotein-1 (Magp1) in vivo reveals roles for microfibrils in vascular development and functionBlood2006107114364437416469878
- WerneckCCVicenteCPWeinbergJSMice lacking the extracellular matrix protein MAGP1 display delayed thrombotic occlusion following vessel injuryBlood200811184137414418281502
- BaumannLSkin ageing and its treatmentJ Pathol2007211224125117200942
- WagenseilJEMechamRPNew insights into elastic fiber assemblyBirth Defects Res C Embryo Today200781422924018228265
- ReinbothBHanssenEClearyEGGibsonMAMolecular interactions of biglycan and decorin with elastic fiber components: biglycan forms a ternary complex with tropoelastin and microfibril-associated glycoprotein 1J Biol Chem200227763950395711723132
- WeinbaumJSTranquilloRTMechamRPThe matrix-binding domain of microfibril-associated glycoprotein-1 targets active connective tissue growth factor to a fibroblast-produced extracellular matrixMacromol Biosci201010111338134420799254
- ClarkeAWWeissASMicrofibril-associated glycoprotein-1 binding to tropoelastin: multiple binding sites and the role of divalent cationsEur J Biochem2004271143085309015233806
- JensenSAReinhardtDPGibsonMAWeissASProtein interaction studies of MAGP-1 with tropoelastin and fibrillin-1J Biol Chem200127643396613966611481325
- TraskBCTraskTMBroekelmannTMechamRPThe microfibrillar proteins MAGP-1 and fibrillin-1 form a ternary complex with the chondroitin sulfate proteoglycan decorinMol Biol Cell20001151499150710793130
- UittoJUnderstanding premature skin agingN Engl J Med199733720146314659358147
- WatsonREGriffithsCECravenNMShuttleworthCAKieltyCMFibrillin-rich microfibrils are reduced in photoaged skin. Distribution at the dermal-epidermal junctionJ Invest Dermatol1999112578278710233772
- BologniaJLBravermanIMRousseauMESarrelPMSkin changes in menopauseMaturitas19891142953042693917
- WatsonREBallSGCravenNMDistribution and expression of type VI collagen in photoaged skinBr J Dermatol2001144475175911298533
- FordJHProtraction of anaphase B in lymphocyte mitosis with ageing: possible contribution to age-related cancer riskMutagenesis201328330731423435012
- FordJHSaturated fatty acid metabolism is key link between cell division, cancer, and senescence in cellular and whole organism agingAge (Dordr)201032223123720431990
- El-DomyatiMAttiaSSalehFIntrinsic aging vs photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skinExp Dermatol200211539840512366692