Abstract
Background
Cellulite is a serious cosmetic concern for most of the 90% of women affected by it.
Objective
To assess the clinical efficacy of a complex integral anti-cellulite gel.
Methods
This double-blind, randomized, placebo-controlled study involved 44 healthy women, aged 25–55 years. Subjects had a normal to slightly overweight body mass index and presented slight to moderate cellulite on their thighs, buttocks, and/or hips at baseline. Subjects were randomly assigned to either the treated or placebo group and accordingly applied the active product or placebo on their hips, stomach, buttocks, and thighs, twice daily for 3 months. Skin tonicity, orange-peel aspect, and stubborn cellulite were assessed at day 0, 28, 56, and 84. A self-evaluation questionnaire was completed by all volunteers.
Results
At the end of the study, an average of 81% of the subjects applying the active product presented improvement in their cellulite condition versus 32% for the placebo group (all descriptors and sites combined). At day 84, skin tonicity, orange-peel appearance, and stubborn cellulite were improved in a significant manner (P<0.05) over placebo, on all studied areas. Skin tonicity improved on average by +41% for buttocks, +35% for hips, and +31% for thighs. Orange peel appearance was reduced on average by −25% for buttocks, −22% for hips, and −22% for thighs. Stubborn cellulite was reduced on average by −19% for buttocks, −24% for hips, and −22% for thighs. Circumference measurements decreased in a significant manner (P<0.05) over placebo, for the abdomen (average value of −1.1 cm) and thighs (average value of −0.8 cm). The product was well tolerated and perceived by the volunteers themselves as better performing than placebo on all criteria.
Conclusion
All results validate the efficacy of the present integral formulation to significantly reduce signs of cellulite and reshape the silhouette.
Introduction
Cellulite refers to a local alteration of the relief of the skin which acquires an orange-peel, or mattress, appearance. The orange-peel appearance results from the bulging of fat lobules out of their connective frame, into the dermis. The phenomenon is most commonly seen on hips, buttocks, and thighs but can also touch other areas, including the abdomen. Up to 90% of woman, over 20 years of age, are affected at various degrees, against only 2% of men.Citation1–Citation3 Cellulite is seen as a normal condition by the medical community, but it is a serious cosmetic concern for most women affected by it.
Although cellulite involves fat cells, it is not a manifestation of obesity, and even young women with a normal body mass index (BMI) may get it.Citation4 However, being overweight aggravates the presence of cellulite. Other risk factors include a predisposing genetic background, hormonal imbalance, medication that causes water retention, a sedentary lifestyle, prolonged periods of immobility, wearing tight clothes, smoking, excessive alcohol intake, unhealthy eating habits, stress, and being Caucasian.Citation5 Some disorders have also been associated with cellulite, such as venous insufficiency, kidney problems, metabolic perturbations, and gastrointestinal alterations.Citation5
The exact etiology of cellulite is still a matter of debate, but most scientists will agree on the involvement of reduced microcirculation, interstitial liquid infiltration (edema), localized hypertrophy of adipocytes, oxidative stress, and persistent low grade inflammation, combined with extracellular matrix alterations.Citation4,Citation6–Citation9 The extensibility, elasticity, and resilience of the skin are also abnormal.Citation10 schematizes all these elements. The condition may start with hormone-induced activation of matrix-metalloproteinases (MMPs), which weakens capillary walls and challenges extracellular matrix integrity.Citation11 As a result, fluid leaks out of vessels, and inflammatory cells are recruited within tissues where they generate inflammation and release additional MMPs. In an effort to heal, the damaged matrix of the septa becomes fibrosclerotic.Citation7 Meanwhile, hormones may also stimulate the metabolic activity of adipocytes, which increase in volume. Hypertrophic fat lobules tend to exert pressure on the surrounding capillaries, therefore adding to their fragility and hampering circulation.Citation10
Figure 1 Major mechanisms involved in cellulite. The exact etiology of cellulite is still a matter of debate but most scientists agree on the involvement of reduced microcirculation, interstitial liquid infiltration (edema), localized hypertrophy of adipocytes, oxidative stress, and persistent low grade inflammation, combined with ECM alterations. Cellulite and skin aging may influence each other.
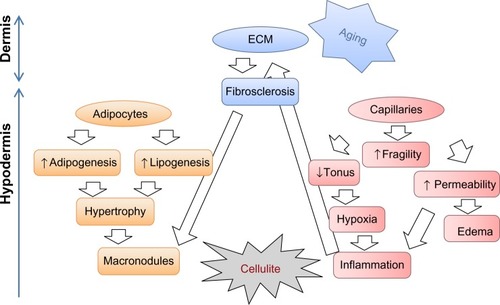
The process is a reminder of what happens with aging in the upper layers of skin (dermis and epidermis) where changes are associated with MMP activation, altered biomechanical properties, reduced vessel integrity, and inflammation. Indeed, a clinical study conducted by Ortonne et alCitation12 confirmed that the presence of cellulite precipitates skin aging in women over 30 years of age. Therefore, it may be advisable to address both conditions simultaneously when treating cellulite. The approach described in this paper follows this lead. The test product is an integral gel, simultaneously addressing skin aging and cellulite. The patent-pendingCitation13 formula combines all active cosmetic ingredients listed in . The final concentration of cosmetic active ingredients in the formulation reaches 25% (weight per weight [w/w]).
Table 1 Actives versus proposed actions on skin. List of all active ingredients found in IDC™ (Immanence IDC Inc, Québec, QC, Canada) anti-cellulite gel, with their proposed actions on skin, according to published literature and/or patent documents
The skin anti-aging aspect of the formulation integrates multiple ingredients addressing all major known mechanisms involved in the process. The components, rationale, and efficacy of this anti-aging approach have been described previously elsewhere.Citation14 For their part, the anti-cellulite ingredients were selected on the basis of their potential complementarities in addressing the cellulite problem on all fronts, according to published literature. They include cosmetic ingredients with well documented anti-cellulite activity, such as caffeine, retinol, forskolin (Coleus forskohlii), sacred lotus (Nelumbo nucifera), carnitine, and escin, among others. For a list of all ingredients present in the formulation and their respective expected action on skin, please refer to .
Many of the ingredients included in this formulation have proven their anti-cellulite efficacy in published human clinical studies. For instance, caffeine is a known stimulator of lipolysis, through inhibition of phosphodiesterase and increased adenosine monophosphate levels in adipocytes,Citation2 and has had its slimming activity clinically confirmed by Lupi et al.Citation15 As is the case for the present formulation, caffeine may be vectorized with phospholipids to facilitate skin absorption.Citation16 As was done here, caffeine may also be mixed with other active ingredients for improved performance. Indeed, a mixture of caffeine and N. nucifera extract was shown by Escudier et al to enhance the benefits of a healthy diet for the treatment of cellulite.Citation17 A synergistic mixture including caffeine, carnitine, forskolin, and retinol was also reported by Roure et alCitation18 to improve several parameters linked to cellulite. Moreover, a mixture of retinol, caffeine, and ruscogenin was able to reduce the orange-peel appearance and increase microcirculation in a clinical study reported by Bertin et al.Citation19 Single ingredients, also found in this formulation have documented anti-cellulite activity as well. This is the case for retinol, which by itself, improves skin thickness in patients with cellulite, as demonstrated clinically by Kligman et al,Citation20 while Piérard-Franchimont et alCitation21 reported effects on tensile properties of skin, in the context of cellulite. Acting to strengthen capillaries and limit edema when applied topically, escin, derived from horse chestnut, is another ingredient of the current gel that has found application in anti-cellulite formulations.Citation22,Citation23
The aim of the present study was to assess the clinical efficacy of a multi-active integral anti-cellulite gel, in comparison with a vehicle placebo gel, on a panel of human volunteers. Both products were evaluated and compared for their effect on tonicity, orange-peel aspect, stubborn cellulite, and their potential for reduction in circumference of areas affected by cellulite, over a period of 84 days.
Materials and methods
Products
The test product (from Immanence IDC Inc, Québec, QC, Canada) and the placebo (vehicle only) were supplied as gels of similar appearance and texture. Upon receipt by the testing laboratory, the samples were blindly assigned a code, before being stored at ambient humidity and temperature, in their original container. The active formulation contained several cosmetic actives selected on the basis of their potential to address all major mechanisms generally recognized as being involved in the development of cellulite (see Introduction and for more details). The total concentration of cosmetic active ingredients in the formulation reached 25% (w/w). The placebo contained the exact formulation as the testing product, only without the active ingredients listed in , and consisted of a basic gel containing mainly water, jellifying agents, and preservatives.
Subjects
Forty-four healthy women, aged 25–55 years (mean age of 39.8 years), were recruited for this study. Twenty-two subjects (mean age of 39.1 years) were randomly assigned to the active product group, while the other 22 (mean age of 40.2 years) formed the placebo group. All subjects presented slight to moderate cellulite on their thighs, buttocks, and/or hips, at baseline. The subjects had a normal to slightly overweight BMI of between 20.0 and 28.0 kg m−2 and agreed to maintain their usual diet and level of physical activity throughout the study. People having taken, within 7 days of study start, medication, treatment, or natural products that could affect the outcome of the study, were excluded from the present protocol. Participants were asked to refrain from applying other anti-cellulite treatments, cosmetic products, or moisturizers to the studied areas for the duration of the study. Participants were neither allowed to receive additional massage treatment, nor to use any massage accessory during the whole length of the study. Participants were also instructed not to take medication or health supplements capable of affecting bodyweight for the length of the study.
Study design
The current study No 12F-0201 was a randomized, parallel-group, double-blind, placebo-controlled study, with one group assigned to the active gel and one group assigned to a placebo gel. Neither the participants nor the evaluators were aware of the nature (active or placebo) of the product being individually used. Subjects were instructed to apply the gel (active or placebo) on their hips, stomach, buttocks, and thighs, on a clean and dry skin, and to gently massage, with the palm only, until complete skin penetration. The procedure was repeated twice a day (morning and evening) for a total of 84 consecutive days (12 weeks). Clinical evaluation was performed in a laboratory room under controlled temperature (22°C±3°C) and relative humidity (30% ±5%), at day 0 (baseline), day 28 (week 4), day 56 (week 8), and day 84 (week 12). The weight of each volunteer was also recorded at each visit to determine their BMI and assess their compliance with protocol. The clinical data obtained at each time-point were compared with baseline for each group and also between groups in the search for statistically relevant differences. A self-assessment questionnaire was filled in on day 14 (week 2), day 28 (week 4), and day 84 (week 12) to document the subjects’ own subjective perception of product efficacy. The full detailed protocol is available from the sponsor of the study (Immanence IDC Inc).
Study location
The study took place in Montréal, Canada, from the end of February to the end of May, for a total of 84 consecutive days (12 weeks) following first application of the product. The study was conducted by an independent contract testing laboratory specialized in claim validation for cosmetic products, under the control of a dermatologist. The testing laboratory was responsible for the selection and randomization of all participants, as well as the gathering and statistical analysis of results.
Clinical assessment
Evaluation of skin tonicity
Skin tonicity was assessed using an analogical scale developed by the testing laboratory responsible for clinical evaluation of the product. For this purpose, a tubular device was designed and filled with layers of foam of increasing density in order to reproduce variations in skin tonicity, on a scale ranging from 1 “minimum firmness” to 7 “maximum firmness” (). Grading of tonicity was performed by comparing the resistance of skin versus the resistance of this dedicated foam-like device, when applying a constant pressure with fingers. The repeatability and reproducibility of the procedure was validated by applying analysis of variance. In the present study, grading was performed by the same trained technician, on a precisely localized area of interest on hips, buttocks, and thighs.
Figure 2 Device for grading skin tonicity. The tubular device is filled with layers of foam of increasing density in order to reproduce variations in skin tonicity, on a scale ranging from 1 “minimum firmness” to 7 “maximum firmness.” Grading is performed by comparing the resistance of skin versus the resistance of this dedicated foam-like device, when applying a constant pressure with fingers. The repeatability and reproducibility of the procedure has been validated by applying analysis of variance.

Evaluation of cellulite appearance
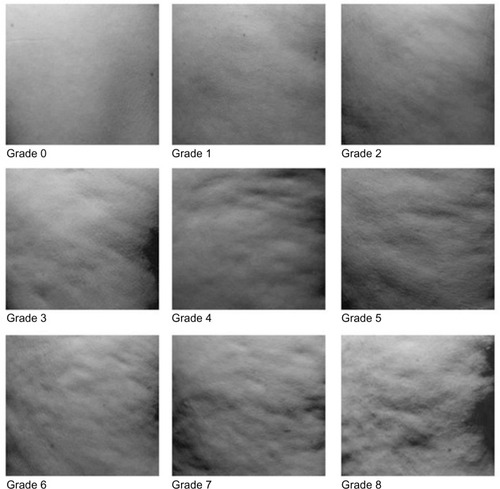
“Orange peel” aspect on relaxed skin and “stubborn cellulite” on pinched skin (thighs and hips) or on contracted buttocks were assessed using an analogical validated scale (from 0 “no intensity” to 8 “maximum intensity”) on hips, buttocks, and thighs (). The scale used in the present study was an adaptation of a scale initially developed by Hexel et al.Citation24 Orange peel and stubborn cellulite evaluation were performed by the same trained technician at each visit, in the same room (under controlled conditions of lighting, temperature, and humidity), and on the same body areas of interest for each subject, with the volunteer standing in a standardized upright position (ground references for feet repositioning).
Figure 3 Visual grading scale for orange-peel appearance. The scale goes from 0 “no intensity” to 8 “maximum intensity.”
Each measurement site was localized precisely, with the help of a graduated rule and a laser beam to determine the site position with respect to the ground and ensure a correct vertical positioning. For reproducibility, the length of the laser beam was recorded at the first visit, and the same length was used at all subsequent visits. Additionally, a mapping of the skin’s surface features (eg, brown spots and scars) for each measurement site on each volunteer was recorded in order to precisely reposition during subsequent measurements.
Circumference measurements
Circumference measurements were obtained using a measuring tape, with the volunteers standing in a standardized upright position. Each measurement site was localized precisely, with the help of a graduated rule and a laser beam to determine the site position with respect to the ground and ensure a correct vertical positioning. For reproducibility, the length of the laser beam was recorded at the first visit, and the same length was used at all subsequent visits. Additionally, a mapping of the skin’s surface features (eg, brown spots and scars) for each measurement site on each volunteer was recorded in order to precisely reposition during subsequent measurements. The circumference of the following sites was measured: abdomen (2–3 cm below the navel), hips/buttocks, and both thighs (in the middle).
Qualitative survey
Treatment efficacy was also qualitatively assessed through a survey. The self-evaluation questionnaire was designed to gauge volunteers’ perception of the overall performance of products (active or placebo). All subjects were requested to fill in a questionnaire pertaining to skin firmness and smoothness on day 14 (week 2), as well as reduction of cellulite, attenuation of “orange skin” appearance, and improvement of skin texture on day 28 (week 4) and day 84 (week 12) of product application. Additionally, volunteers were asked to evaluate the perceived slimming effect after 84 days of twice-daily treatment. For a list of all evaluation criteria, please refer to .
Table 2 Questionnaire and schedule for self-evaluation of product efficacy
Statistical analysis
Statistical analysis was carried out on all pertinent parameters. Results obtained at day 28, 56, and 84 for both treatments (test product and placebo) were compared with baseline results (day 0) using the Student’s t-test (paired two-sample t-test for means), allowing the evaluation of the effect of each treatment. Whenever appropriate, results were expressed as the mean of measurements obtained from all volunteers within each group. All relevant anti-cellulite results were noted and analyzed using a hypothesis test (two-sample t-test, assuming equal or unequal variance), allowing the comparison of the mean value of both groups at day 28 (week 4), day 56 (week 8), and day 84 (week 12), in order to determine whether there was any significant difference between the two treatments.
Ethics
The standard procedure and associated documents were reviewed and approved by the ethics committee of Evalulab Inc. prior to commencement of the clinical trial. The ethics committee was an independent organization whose members’ responsibility was to ensure the protection of the rights, security, and wellbeing of the volunteers participating in the study. Written informed consent was obtained from all participants prior to any trial procedure. This study was conducted in accordance with the ethical standards formulated in the 1964 Declaration of Helsinki and its later amendments.
Results
Participants
Of the 44 volunteers initially recruited, 40 completed the study. Two participants from each group (active and placebo) did not complete the study, the reason being unanticipated schedule incompatibilities. The remaining 40 volunteers completed the study without any adverse event and were included in the statistical analysis of the results by original assigned groups.
Efficacy results
Bodyweight evolution
The BMI of all volunteers did not vary significantly throughout the study. For the active product group, the average BMI was 24.8 kg m−2 at day 0 and 28, 24.6 kg m−2 at day 56, and 24.7 kg m−2 at day 84. For the placebo group, the average BMI was 24.5 kg m−2 at day 0, 24.7 kg m−2 at day 28, 24.6 kg m−2 at day 56, and 24.5 kg m−2 at day 84. Therefore, it is considered that all participants adhered to the protocol by maintaining their weight and lifestyle.
Evaluation of skin tonicity
After 84 days of twice-daily treatment the active gel significantly (P<0.05) improved skin tonicity, over baseline, on all studied areas (). When compared with baseline, results reached average values of +9% at day 28, +39% at day 56, and +41% at day 84 on the buttocks. For hips, results gave average values of +10% at day 28, +17% at day 56, and +35% at day 84. For thighs, results were on average +9% at day 28, +17% at day 56, and +31% at day 84. Placebo treatment resulted in limited improvement of skin tonicity ().
Figure 4 Means of the evolution of skin tonicity at (A) day 28, (B) day 56, and (C) day 84 for both treated and placebo groups.
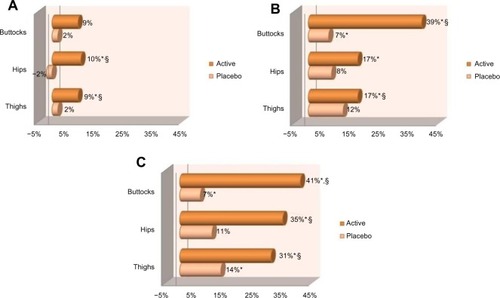
At day 84, statistical analysis (P<0.05) on all studied areas demonstrated that the active gel was better performing than placebo at increasing skin tonicity (). Also, at the end of the study, a larger number of subjects presented improvement in skin tonicity when applying the active product, compared with placebo (95% versus 55% on thighs, 70% versus 40% on hips, and 70% versus 30% on buttocks) ().
Table 3 Percentage of volunteers with improvement at the end of the study (day 84)
Evaluation of orange-peel appearance
After 84 days of product use, treatment with the active gel significantly (P<0.05) reduced the orange-peel appearance of the skin (no pinching), over baseline, on all studied areas (). When compared with baseline, results obtained for buttocks reached average values of −9% at day 28, −16% at day 56, and −25% at day 84. For thighs, results were on average −5% at day 28, −11% at day 56, and −22% at day 84. For hips, results gave average values of −8% at day 56 and −22% at day 84. Placebo treatment resulted in limited improvement of orange-peel appearance ().
Figure 5 Means of the evolution of orange-peel appearance at (A) day 28, (B) day 56, and (C) day 84 for both treated and placebo groups.
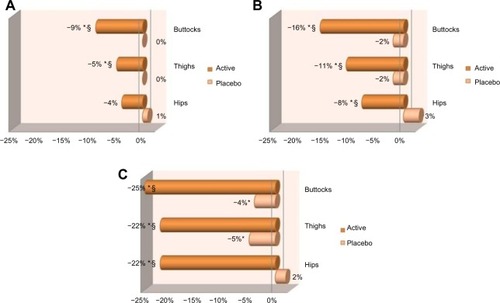
At day 84, statistical analysis (P<0.05) on all studied areas demonstrated that treatment with the active gel was better performing at reducing the orange-peel appearance than placebo treatment (). Also, at the end of the study, a larger number of subjects presented improvement in orange-peel appearance when applying the active gel, compared with placebo (95% versus 40% on thighs, 65% versus 10% on hips, and 80% versus 20% on buttocks) ().
Evaluation of stubborn cellulite appearance
After 84 days of product use, treatment with the active gel significantly (P<0.05) reduced stubborn cellulite (with pinching), over baseline, on all studied areas (). When compared with baseline, results obtained for hips gave average values of −6% at day 28, −17% at day 56, and −24% at day 84. For thighs, the average values were −6% at day 28, −15% at day 56, and −22% at day 84. For buttocks, the average values were −9% at day 28, −15% at day 56, and −19% at day 84. Placebo treatment resulted in limited improvement of stubborn cellulite ().
Figure 6 Means of the evolution of stubborn cellulite (with pinching) at (A) day 28, (B) day 56, and (C) day 84 for both treated and placebo groups.
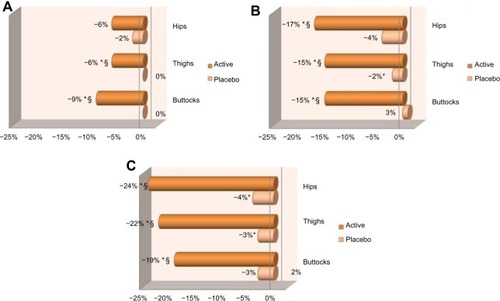
At day 84, statistical analysis (P<0.05) on all studied areas demonstrated that treatment with the active gel was better performing at reducing stubborn cellulite than placebo treatment (). Also, at the end of the study, a larger number of subjects presented improvement in stubborn cellulite appearance when applying the active gel, compared with placebo (95% versus 40% on thighs, 75% versus 20% on hips, and 85% versus 35% on buttocks) ().
Circumference measurements
After 84 days of product use, treatment with the active gel significantly (P<0.05) reduced, over baseline, the circumference of all studied areas. When compared with baseline, results obtained for the abdomen gave average values of −0.4 cm at day 28, −0.9 cm at day 56, and −1.1 cm at day 84. For the right thigh, the average values were −0.3 cm at day 28, −0.6 cm at day 56, and −0.8 cm at day 84. For the left thigh, the average values were −0.1 cm at day 28, −0.4 cm at day 56, and −0.8 cm at day 84. For buttocks, the average values were −0.4 cm at day 28, −0.7 cm at day 56, and −0.8 cm at day 84 (). Placebo treatment resulted in limited improvement of circumference measurements ().
Table 4 Means of the evolution (Dx–D0) of circumference measurements (in cm)
At day 84, statistical analysis (P<0.05) demonstrated that treatment with the active gel was better performing than placebo at reducing the circumference of the abdomen and thigh areas (). Also, at the end of the study, a larger number of subjects presented a reduction in circumference measurements on all studied areas when applying the active gel, compared with placebo (80% versus 35% on the abdomen, 45% versus 35% on the right thigh, and 70% versus 35% on the left thigh) (results not shown).
Qualitative survey
The overall scores for perceived performance of the test product (active gel or placebo) collected from the self-evaluation questionnaires completed by all volunteers are presented in .
Figure 7 Means of the evolution of self-perception of product efficacy at (A) day 14, (B) day 28, and (C) day 84 for both treated and placebo groups.
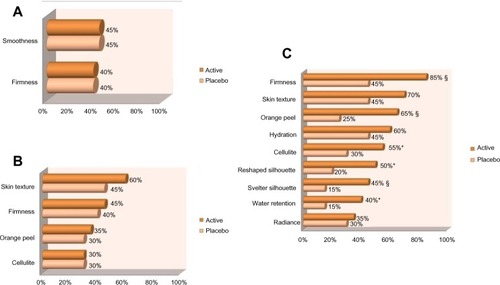
At day 14 and 28, statistical analysis of the data did not demonstrate any significant difference between the two groups in the perception of treatment efficacy.
At the end of the study (day 84), statistical analysis showed that the active gel performed significantly better than the placebo, on the following parameters ().
Firmness: 85% versus 45% (P<0.05)
Orange peel appearance: 65% versus 25% (P<0.05)
Silhouette seems more svelte: 45% versus 15% (P<0.05)
At day 84, results reached near significance on the following parameters ().
Silhouette seems reshaped: 50% versus 20% (P<0.1)
Less water retention: 40% versus 15% (P<0.1)
Signs of cellulite are visibly reduced: 55% versus 30% (P<0.1)
No statistical difference was observed for the other parameters.
Discussion
The present study was rigorously designed on a pharmaceutical model. This was a double-blind, parallel group, randomized, placebo-controlled study. The study establishes the efficacy of the test product (from Immanence IDC Inc) to improve the appearance of cellulite and reduce the circumference of the affected areas. At the end of the study period, statistical analysis on all pertinent parameters clearly demonstrated significant performance superiority for the active product over placebo. The test product was a gel integrating several cosmetic active ingredients (listed in ) selected on the basis of their potential to address all major mechanisms generally recognized as being involved in the development of cellulite ( and ), according to published literature and/or patent documents. The formulation also covers all major skin aging mechanisms,Citation14 since skin aging and cellulite may influence each other,Citation12 as outlined in the Introduction.
By the end of the clinical trial (day 84), following twice-daily application of the test product, all studied parameters relating to cellulite, including skin tonicity, orange-peel appearance, and stubborn cellulite, were statistically improved over placebo (P<0.05) on all studied areas, ie, buttocks, thighs, and hips. Results obtained for skin tonicity reached average values of +41% for buttocks, +35% for hips, and +31% for thighs. Results obtained for orange-peel appearance (no pinching) reached average values of −25% for buttocks, −22% for hips, and −22% for thighs. Results obtained for stubborn cellulite (with pinching or on contracted buttocks) reached average values of −19% for buttocks, −24% for hips, and −22% for thighs. For the treated group, benefits were already seen on all parameters by day 28, improving constantly over time until the end of the study. The absence of a plateau effect suggests that the full potential for improvement had not been reached within 84 days (12 weeks) of twice-daily application of the test product, and that further amelioration might be seen with longer application periods.
At the end of the study (day 84), an average of 81% of the subjects applying the active gel presented improvement in their cellulite condition versus 32% for the placebo group (all descriptors and sites combined). The slight benefits obtained with the placebo gel are most likely related to a massaging effect upon application of the gel. Massaging is known to impact positively on cellulite appearance possibly by improving microcirculation and drainage in the affected area.Citation25 In support of that, a combination of mechanical and manual lymphatic drainage has been reported to reduce body measurements in areas with cellulite.Citation26 However, in the present study, the potential benefits from massaging are expected to be comparable for both groups since the placebo and treatment products contained the exact same gel base, had similar rheological characteristics, and were applied in the same manner.
The effect of the product on the appearance of the silhouette was assessed through circumference measurements of the abdomen, thighs, and hips/buttocks areas. By the end of the study (day 84), a significant reduction in circumference was observed over placebo (P<0.05) for the abdomen (mean of −1.1 cm) and for both thighs (mean of −0.8 cm). Again, limited benefits were obtained with the placebo gel; we believe this to reflect the contribution of massaging upon application. Importantly, all volunteers maintained a constant BMI throughout the study, attesting that the reduction in circumference and remodeling effects were not due to weight loss but most likely to better fluid drainage of the cellulite-affected areas and possibly also through a reduction in aging symptoms.
Treatment efficacy was also evaluated by the volunteers themselves. As could be expected, there was no difference in efficacy perception between the active product and the placebo group at day 14. Cellulite is a complex condition that cannot improve rapidly. However, slight differences between the two groups started emerging at day 28, and were neatly confirmed at day 84, with better performance for the active product over placebo. This progression in efficacy perception mirrors the progression documented through trained specialist evaluation. At the end of the study, statistically significant difference (Δ%) in terms of criteria appreciation between groups was seen for skin firmness, orange-peel appearance, and reshaped silhouette ().
Conclusion
All results validate the efficacy of the present integral formulation to significantly reduce the signs of cellulite and reshape the silhouette, but do not provide information on the performance of individual ingredients within it. Cellulite is a complex phenomenon that requires a complex approach, and it is likely that no single ingredient is solely responsible for the benefits reported here. In support of this, synergistic action of anti-cellulite ingredients has been described in the literature previously.Citation17–Citation19,Citation27,Citation28 In fact, a multi-target/multi-component strategy is increasingly seen as the best approach to improve the appearance of cellulite.
Another limitation of the present study comes from the fact that it does not allow evaluating the contribution of anti-aging actives, found in the formulation, to the overall anti-cellulite effects. This could be the subject of future studies. The concept of fighting the appearance of cellulite by including both anti-aging and anti-cellulite actives in one integral formula is an interesting and promising approach that certainly deserves a closer look.
Yet another limitation of the study is linked to the fact that it was stopped before any plateau effect was reached. The maximum efficacy of the gel remains unknown, as well as its sustainability in time. More prolonged studies may be advisable in the future when assessing the effect of anti-cellulite products.
Nevertheless, the clear anti-cellulite beneficial effects of the blend of actives tested here support the use of a combination of ingredients exploiting different mechanisms of action and complementarily working to improve the condition.
Disclosure
ED owns Immanence IDC Inc (Québec, QC, Canada), the company that provided the test product and funded this research. JG is employed by the sponsor company, and DB is a paid consultant for the sponsor company. The other authors report no conflicts of interest in this work.
References
- EmanueleEBertonaMGeroldiDA multilocus candidate approach identifies ACE and HIF1A as susceptibility genes for celluliteJ Eur Acad Dermatol Venereol201024893093520059631
- RawlingsAVCellulite and its treatmentInt J Cosmet Sci200628317519018489274
- MirrashedFSharpJCKrauseVMorganJTomanekBPilot study of dermal and subcutaneous fat structures by MRI in individuals who differ in gender, BMI, and cellulite gradingSkin Res Technol200410316116815225265
- KruglikovIThe pathophysiology of cellulite: can the puzzle eventually be solved?J Cosmet Dermatol Sci Appl20122117
- RossiABVergnaniniALCellulite: a reviewJ Eur Acad Dermatol Venereol200014425126211204512
- TerranovaFBerardescaEMaibachHCellulite: nature and aetiopathogenesisInt J Cosmet Sci200628315716718489272
- KhanMHVictorFRaoBSadickNSTreatment of cellulite: Part I. PathophysiologyJ Am Acad Dermatol201062336137020159304
- HexselDSoirefmannMCosmeceuticals for CelluliteSemin Cutan Med Surg201130316717021925371
- de la Casa AlmeidaMSuarez SerranoCRebollo RoldánJJiménez RejanoJJCellulite’s aetiology: a reviewJ Eur Acad Dermatol Venereol201327327327822758934
- DraelosZDThe disease of celluliteJ Cosmet Dermatol20054422122217168866
- PugliesePTThe pathogenesis of cellulite: a new conceptJ Cosmet Dermatol20076214014217524132
- OrtonneJPZartarianMVerschooreMQueille-RousselCDuteilLCellulite and skin aging: is there any interaction?J Eur Acad Dermatol Venereol200822782783418312331
- DupontESamsonMGalderisiAinventorsImmanence Integral Dermo Correction Inc, assigneeSkin care compositions and method of use thereof United States patent US 201101589226302011
- DupontEGomezJLéveilléCBilodeauDFrom hydration to cell turnover: an integral approach to anti-agingCosmet Toiletries201012535060
- LupiOSemenovitchIJTreuCBottinoDBouskelaEEvaluation of the effects of caffeine in the microcirculation and edema on thighs and buttocks using the orthogonal polarization spectral imaging and clinical parametersJ Cosmet Dermatol20076210210717524126
- KimCShimJHanSChangIThe skin-permeation-enhancing effect of phosphatidylcholine: caffeine as a model active ingredientJ Cosmet Sci200253636337412512013
- EscudierBFanchonCLabrousseEPellaeMBenefit of a topical slimming cream in conjunction with dietary adviceInt J Cosmet Sci201133433433721284660
- RoureROddosTRossiAVialFBertinCEvaluation of the efficacy of a topical cosmetic slimming product combining tetrahydroxypropyl ethylenediamine, caffeine, carnitine, forskolin and retinol, In vitro, ex vivo and in vivo studiesInt J Cosmet Sci20113361820546050
- BertinCZuninoHPittetJCA double-blind evaluation of the activity of an anti-cellulite product containing retinol, caffeine, and ruscogenine by a combination of several non-invasive methodsJ Cosmet Sci200152419921011479653
- KligmanAMPagnoniAStoudemayerTTopical retinol improves celluliteJ Dermatolog Treat1999102119125
- Piérard-FranchimontCPiérardGEHenryFVroomeVCauwenberghGA randomized, placebo-controlled trial of topical retinol in the treatment of celluliteAm J Clin Dermatol20001636937411702613
- SirtoriCRAescin: pharmacology, pharmacokinetics and therapeutic profilePharmacol Res200144318319311529685
- IncandelaLBelcaroGNicolaidesANGeroulakosGCesaroneMRDe SanctisMTMicrocirculation after standardized application of Essaven gel on normal skin – a placebo-controlled, randomized studyAngiology200152Suppl 3S5S1011775650
- HexselDDal’fornoTHexselCLA validated photonumeric cellulite severity scaleJ Eur Acad Dermato Venereol2009235523528
- Bayrakci TunayVAkbayrakTBakarYKayihanHErgunNEffects of mechanical massage, manual lymphatic drainage and connective tissue manipulation techniques on fat mass in women with celluliteJ Eur Acad Dermatol Venereol201024213814219627407
- de GodoyJMde Godoy MdeFTreatment of cellulite based on the hypothesis of a novel physiopathologyClin Cosmet Investig Dermatol201145559
- Al-BaderTByrneAGillbroJEffect of cosmetic ingredients as anticellulite agents: synergistic action of actives with in vitro and in vivo efficacyJ Cosmet Dermatol2012111172622360330
- VogelgesangBBonnetIGodardNSohmBPerrierEIn vitro and in vivo efficacy of sulfo-carrabiose, a sugar-based cosmetic ingredient with anti-cellulite propertiesInt J Cosmet Sci201133212012520807262
- VallsMDCronsteinBNMontesinosMCAdenosine receptor agonists for promotion of dermal wound healingBiochem Pharmacol20097771117112419041853
- BoumaMGJeunhommeTMBoyleDLAdenosine inhibits neutrophil degranulation in activated human whole blood: involvement of adenosine A2 and A3 receptorsJ Immunol199715811540054089164961
- ShecterleLMSt CyrJADermal benefits of topical D-riboseClin Cosmet Investig Dermatol20092151152
- BorelMCalmonEBezivinCPletschSSt CyrJAD-ribose enhances basal and mitochrondrial respiratory rates in human dermal fibroblastsFASEB J2007216A835
- KawashimaMMizunoAMurataYImprovement of hyperpigmentation based on accelerated epidermal turnover: clinical effects of disodium adenosine monophosphate in patients with melasmaJpn J Clin Dermatol200862250257
- FurukawaFKaneharaSHaranoFEffects of adenosine 5′-monophosphate on epidermal turnoverArch Dermatol Res2008300948549318758798
- AndoHMatsuiMSIchihashiMQuasi-drugs developed in Japan for the prevention or treatment of hyperpigmentary disordersInt J Mol Sci2010112566257520640168
- BurnstockGKnightGEGreigAVPurinergic signaling in healthy and diseased skinJ Invest Dermatol20121323 Pt 152654622158558
- CronsteinBMAdenosine receptors and wound healing, revisedScientificWorldJournal2006698499116921444
- ThibodeauAProtecting the skin from environmental stresses with an exopolysaccharide formulationCosmet Toiletries2005120128189
- NusgensBVHumbertPRougierATopically applied vitamin C enhances the mRNA level of collagens I and III, their processing enzymes and tissue inhibitor of matrix metalloproteinase 1 in the human dermisJ Invest Dermatol2001116685385911407971
- KameyamaKSakaiCKondohSInhibitory effect of magnesium L-ascorbyl-2-phosphate (VC PMG) on melanogenesis in vitro and in vivoJ Am Acad Dermatol199634129338543691
- SauermannKJaspersSKoopUWenckHTopically applied vitamin C increases the density of dermal papillae in aged human skinBMC Dermatol2004411315456516
- BoyeraNGaleyIBernardBAEffect of vitamin C and its derivatives on collagen synthesis and cross-attachment linking by normal human fibroblastsInt J Cosmet Sci199820315115818505499
- XieYChenXStructures required of polyphenols for inhibiting advanced glycation end products formationCurr Drug Metab201314441443123330933
- CaengprasathNNgamukoteSMäkynenKAdisakwattanaSThe protective effects of pomelo extract (Citrus grandis L. Osbeck) against fructose-mediated protein oxidation and glycationEXCLI J201312491502
- Leite GdeOLeiteLHSampaio RdeS(−)-α-Bisabolol attenuates visceral nociception and inflammation in miceFitoterapia201182220821120875845
- KamatouGPPViljoenAMA review of the application and pharmacological properties of α-bisabolol and α-bisabolol-rich oilsJ Am Oil Chem Soc20108717
- MamalisANguyenDHBrodyNJagdeoJThe active natural antioxidant properties of chamomile, milk thistle, and halophilic bacterial components in human skin in vitroJ Drugs Dermatol201312778078423884490
- HerrmannMMeyerIJoppeHVielhaberGThe Syngeristic Anti-irritant Effects of (−)-α-Bisabolol and GingerCosmetics and Toiletries2007121227
- KimSLeeJJungEMechanisms of depigmentation by alpha-bisabololJ Dermatol Sci200852321922218692366
- LeeJJunHJungEHaJParkDWhitening effect of alpha-bisabolol in Asian women subjectsInt J Cosmet Sci201032429930320642768
- HermanAHermanAPCaffeine’s mechanisms of action and its cosmetic useSkin Pharmacol Physiol201326181423075568
- JagdeoJBrodyNComplementary antioxidant function of caffeine and green tea polyphenols in normal human skin fibroblastsJ Drugs Dermatol201110775376121720657
- DayanNSivalenkaRChaseJSkin moisturization by hydrogenated polyisobutene – quantitative and visual evaluationJ Cosmet Sci2009601152419296892
- HexselDZechmeister do PradoDRaoJGoldmanMPTopical management of celluliteGoldmanMPBacciPALeibaschoffGHexselDAngeliniiFCellulite: Pathophysiology and TreatmentNew YorkTaylor and Francis Group2006159170
- NayakBSRamdeenRAdogwaARamsubhagAMarshallJRWound-healing potential of an ethanol extract of Carica papaya (Caricaceae) seedsInt Wound J20129665065522296524
- DayanandCDKrishnamurthyNAshakiranSShashidharKNCarnitine: a novel health factor – an overviewInt J Pharm Biomed Res2011227989
- KarsidagTAsensioJAKabukcuogluFTuzunSPreliminary study comparing the effects of locally and systemically applied L-carnitine on the healing of full-thickness skin defectsScand J Surg201099314715221044932
- WallimannTTokarska-SchlattnerMSchlattneUThe creatine kinase system and pleiotropic effects of creatineAmino Acids20114051271129621448658
- OsamuTAkinoriHThe efficacy of L-carnitine on topical application for dry skin. The development of new active ingredient for dry skin as quasi-drugFragr J20053388285
- PillichRTScarsellaGRisuleoGReduction of apoptosis through the mitochondrial pathway by the administration of acetyl-L-carnitine to mouse fibroblasts in cultureExp Cell Res200530611815878327
- ParameshwaraiahSShivakumarHGEvaluation of topical formulations of aqueous extract of Centella asiatica on open wounds in ratsIndian J Exp Biol19983665695729731470
- HaftekMMac-MarySLe BitouxMAClinical, biometric and structural evaluation of the long-term effects of a topical treatment with ascorbic acid and madecassoside in photoaged human skinExp Dermatol2008171194695218503551
- PaolinoDCoscoDCilurzoFImproved in vitro and in vivo collagen biosynthesis by asiaticoside-loaded ultradeformable vesiclesJ Control Release2012162114315122698941
- ShuklaARasikAMDhawanBNAsiaticoside-induced elevation of antioxidant levels in healing woundsPhytother Res1999131505410189951
- GarciaCStoltzCinventorsSEPPIC, assigneeUse of quinoa extract as cosmetic and pharmaceutic slimming agent and/or as an agent preventing the formation of new fats in the human body United States patent US 20100061945 A13112010
- GreenwayFLBrayGAHeberDTopical fat reductionObes Res19953Suppl 4561S568S8697059
- PasseronTNamikiTPasseronHJLe PapeEHearingVJForskolin protects keratinocytes from UVB-induced apoptosis and increases DNA repair independent of its effects on melanogenesisJ Invest Dermatol2009129116216618580960
- PeiranoRIAchterbergVDüsingHJDermal penetration of creatine from a face-care formulation containing creatine, guarana and glycerol is linked to effective antiwrinkle and antisagging efficacy in male subjectsJ Cosmet Dermatol201110427328122151935
- LenzHSchmidtMWelgeVThe creatine kinase system in human skin: Protective effects of creatine against oxidative and UV damage in vitro and in vivoJ Invest Dermatol2005124244345215675966
- KnottAKoopUMielkeHA novel treatment option for photoaged skinJ Cosmet Dermatol200871152218254806
- BlattTLenzHKoopUStimulation of skin’s energy metabolism provides multiple benefits for mature human skinBiofactors2005251–417918516873944
- KobayashiAShibazakiTManufacture and physiological activities of hydroxyprolineFragr J20033133743
- McNultyAKRhodesTJinventorsMcNultyAKRhodesTJassigneesProtease inhibitor compositions for prevention and treatment of skin conditions United States patent US 20050048105 A1332005
- NagaoSUeharaKApplication to the cosmetics of amino acid derivativesFragr J20043275158
- HashizumeENakanoTKamimuraAMorishitaKTopical effects of N-acetyl-L-hydroxyproline on ceramide synthesis and alleviation of pruritusClin Cosmet Investig Dermatol201364349
- LintnerKinventorSederma,assigneeCosmetic or dermopharmaceutical compositions which are used to reduce bags and circles under the eyes European patent EP 1474100 B18142013
- FacinoRMCariniMStefaniRAldiniGSaibeneLAnti-elastase and anti-hyaluronidase activities of saponins and sapogenins from Hedera helix, Aesculus hippocastanum, and Ruscus aculeatus: factors contributing to their efficacy in the treatment of venous insufficiencyArch Pharm (Weinheim)1995328107207248554461
- LuzziRFeragalliBBelcaroGAescin: microcirculatory activity. Effects of accessory components on clinical and microcirculatory efficacyPanminerva Med2011533 Suppl 1515522108477
- HexselDOrlandiCZechmeister do PradoDBotanical extracts used in the treatment of celluliteDermatol Surg2005317 Pt 286687216029680
- FujimuraTTsukaharaKMoriwakiSHottaMKitaharaTTakemaYA horse chestnut extract, which induces contraction forces in fibroblasts, is a potent anti-aging ingredientJ Cosmet Sci200657536937617111071
- RosenthalRAFishBHillRPSalen Mn complexes mitigate radiation injury in normal tissuesAnticancer Agents Med Chem201111435937221453241
- DeclercqLSenteIHellemansLCorstjensHMaesDUse of the synthetic superoxide dismutase/catalase mimetic EUK-134 to compensate for seasonal antioxidant deficiency by reducing pre-existing lipid peroxides at the human skin surfaceInt J Cosmet Sci200426525526318492138
- DecraeneDSmaersKGanDA synthetic superoxide dismutase/catalase mimetic (EUK-134) inhibits membrane-damage-induced activation of mitogen-activated protein kinase pathways and reduces p53 accumulation in ultraviolet B-exposed primary human keratinocytesJ Invest Dermatol2004122248449115009734
- ThibodeauAThe crucial role of metalloproteinase inhibitors and regenerating antioxidants in the age-related alterations of the skinSOFW J200513141020
- ParomovVKumariSBrannonMProtective effect of liposome-encapsulated glutathione in a human epidermal model exposed to a mustard gas analogJ Toxicol2011201110951621776256
- VillaramaCDMaibachHIGlutathione as a depigmenting agent: an overviewInt J Cosmet Sci200527314715318492181
- FluhrJWDarlenskiRSurberCGlycerol and the skin: holistic approach to its origin and functionsBr J Dermatol20081591233418510666
- DraelosZDActive agents in common skin care productsPlast Reconstr Surg2010125271972420124857
- MoonMHJeongJKLeeYJ18β-Glycyrrhetinic acid inhibits adipogenic differentiation and stimulates lipolysisBiochem Biophys Res Commun2012420480581022465130
- ArmaniniDNacamulliDFrancini-PesentiFBattaginGRagazziEFioreCGlycyrrhetinic acid, the active principle of licorice, can reduce the thickness of subcutaneous thigh fat through topical applicationSteroids200570853854215894038
- AfnanQAdilMDNissar-UlAGlycyrrhizic acid (GA), a triterpenoid saponins glycoside alleviates ultraviolet-B irradiation-induced photoaging in human dermal fibroblastsPhytomedicine201219765866422516896
- AslMNHosseinzadehHReview of pharmacological effects of Glycyrrhiza sp and its bioactive compoundsPhytother Res200822670972418446848
- GrafJHerbal anti-inflammatory agents for skin diseaseSkin Therapy Lett2000543510785407
- SaeediMMorteza-SemnaniKGhoreishiMRThe treatment of atopic dermatitis with licorice gelJ Dermatolog Treat200314315315714522625
- CohenDHeidaryNTreatment of irritant and allergic contact dermatitisDermatol Ther200417433434015327479
- CallenderVDSt Surin-LordSDavisECMaclinMPostinflammatory hyperpigmentation: etiologic and therapeutic considerationsAm J Clin Dermatol2011122879921348540
- GoldmanMPBacciPALeibaschoffGHexselDAngeliniiFCellulite: Pathophysiology and TreatmentNew YorkTaylor and Francis Group2006
- RaiAThe antiinflammatory and antiarthritic properties of ethanol extract of Hedera helixIndian J Pharm Sci20137519910223901168
- GülçinIMshvildadzeVGepdiremenAEliasRAntioxidant activity of saponins isolated from ivy: alpha hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside-FPlanta Med200470656156315241892
- KoyuncuHBerkardaBBaykutFPreventive effect of hesperidin against inflammation in CD-1 mouse skin caused by tumor promoterAnticancer Res1999194B3237324110652617
- SilvaARMenezesPFMartinelloTNovakovichGFPraesCEFefermanIHAntioxidant kinetics of plant-derived substances and extractsInt J Cosmet Sci2010321738019818087
- HouMManMManWTopical hesperidin improves epidermal permeability barrier function and epidermal differentiation in normal murine skinExp Dermatol201221533734022509829
- JinSZhouBLuoDHesperidin promotes cyclobutane pyrimidine dimer repair in UVB-exposed mice epidermisIr J Med Sci2011180370971420535600
- ZhuWGaoJThe use of botanical extracts as topical skin-lightening agents for the improvement of skin pigmentation disordersJ Investig Dermatol Symp Proc20081312024
- LiDMitsuhashiSUbukataMProtective effects of hesperidin derivatives and their stereoisomers against advanced glycation end-products formationPharm Biol201250121531153522954318
- Leite e SilvaVRSchulmanMAFerelliCHydrating effects of moisturizer active compounds incorporated into hydrogels: in vivo assessment and comparison between devicesJ Cosmet Dermatol200981323919250164
- LuMDugganMMenonGTheophilusEDokkaSWangHinventorsAvon Prod Inc, assigneeTopical cosmetic composition with skin rejuvenation benefits European patent EP 1441686 B1722008
- AgyareCDwobengASAgyepongNAntimicrobial, antioxidant, and wound healing properties of Kigelia africana (Lam) Beneth and Strophanthus hispidus DCAdv Pharmacol Sci2013201369261323662099
- PicernoPAutoreGMarzoccoSMeloniMSanogoRAquinoRPAnti-inflammatory activity of verminoside from Kigelia africana and evaluation of cutaneous irritation in cell cultures and reconstituted human epidermisJ Nat Prod200568111610161416309308
- OyedejiFOBankole-OjoOSQuantitative evaluation of the antipsoriatic activity of sausage tree (Kigelia africana)AJPAC2012613214218
- MorvanPYL PentecouteauLOffredoHValléeRAnti-ageing and slimming potential of a lotus maritimus extract via its effect on mitochondrial sirtuins on human skin cellsProceedings of the 26th IFSCC CongressSeptember 20–23, 2010Buenos-Aires, Argentina
- PaufiqueJinventorSILAB, assigneeUse of active ingredient comprising combination of active ingredient obtained from medicago sativa and active ingredient from Lupinus albus, to prepare cosmetic and/or dermopharmaceutical composition to fight against bags under the eyes French patent FR 2921836-A11052007
- GaultierFFoucault-BertaudALamyEEffects of a vegetable extract from Lupinus albus (LU105) on the production of matrix metalloproteinases (MMP1, MMP2, MMP9) and tissue inhibitor of metalloproteinases (TIMP1, TIMP2) by human gingival fibroblasts in cultureClin Oral Investig200374198205
- SigerACzubinskiJKachlickiPDwieckiKLampart-SzczapaENogala-KaluckaMAntioxidant activity and phenolic content in three Lupin speciesJ Food Comp Anal2012252190197
- PaufiqueJinventorSILAB, assigneeActive ingredient obtained from powdered Medicago sativa seeds European patent EU 1559417A1832005
- PaufiqueJinventorSILAB, assigneeMethod for obtaining a slimming cosmetic active agent, active ingredient obtained and composition including same World patent WO2008087361 A27242008
- ChoulotJCinventorCaster, assigneeComposition topique à visée amincissante European patent EP 2604317 A16192013
- HuangBZhuLLiuSIn vitro and in vivo evaluation of inhibition activity of lotus (Nelumbo nucifera Gaertn.) leaves against ultraviolet B-induced phototoxicityJ Photochem Photobiol B20131211523474526
- LupoMPPeptides for facial skin agingShiffmanMAMirrafatiSJLamSMCueteauxCGSimplified Facial Rejuvenation, Part IIIBerlinSpringer Berlin Heidelberg20087981
- MaquartFXPickartLLaurentMGilleryPMonboisseJCBorelJPStimulation of collagen synthesis in fibroblast cultures by the tripeptide-copper complex glycyl-L-histidyl-Llysine-Cu2+FEBS Lett198823823433463169264
- MaquartFXBellonGPascoSMonboisseJCMatrikines in the regulation of extracellular matrix degradationBiochimie2005873–435336015781322
- PickartLThe human tri-peptide GHK and tissue remodelingJ Biomater Sci Polym Ed200819896998818644225
- ChoiHRKangYARyooSJStem cell recovering effect of copper-free GHK in skinJ Pept Sci2012181168569023019153
- Mas-ChamberlinCMondonPLamyFPeschardOLintnerKReduction of hair-loss: matrikines and plant molecules to the rescueKrisdaphongPProceedings of the 7th Scientific Conference of the Asian Society of Cosmetic Chemists (ASCS): Toward a New Horizon: Uniting Cosmetic Science with Oriental WisdomMarch 7-9, 2005Bangkok, ThailandSociety of Cosmetic Chemists of Thailand2005
- LintnerKinventorSederma, assigneeUtilisation cosmétique ou pharmaceutique de peptides pour la régulation des dysfonctionnements immunologiques et dans l’inflammation cutanée World patent WO2000043417 A17272000
- dal FarraCDomlogeNBottoJMinventorsISP, assigneeDermatological and/or cosmetic composition containing polypeptides United States patent US 20080171076 A17172008
- dal FarraCBerghiAObertoGDomlogeNSignificant in vivo slimming properties observed in new UCP-like peptideJ Am Acad Dermatol2007562AB88P1015
- RiosLDelattreCChaisemartinLFavre-MercuretMBerthonJYA new generation of slimming productsSOFW J201213811111
- RiosLDelattreCPatriarcaPFavre-MercuretMBerthonJYA polyglucuronic acid to target the fiaf adipokine for slimming effectsCosmet Toiletries20111263196206
- DarlenskiRSurberCFluhrJWTopical retinoids in the management of photodamaged skin: from theory to evidence-based practical approachBr J Dermatol201016361157116520633013
- KligmanAMDogadkinaDLavkerRMEffects of topical tretinoin on non-sun-exposed protected skin of the elderlyJ Am Acad Dermatol199329125337686187
- VaraniJWarnerRLGharaee-KermaniMVitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skinJ Invest Dermatol2000114348048610692106
- KangSThe mechanism of action of topical retinoidsCutis200575Suppl 2101315773538
- WolfJEJrPotential anti-inflammatory effects of topical retinoids and retinoid analoguesAdv Ther200219310911812201351
- BellemèreGVon StettenOOddosTRetinoic acid increases aquaporin 3 expression in normal human skinJ Invest Dermatol2008128354254817943189
- MurphyGFKatzSKligmanAMTopical tretinoin replenishes CD1a-positive epidermal Langerhans cells in chronically photodamaged human skinJ Cutan Pathol199825130349508341
- OrtonneJPRetinoid therapy of pigmentary disordersDermatol Ther200619528028817014483
- BellemèreGStamatasGNBruèreVBertinCIssacharNOddosTAntiaging action of retinol: from molecular to clinicalSkin Pharmacol Physiol200922420020919648781
- SabancilarEAydinFBekYTreatment of melasma with a depigmentation cream determined with colorimetryJ Cosmet Laser Ther201113525525921774660
- MerinvilleEByrneAJVisdal-JohnsenLClinical evaluation of a dioic acid-based formulation on facial skin in an Indian populationInt J Cosmet Sci201234657558122994950
- MaswadehHMSemreenMHNaddafARAnti-inflammatory activity of Achillea and Ruscus topical gel on carrageenan-induced paw edema in ratsActa Pol Pharm200663427728017203864
- SharmaSKaurIPDevelopment and evaluation of sesamol as an antiaging agentInt J Dermatol200645320020816533216
- HuQXuJChenSYangFAntioxidant activity of extracts of black sesame seed (Sesamum indicum L.) by supercritical carbon dioxide extractionJ Agric Food Chem200452494394714969554
- SujaKPJayalekshmyAArumughanCFree radical scavenging behavior of antioxidant compounds of sesame (Sesamum indicum L.) in DPPH(*) systemJ Agric Food Chem200452491291514969550
- KiranKAsadMWound healing activity of Sesamum indicum L seed and oil in ratsIndian J Exp Biol2008461177778219090349
- SchlesingerTEPowellCREfficacy and tolerability of low molecular weight hyaluronic acid sodium salt 0.2% cream in rosaceaJ Drugs Dermatol201312666466723839183
- PavicicTGauglitzGGLerschPEfficacy of cream-based novel formulations of hyaluronic acid of different molecular weights in anti-wrinkle treatmentJ Drugs Dermatol2011109990100022052267
- GariboldiSPalazzoMZanobbioLLow molecular weight hyaluronic acid increases the self-defense of skin epithelium by induction of beta-defensin 2 via TLR2 and TLR4J Immunol200818132103211018641349
- DraelosZDNew treatments for restoring impaired epidermal barrier permeability: skin barrier repair creamsClin Dermatol201230334534822507050
- MummertMEImmunologic roles of hyaluronanImmunol Res200531318920615888911
- ChenWYAbatangeloGFunctions of hyaluronan in wound repairWound Repair Regen199972798910231509
- MerinvilleEByrneAJRawlingsAVMuggletonAJLaloeufACThree clinical studies showing the anti-aging benefits of sodium salicylate in human skinJ Cosmet Dermatol2010317418420883290
- WeimarVPolymorphonuclear invasion of wounded corneas; inhibition by topically applied sodium salicylate and soybean trypsin inhibitorJ Exp Med1957105214115213406174
- BairWB3rdHartNEinspahrJInhibitory effects of sodium salicylate and acetylsalicylic acid on UVB-induced mouse skin carcinogenesisCancer Epidemiol Biomarkers Prev200211121645165212496056
- LinANNakatsuiTSalicylic acid revisitedInt J Dermatol19983753353429620477
- HuangZRLinYKFangJYBiological and pharmacological activities of squalene and related compounds: potential uses in cosmetic dermatologyMolecules200914154055419169201
- WołosikKKnaśMZalewskaANiczyporukMPrzystupaAWThe importance and perspective of plant-based squalene in cosmetologyJ Cosmet Sci20131596623449131
- KimSKBhatnagarIPhysical, chemical, and biological properties of wonder kelp-LaminariaAdv Food Nutr Res201164859622054940
- AntonJGPucheJCVallesAGPasseriniEinventorsLipotecSAassigneeComposition for the prevention and treatment of cellulitis United States patent US 20070031516 A1282007
- Cals-GriersonMMModulation of activity of the adipocyte aquaglyceroporin channel by plant extractsInt J Cosmet Sci200729171418489306
- MitaniHRyuASuzukiTYamashitaMArakaneKKoideCTopical application of plant extracts containing xanthine derivatives can prevent UV-induced wrinkle formation in hairless micePhotodermatol Photoimmunol Photomed2007232–3869417523930
- RamiroEFranchACastelloteCFlavonoids from Theobroma cacao down-regulate inflammatory mediatorsJ Agric Food Chem200553228506851116248545
- SanbongiCOsakabeNNatsumeMTakizawaTGomiSOsawaTAntioxidative polyphenols isolated from Theobroma cacaoJ Agric Food Chem199846245445710554262
- GorouhiFMaibachHIRole of topical peptides in preventing or treating aged skinInt J Cosmet Sci200931532734519570099
- GruchlikAJurzakMChodurekEDzierzewiczZEffect of Gly-Gly-His, Gly-His-Lys and their copper complexes on TNF-alpha-dependent IL-6 secretion in normal human dermal fibroblastsActa Pol Pharm20126961303130623285694
- HussainMGoldbergDJTopical manganese peptide in the treatment of photodamaged skinJ Cosmet Laser Ther20079423223618236243
- KangYAChoiHRNaJICopper-GHK increases integrin expression and p63 positivity by keratinocytesArch Dermatol Res2009301430130619319546
- ZhangLFallaTJCosmeceuticals and peptidesClin Dermatol200927548549419695481
- ThieleJJEkanayake-MudiyanselageSVitamin E in human skin: organ-specific physiology and considerations for its use in dermatologyMol Aspects Med2007285–664666717719081
- KatoETakahashiNImprovement by sodium dl-α-tocopheryl-6-O-phosphate treatment of moisture-retaining ability in stratum corneum through increased ceramide levelsBioorg Med Chem201220123837384222579618
- De PascaleMCBassiAMPatroneVVillacortaLAzziAZinggJMIncreased expression of transglutaminase-1 and PPARgamma after vitamin E treatment in human keratinocytesArch Biochem Biophys200644729710616530159
- JainSKPalmerMThe effect of oxygen radicals metabolites and vitamin E on glycosylation of proteinsFree Radic Biol Med19972245935969013122
- Muta-TakadaKTeradaTYamanishiHCoenzyme Q10 protects against oxidative stress-induced cell death and enhances the synthesis of basement membrane components in dermal and epidermal cellsBiofactors200935543544119753652
- InuiMOoeMFujiiKMatsunakaHYoshidaMIchihashiMMechanisms of inhibitory effects of CoQ10 on UVB-induced wrinkle formation in vitro and in vivoBiofactors2008321–423724319096121
- HoppeUBergemannJDiembeckWCoenzyme Q10, a cutaneous antioxidant and energizerBiofactors199992–437137810416055
- PrahlSKueperTBiernothTAging skin is functionally anaerobic: importance of coenzyme Q10 for anti aging skin careBiofactors2008321–424525519096122
- Grether-BeckSFelsnerIBrendenHUrea uptake enhances barrier function and antimicrobial defense in humans by regulating epidermal gene expressionJ Invest Dermatol201213261561157222418868
- HagemannIProkschETopical treatment by urea reduces epidermal hyperproliferation and induces differentiation in psoriasisActa Derm Venereol19967653533568891006
- ZhuKZhouHQianHAntioxidant and free radical-scavenging activities of wheat germ protein hydrolysates (WGPH) prepared with alcalaseProcess Biochemistry200641612961302
- SarafSSahuSKaurCDSarafSComparative measurement of hydration effects of herbal moisturizersPharmacognosy Res20102314615121808557
