Abstract
Introduction
Skin changes are among the most visible signs of aging. Skin properties such as hydration, elasticity, and antioxidant capacity play a key role in the skin aging process. Skin aging is a complex process influenced by heritable and environmental factors. Recent studies on twins have revealed that up to 60% of the skin aging variation between individuals can be attributed to genetic factors, while the remaining 40% is due to non-genetic factors. Recent advances in genomics and bioinformatics approaches have led to the association of certain single nucleotide polymorphisms (SNPs) to skin properties. Our aim was to classify individuals based on an ensemble of multiple polymorphisms associated with certain properties of the skin for providing personalized skin care and anti-aging therapies.
Methods and results
We identified the key proteins and SNPs associated with certain properties of the skin that contribute to skin aging. We selected a set of 13 SNPs in gene coding for these proteins which are potentially associated with skin aging. Finally, we classified a sample of 120 female volunteers into ten clusters exhibiting different skin properties according to their genotypic signature.
Conclusion
This is the first study that describes the actual frequency of genetic polymorphisms and their distribution in clusters involved in skin aging in a Caucasian population. Individuals can be divided into genetic clusters defined by genotypic variables. These genotypic variables are linked with polymorphisms in one or more genes associated with certain properties of the skin that contribute to a person’s perceived age. Therefore, by using this classification, it is possible to characterize human skin care and anti-aging needs on the basis of an individual’s genetic signature, thus opening the door to personalized treatments addressed at specific populations. This is part of an ongoing effort towards personalized anti-aging therapies combining genetic signatures with environmental and life style evaluations.
Introduction
Skin aging
As developed countries grow older, scientists are trying to gain insight into the molecular and physiological events involved in the skin aging process. Skin changes are among the most visible signs of senescence, and have garnered more interest in a society that values youth and beauty.Citation1 In this scenario, the term “perceived age” refers to the age that someone is visually estimated to have in relation to their chronological age. This concept has implications that go beyond mere aesthetics: perceived age is a robust biomarker of aging that predicts survival in certain groups of patientsCitation2 and correlates with important functional and molecular aging phenotypes.Citation3
Research on same-sex twins suggests that up to 60% of the variation in perceived age is influenced by genetics, while the remaining 40% can be explained by non-genetic factors.Citation3,Citation4 Among the latter, smoking and sun exposure are the environmental factors of major importance for premature skin wrinkling or facial aging.Citation5 Furthermore, a direct effect of both smoking and light exposure on overall health status has been documented.Citation6
Ethnicity and sex provide obvious evidence of genetic influence on skin aging. Wrinkling in Asians tends to occur later and with less severity than in Caucasians,Citation7 while another study revealed that dryness of the skin is higher in African-American and Caucasian women.Citation8 But the most explicit difference between ethnic groups is skin color, with more darkly pigmented subjects retaining younger skin properties.Citation9 On the other hand, physiological dissimilarities between men and women also highlight the role of genetics in skin aging. For example, male skin is more sensitive to environmental aggressors, particularly ultraviolet light exposure.Citation10
Intrinsic and extrinsic factors converge in modifying certain biochemical properties that, in the end, are responsible for progressive skin decay. Significant changes driving this process are oxidative stress,Citation11 lack of elasticity, and reduced hydration.Citation12 Oxidative damage arises as a consequence of sun exposure and metabolic generation of free radicals and leads to loss of dermal cells and the extracellular matrix. Although elastic proteins in the matrix are long-lived, they also accumulate damage via crosslinking and other mechanisms. With the degeneration of glycolipids that maintain skin hydration, skin suffers severe loss of water.
Sequencing of the human genome and subsequent developments in genomics have provided new tools with which to investigate these factors at the molecular level, while bioinformatics allows an integrated analysis of the huge amount of data that are being generated.Citation13 At the same time, new techniques in genomic expression profiling are able to characterize individual genotypes, thereby opening the door to personalized dermatologic therapy.Citation14
Single nucleotide polymorphisms (SNPs) occur when single bases in genes are changed or deleted, which may result in an amino acid change at a specific position and a change in phenotype. Numerous physiological states or diseases have been correlated with occurrence of SNPs associated with particular genes in the genome of a human who exhibits the physiological states or disease. For example, manganese superoxide dismutase (SOD2V16A) polymorphism has been shown to be associated with cancer risk,Citation15 and matrix metalloproteinases-2 (735C/T and 1306C/T) polymorphisms with the development of glaucoma.Citation16 SNPs have also been associated with skin features. Han et al identified novel SNPs associated with skin pigmentation and hair color.Citation17 Further supporting this, a recent genome-wide association study reported the role of STXBP5L gene variants in photoagingCitation18 or the association of mutations in genes coding for antioxidant response with the predisposition to higher oxidative stress, leading to accelerated deterioration.Citation19 But, we still lack information regarding the identification, frequency, and clustering of gene variants related to main functions of aging skin, such as antioxidative capacity, collagen metabolism and structure (elasticity), and natural hydration capacity in a healthy Caucasian population.
Taking advantage of the recent advances in genomics and bioinformatics, we describe herein how main skin functions can be classified according to genotypic signature and how pertinent personalized corrective treatments could be designed. With this aim, we classified a normal population into clusters according to the distribution of polymorphisms in genes associated with antioxidant capacity, hydration, and elasticity of the skin. As more precise methods providing personalized dermatological and anti-aging treatments are needed, skin therapies adapted to each one of these clusters could then be specifically designed. This work represents part of the technologies developed by the authors towards genetically personalized dermocosmetics treatments.
Methods and results
Characterization of skin aging at the gene and protein level
Aged skin is characterized, among other features, by dehydration, loss of elasticity, and increased oxidative damage.Citation11,Citation12 These three main skin properties were selected because they are strongly related to well-known metabolic pathways, and because many other apparent skin properties, such as wrinkle extension and depth, can be attributed to one of these three (in this case, wrinkles can be attributed to loss of elasticity and collagen metabolism). Accordingly, through an extensive literature search, we identified the genes that encode proteins involved in skin hydration, skin elasticity, and antioxidant capacity of the skin. A total of 72 proteins were identified: the group of proteins related to skin elasticity is composed of 39 proteins, including matrix metalloproteases involved in collagen and elastin metabolism; the hydration group is composed of six proteins, mainly aquaporins and proteoglycans; and the group related to antioxidant capacity of the skin is composed of 27 proteins, including superoxide dismutase, catalase, and nicotinamide adenine dinucleotide phosphate-oxidase.
Selection of polymorphisms associated with skin aging
Among all the 72 proteins involved in skin aging, we further identified which proteins have associated SNPs in their genes that influence the corresponding protein product expression or functionality. A total of 13 SNPs in ten proteins were selected, NAD(P)H dehydrogenase [quinone] 1,Citation20 matrix metalloproteinase-1,Citation21 superoxide dismutase II,Citation22,Citation23 nuclear factor erythroid 2-related factor 2,Citation24–Citation26 glutathione peroxidase 1,Citation27 catalase,Citation28 matrix metalloproteinase-3,Citation29 interleukin-6,Citation30 matrix metalloproteinase-9,Citation31 and aquaporin-3.Citation32 The effect of the SNP on protein expression or activity was categorized as +1, which indicates that the SNP increases the protein expression or activity, or −1, which indicates that the SNP decreases protein expression or activity ().
Table 1 List of selected polymorphisms in proteins associated with skin aging
Moreover, in order to study SNPs that are frequent in Caucasian populations, we checked the previously described population frequencies for these SNPs in NCBI database (http://www.ncbi.nlm.nih.gov/) in a Caucasian population. Genetic variants were selected as having a described population frequency higher than 10%.
Study population
A noninterventional, epidemiological, cross-sectional study was conducted in 120 female volunteers. The epidemiologic study was conducted at the Hospital of Nisa, Valencia, Spain. The study received the approval of the Autonomic Ethics Committee of Clinical Studies of Drugs and Medical Devices of the Valencian Community in Spain (CAEC).
Eligibility criteria for the study
Participants in the study were women aged between 41 and 49 years old, of any skin type, who regularly attended the dermatology clinic for dermo-aesthetic purposes, and who had a predominantly urban lifestyle. They had to have full capability to understand and sign the informed consent document with free will. Prospective participants were excluded if they were exposed to external factors that posed a serious risk for skin health or suffered from severe skin disorders.
Sample collection
Saliva samples were collected from study subjects by using DNA collection tubes (DNA Genotek Inc., Ottawa, ON, Canada). The samples were sent to Progenika Biopharma SA (Vizcaya, Spain) and to Centro Nacional de Genotipado (Santiago de Compostela, Spain) for the genotyping of selected SNPs by using Affymetrix (Santa Clara, CA, USA) and Sequenom, Inc. (San Diego, CA, USA) technologies.
In brief, DNA was extracted using ORAGENE (DNA Genotek Inc.) extraction reagent following the indications of the supplier. Quality and quantity DNA from saliva samples were assessed in a NanoDrop (Thermo Fisher Scientific, Waltham, MA, USA) spectrophotometer. Allele-specific probes were designed in order to discriminate different genotypes by means of fluorescence detection in real-time quantitative polymerase chain reaction for every SNP, except for rs3918242. The rs3918242 polymorphism was analyzed by polymerase chain reaction followed by restriction fragment length polymorphism analysis as described.Citation33
Classification of individuals based on an ensemble of multiple genetic polymorphisms associated with certain properties of the skin
A total of 13 SNPs in genes associated with certain properties of the skin (phenotypes) were considered for the analysis (). The detailed population frequency of the analyzed SNPs is as described in .
Table 2 Single nucleotide polymorphisms and variant frequencies in the sample population
Our next goal was to identify if the selected SNPs could define genetic groups or clusters in the study sample, and to see if these genetic groups could be linked to biochemical and metabolic skin properties. As it has been described, each SNP gives rise to two different genotypes with phenotype consequences, and we assigned a binary effect to each SNP. Thus, the variant contributing positively to the skin property was given a value of +1, whereas the variant not contributing positively to the skin property was given a value of −1.
The genetic variants were clustered using the following criteria: 1) obtaining a total number of clusters between two and 20; and 2) identifying genetic clusters that explain the differences in the effects of the SNP variants on the biochemical and metabolic properties of the skin.
The genetic clustering analysis was conducted by using the k-means technique for genetic cluster analysis as described elsewhere.Citation34
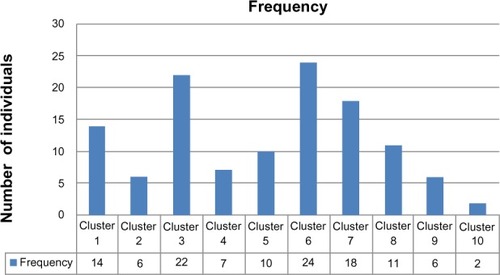
The conducted analysis shows that the sample can be optimally classified into ten genetic clusters, each with its own genetic signature, as shown in . Each one of the SNPs exerts a specific and differential contribution on each of the ten genetic clusters identified. For example, SNP rs1800566 is very relevant for the definition of clusters 9 and 10 (contribution of 100% to both), whereas it is less relevant for the definition of clusters 4 and 5 (contribution of 16% and 29%, respectively). Each genetic cluster has its own combination of relevant SNPs. For example, cluster 1 is different from cluster 2, mainly because of the differential contribution of SNPs rs6721961 (46% versus 100%), rs1050450 (35% versus 76%), rs1001179 (18% versus 76%), rs1800795 (73% versus 25%), or rs17553719 (50% versus 100%). A given individual can then be classified into one of the ten genetic clusters according to his/her genetic signature. The frequency distribution of the 120 females of the study population in the ten genetic clusters is shown in .
Table 3 Contribution of individual polymorphisms to the definition of each genetic cluster
As shown in , each of the SNPs contributes to the expression and/or activity of certain proteins linked with skin properties, with an effect described as +1 (positive for skin property) or −1 (negative for skin property). Consequently, we were able to describe the contribution of the genetic profile of each cluster to each one of the three skin properties (antioxidant capacity, elasticity and collagen structure, and natural hydration ability). To conduct this calculation, we considered only polymorphisms with a contribution of over 75% to the group, and SNPs that exhibited variability between genotypic groups. For that reason, SNP rs1141718 was discarded for further analysis as it was predominant in all the study population. The values in for each skin property and each cluster show the magnitude of the effect, by simple addition of the individual effect values of the relevant SNPs in the genetic group to that property. The plus sign indicates that the ensemble of the genotypic variants in the group has a positive effect on the skin property, and the minus sign indicates that it has a negative effect. The higher the value, the higher will be the effect on that skin property. It should be noted that certain SNP variants in different polymorphisms for the same property can cancel each other out. For example, if the variant C:C of rs1800566 (effect +1) and the variant T:T of rs4880 (effect −1) appear as relevant in the same cluster, the net effect value for antioxidant capacity is 0.
Table 4 Overall contribution of the set of polymorphisms that define a genotypic group on skin properties involved in aging
Based on the SNP contribution and particular effect to each one of the genetic groups, we can further describe the genetic clusters as follows (only relevant features are highlighted for each group):
Cluster 1: genetic susceptibility to loss of elasticity of the skin due to polymorphisms in genes that encode matrix metalloproteinase-3 and matrix metalloproteinase-9.
Cluster 2: genetic susceptibility to oxidative damage mainly due to polymorphisms in genes that encode NAD(P)H dehydrogenase [quinone] 1, nuclear factor erythroid 2-related factor 2, glutathione peroxidase 1, and catalase, together with a genetic capability to maintain the appropriate hydration levels of the skin due to polymorphism in genes for aquaporin-3.
Cluster 3: genetic capability to maintain the appropriate hydration levels of the skin due to polymorphism in genes for aquaporin-3.
Cluster 4: genetic susceptibility to oxidative stress damage of the skin due to polymorphism in genes that encode glutathione peroxidase 1 protein, together with a genetic capability for appropriate elasticity of the skin due to polymorphism in genes for matrix metalloproteinase-9 and matrix metalloproteinase-3.
Cluster 5: genetic susceptibility to oxidative stress damage of the skin due to polymorphism in genes for nuclear factor erythroid 2-related factor 2.
Cluster 6: genetic susceptibility to loss of elasticity of the skin due to polymorphisms in genes that encode matrix metalloproteinase-3.
Cluster 7: genetic capability for appropriate skin elasticity and antioxidant capacity due to polymorphisms in genes that encode matrix metalloproteinase-9, superoxide dismutase II, and nuclear factor erythroid 2-related factor 2.
Cluster 8: genetic susceptibility to oxidative stress damage of the skin due to polymorphism in genes that encode nuclear factor erythroid 2-related factor 2, NAD(P)H dehydrogenase [quinone] 1 protein, glutathione peroxidase 1, and catalase.
Cluster 9: genetic capability for good skin elasticity due to polymorphisms in genes for matrix metalloproteinase-9.
Cluster 10: genetic capability against oxidative damage due to polymorphisms in genes that encode nuclear factor erythroid 2-related factor 2, superoxide dismutase II, and catalase, together with a genetic susceptibility for loss of elasticity and hydration of the skin due to polymorphisms in genes that encode matrix metalloproteinase-9, interleukin-6, matrix metalloproteinase-1, and aquaporin-3.
Discussion
This is the first study that describes the actual frequency of genetic polymorphisms involved in skin aging in a Caucasian population. We identified 13 SNPs in genes coding for proteins that play a role in skin properties associated with aging, namely, oxidative stress, elasticity, and hydration. The genetic polymorphism frequency and the SNP clustering analysis in the study sample allowed us to classify the 120 female volunteers into ten genotypic groups or clusters. Taking into account the different protein functionalities from each one of the genetic variants as described in the literature (), a global effect was assigned to each cluster on the basis of the collective contribution of the set of polymorphisms to the biochemical and metabolic properties of the skin.
This clustering analysis suggests that different skin care needs depend on the naturally occurring single genetic variants present in each one of the genetic clusters. For example, cluster 8 shows the worst combination of antioxidant genes, having the weakest versions of NAD(P)H dehydrogenase [quinone] 1 protein, glutathione peroxidase 1, and catalase, which are enzymes linked with the antioxidant pathways, whereas cluster 10 shows a good genetic capability against oxidative damage due to good versions of genes for nuclear factor erythroid 2-related factor 2, superoxide dismutase II, and catalase. Thus, cosmetic products or therapies for individuals from cluster 8 should be more concentrated in antioxidative ingredients than the cosmetics aimed at those in cluster 10 – not only because cluster 8 seems to present a reduced natural antioxidative potential, which is why they would need an antioxidative supplement, but because the skin of individuals in cluster 10, which has a good natural antioxidative capacity, could be damaged by an excess of antioxidative ingredients (the genetic natural plus using cosmetics with even more antioxidative capacity). It is clear, then, that each one of the different genetic clusters shows a unique combination of needs for each one of the three main studied features. We suggest that it is not only necessary to supplement the lack or low activity of certain skin functions, but also that the excess of active ingredients in cosmetics or skin therapies can even be harmful for those individuals whose genetic background already provides strong natural capabilities.
In consequence, we suggest that genomic analysis can contribute to the characterization of human skin care and anti-aging needs, by conducting a simple genetic test and assigning an individual to one of the ten genetic groups.
Study limitations
This study presents the following limitation: only genetic variants with sufficiently described effects on skin properties were included for analysis. Other well-known variants related to skin aging but which do not have a strong genetic background or expected variability in a normal population have not been considered; for example, synthesis of elastic fibers or hyaluronan, or other skin matrix or cellular components known to be linked with skin aging processes. Once well-established genetic and phenotypic information about these processes are available, they could be included in further analyses.
This study nevertheless, for the first time, sets the ground for the description of the actual prevalence of a number of the main relevant genetic variants in a normal Caucasian population. We suggest that, in order to complete the skin aging evaluation and to apply corrective measures when needed, it is necessary to combine genetic analysis with direct evaluation of skin needs.
Conclusion
The use of genetic signature for the identification of skin care individual requirements opens the door to personalized treatments for specific populations. With genotypic services becoming increasingly affordable, this perspective could be a reality in the not-so-distant future. Suggestions for further research include the discovery of new proteins associated with skin aging, additional polymorphisms that modify their activity or expression, and epigenetic modifications of DNA affecting gene regulation. One can even envisage having the complete DNA sequence of an individual available as an aid to designing a personalized skin care and anti-aging treatment. Authors are currently developing new avenues for personalized dermo-aesthetics by exploring new genetic signatures combined with enviromental and lifestyle factors.
Disclosure
The authors report no conflicts of interest in this work.
References
- SarwerDBGrossbartTADidieERBeauty and societySemin Cutan Med Surg2003222799212877227
- SaeedMBerlinRMCruzTDExploring the utility of genetic markers for predicting biological ageLeg Med (Tokyo)201214627928522770678
- ChristensenKThinggaardMMcGueMPerceived age as clinically useful biomarker of ageing: cohort studyBMJ2009339b526220008378
- ShekarSNLucianoMDuffyDLMartinNGGenetic and environmental influences on skin pattern deteriorationJ Invest Dermatol200512561119112916354181
- RexbyeHPetersenIJohansensMKlitkouLJeuneBChristensenKInfluence of environmental factors on facial ageingAge Ageing200635211011516407433
- FarageMAMillerKWElsnerPMaibachHIIntrinsic and extrinsic factors in skin ageing: a reviewInt J Cosmet Sci2008302879518377617
- MakrantonakiEBekouVZouboulisCCGenetics and skin agingDermatoendocrinol20124328028423467395
- DiridollouSde RigalJQuerleuxBLeroyFHolloway BarbosaVComparative study of the hydration of the stratum corneum between four ethnic groups: influence of ageInt J Dermatol200746Suppl 1111417919198
- RawlingsAVEthnic skin types: are there differences in skin structure and function?Int J Cosmet Sci2006282799318492142
- OblongJEMale skin care: shaving and moisturization needsDermatol Ther201225323824322913441
- PoljšakBDahmaneRGGodićAIntrinsic skin aging: the role of oxidative stressActa Dermatovenerol Alp Panonica Adriat20122123336
- NaylorECWatsonRESherrattMJMolecular aspects of skin ageingMaturitas201169324925621612880
- RobinsonMKBinderRLGriffithsCEGenomic-driven insights into changes in aging skinJ Drugs Dermatol20098Suppl 7s8s1119623778
- RizzoAEMaibachHIPersonalizing dermatology: the future of genomic expression profiling to individualize dermatologic therapyJ Dermatolog Treat201223316116721254882
- KangDLeeKMParkSKFunctional variant of manganese superoxide dismutase (SOD2 V16A) polymorphism is associated with prostate cancer risk in the prostate, lung, colorectal, and ovarian cancer studyCancer Epidemiol Biomarkers Prev20071681581158617646272
- KaminskaABanas-LezanskaPPrzybylowskaKThe protective role of the -735C/T and the -1306C/T polymorphisms of the MMP-2 gene in the development of primary open-angle glaucomaOphthalmic Genet2014351414623725205
- HanJKraftPNanHA genome-wide association study identifies novel alleles associated with hair color and skin pigmentationPLoS Genet200845e100007418483556
- Le ClercSTaingLEzzedineKA genome-wide association study in Caucasian women points out a putative role of the STXBP5L gene in facial photoagingJ Invest Dermatol2013133492993523223146
- OsborneRHakozakiTLaughlinTFinlayDRApplication of genomics to breakthroughs in the cosmetic treatment of skin ageing and discolorationBr J Dermatol2012166Suppl 2161922670614
- FischerASchmelzerCRimbachGNiklowitzPMenkeTDöringFAssociation between genetic variants in the Coenzyme Q10 metabolism and Coenzyme Q10 status in humansBMC Res Notes2011424521774831
- RutterJLMitchellTIButticèGA single nucleotide polymorphism in the matrix metalloproteinase-1 promoter creates an Ets binding site and augments transcriptionCancer Res19985823532153259850057
- HiroiSHaradaHNishiHSatohMNagaiRKimuraAPolymorphisms in the SOD2 and HLA-DRB1 genes are associated with nonfamilial idiopathic dilated cardiomyopathy in JapaneseBiochem Biophys Res Commun1999261233233910425186
- BorgstahlGEPargeHEHickeyMJHuman mitochondrial manganese superoxide dismutase polymorphic variant Ile58Thr reduces activity by destabilizing the tetrameric interfaceBiochemistry19963514428742978605177
- ArisawaTTaharaTShibataTThe influence of promoter polymorphism of nuclear factor-erythroid 2-related factor 2 gene on the aberrant DNA methylation in gastric epitheliumOncol Rep200819121121618097597
- GuanCPZhouMNXuAEThe susceptibility to vitiligo is associated with NF-E2-related factor2 (Nrf2) gene polymorphisms: a study on Chinese Han populationExp Dermatol200817121059106218537816
- MarzecJMChristieJDReddySPFunctional polymorphisms in the transcription factor NRF2 in humans increase the risk of acute lung injuryFASEB J20072192237224617384144
- ShuvalovaYAKaminnyiAIMeshkovANKukharchukVVPro198Leu polymorphism of GPx-1 gene and activity of erythrocytic glutathione peroxidase and lipid peroxidation productsBull Exp Biol Med2010149674374521165435
- ForsbergLLyrenäsLde FaireUMorgensternRA common functional C-T substitution polymorphism in the promoter region of the human catalase gene influences transcription factor binding, reporter gene transcription and is correlated to blood catalase levelsFree Radic Biol Med200130550050511182520
- SouslovaVTownsendPAMannJAllele-specific regulation of matrix metalloproteinase-3 gene by transcription factor NFkappaBPLoS One201053e990220360864
- FishmanDFauldsGJefferyRThe effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritisJ Clinical Invest19981027136913769769329
- ZhangBYeSHerrmannSMFunctional polymorphism in the regulatory region of gelatinase B gene in relation to severity of coronary atherosclerosisCirculation199999141788179410199873
- HaraMMaTVerkmanASSelectively reduced glycerol in skin of aquaporin-3-deficient mice may account for impaired skin hydration, elasticity, and barrier recoveryJ Biol Chem200227748466164662112270942
- ChenHYLinWYChenYHChenWCTsaiFJTsaiCHMatrix metalloproteinase-9 polymorphism and risk of pelvic organ prolapse in Taiwanese womenEur J Obstet Gynecol Reprod Biol2010149222222420144500
- BishopCMNeural Networks for Pattern RecognitionOxfordClarendon Press1995