Abstract
Background
Pyoderma gangrenosum (PG) is a rare dermatological condition characterized by the rapid progression of a painful, necrolytic ulcer with an irregular, undermined border and commonly affects the lower extremities, mainly in the pretibial area. The diagnosis of PG is not easy. Due to lack of diagnostic laboratory test and histopathological findings indicative of PG, it is often misdiagnosed as an infection. This results in delayed or inappropriate treatment of the condition, which leads to devastating consequences such as limb amputation and death.
Main observations
We report a rare case of a 51-year-old female who was initially diagnosed as having infected ulcers and underwent serial debridements, which resulted in extensive PG at three different sites (abdominal, left thigh, and sacral).
Conclusion
This case highlights the challenges in diagnosing PG, emphasizes the key clinical features to aid diagnosis, and the clinical consequences of delayed or misdiagnosis of this condition.
Introduction
Pyoderma gangrenosum (PG) is a rare dermatological condition characterized by the rapid progression of a painful, necrolytic ulcer with an irregular, undermined border and commonly affects the lower extremities, mainly in the pretibial area.Citation1 In 70% of cases, PG is associated with an underlying disease such as inflammatory bowel disease, inflammatory arthritis, and hematological malignancies.Citation2–Citation4 The etiology of PG is unclear but is thought to be a reactive inflammatory dermatosis and part of the spectrum of neutrophilic dermatosis. More recently, new disease entities such as PASH syndrome (PG, acne, and suppurative hidradenitis) and PAPA syndrome (pyogenic arthritis, PG, and acne) are described in the literature as part of the spectrum of autoinflammatory disorders distinct from allergic, autoimmune, and infectious processes.Citation5 PAPA syndrome is associated with genetic mutations involved in innate immune response regulation,Citation6 while no genetic links have been detected in patients with PASH syndrome.Citation5
PG is a diagnosis of exclusion and can only be made after common causes of ulcers such as infection and malignancy have been ruled out. However, the management of suspected infected or malignant ulcers may require debridement or excision, which can exacerbate PG through a process called pathergy (a term used to describe an exaggerated skin injury occurring after trauma).Citation1 We report a case of a patient who developed extensive PG at multiple sites as a result of serial debridements for suspected infected ulcers, highlighting the challenges in diagnosing PG and the clinical consequences of misdiagnosis or delayed diagnosis.
Case report
A 51-year-old female initially presented with infected abdominal, thigh, and sacral ulcers. Her ulcers developed as a result of poor self-care and hygiene. Her past medical history included type 2 diabetes, hypertension, hypercholesterolemia, and back pain. Physical examination revealed an abdominal ulcer measuring 4 cm × 2 cm in the right iliac fossa with surrounding erythema, a small ulcer on her left thigh (1 cm × 1 cm), and two sacral ulcers (7 cm × 3 cm and 4.5 cm × 2 cm, respectively). Ulcer cultures revealed skin flora. Although she did not have any systemic features of infection, blood investigations on admission showed an elevated white cell count of 14.9×109/L (reference interval, 4.0×09−11.0×109/L) and C-reactive protein of 120 mg/L (reference interval, 0–10 mg/L). She was subsequently commenced on 2 g of empirical intravenous flucloxacillin four times daily.
In the next few weeks, all her wounds worsened despite wound dressings and antibiotics. Her abdominal and thigh ulcers had increased in size. The size of her two sacral ulcers remained the same but had increased wound necrosis. One month after admission, her abdominal ulcer measured 21 cm × 7 cm, and the thigh ulcer was 4 cm × 1 cm. In view of this, surgical excisions of the necrotic areas were performed. Vacuum-assisted closure dressings were applied to the anterior abdomen and sacrum.
Three days later, the patient underwent a routine vacuum-assisted closure dressing change. It was noted that her wounds had worsened rapidly since the last debridement. The once healthy wound edges had become necrotic. Further debridement was performed. Excised tissues sent off for microscopy and culture grew methicillin-sensitive Staphylococcus aureus and Enterobacter aerogenes. Intravenous tazocin (4.5g 8 hourly) was added for suspected postoperative wound infection.
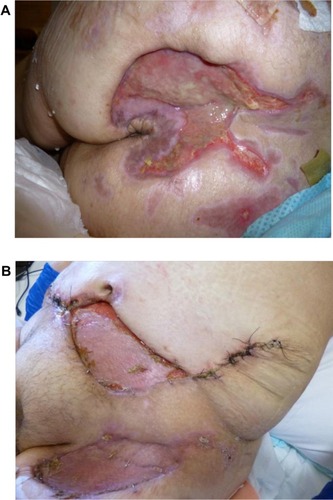
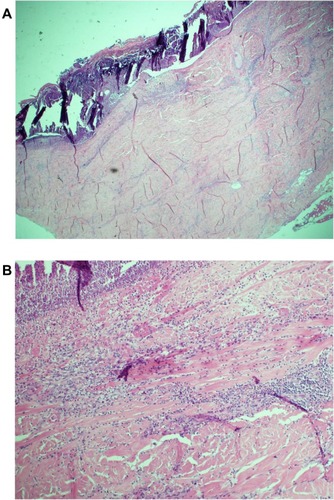
The patient’s wounds continued to deteriorate with antibiotics and further debridements. After the third debridement, the abdominal ulcer had increased to a size of 32 cm × 11 cm, and the two sacral ulcers became a huge sacral defect extending into the perianal region ( and ). Cultures of subsequently debrided tissue were negative for bacteria and fungi. Repeated histological sections of sacral and abdominal tissues revealed neutrophilic infiltrates extending into deep dermis and no evidence of malignancy ( and ). Antinuclear antibodies, extractable nuclear antigens, rheumatoid factor, and anti-neutrophil cytoplasmic antibodies were all negative. A diagnosis of PG was made, and our patient was commenced on 50 mg of prednisolone daily. After 1 week, her wounds showed slow improvement, with resolution of necrotic wound edges and formation of granulation tissue. Two weeks later, she developed steroid-induced hyperglycemia, and 500 mg twice daily of mycophenolate mofetil was added as a steroid sparing agent. Her prednisolone dose was gradually tapered by 5 mg every 2 weeks, and the dose of mycophenolate mofetil increased to 1 g twice daily. Four months after admission, the patient underwent successful split skin grafts and primary closure to her abdominal and sacral ulcers while on immunosuppressive therapy ( and ). At 3-month follow-up, there was no recurrence of the disease.
Figure 1 (A) Sacral pyoderma gangrenosum after multiple debridements. (B) Huge abdominal and left thigh wound defect with typical violaceous borders post-debridement.
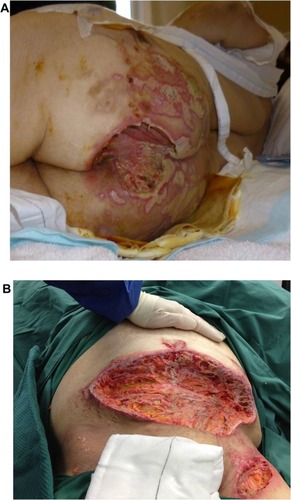
Figure 2 Histology of incisional biopsy of abdominal ulcer. (A) Extensive skin ulceration is shown (H&E stain; original magnification ×10). (B) Higher-powered view of ulcer being covered by inflamed crust and infiltrated by a heavy collection of acute inflammatory cells (neutrophils) (H&E stain; ×20).
Discussion
PG was first described more than a century ago by Louis Brocq, a French dermatologist.Citation7 He reported a series of patients with typical features of PG and described the appearance of their ulcers. Today, PG still remains a clinical diagnosis. There are no diagnostic laboratory investigations. Histology findings are not specific but can serve to exclude infection, vasculitis, and malignancy.Citation1
To aid the recognition of PG, Su et alCitation1 proposed a set of diagnostic criteria for ulcerative PG. Diagnosis requires both major criteria and at least two minor criteria. The major criteria include the characteristic appearance of a painful, irregular ulcer with a violaceous border, and exclusion of other causes of ulceration such as malignancy, vasculitis, primary infection, and drugs. The minor criteria include history of pathergy, associated systemic illness, histology findings of dermal neutrophilia, and response to steroid treatment. Other diagnostic criteria for variants of PG, namely bullous, pustular, and vegetative PG, have also been described.Citation4,Citation8,Citation9
A majority of the PG cases were often misdiagnosed as infection resulting in repeated surgical debridement.Citation10–Citation12 Unfortunately, surgical debridement worsens PG through a phenomenon called pathergy. Diagnosis of PG is often made after failure of initial treatment and absence of Gram stain on culture. Furthermore, the presence of secondary infections can obscure the diagnosis of this condition.
Diagnosis of PG in our patient was extremely difficult, made more complicated by the fact that her initial tissue cultures grew Staphylococcus and Enterobacter. As a result, she was placed on antibiotics and underwent repeated surgical debridement for a presumed diagnosis of a wound infection. Diagnosis of PG was considered only after repeated biopsies revealed dermal neutrophilia, failure of treatment with broad-spectrum antibiotics, and most importantly, the wound exhibited pathergy following debridement. However, multiple debridements of her ulcers and the delay in instituting appropriate treatment led to extensive ulcerations and prolonged recovery for the patient.
Currently, there are no standard guidelines for the treatment of PG. Marzano et alCitation13 recently published a therapeutic algorithm based on the clinical extent of the lesions. However, due to its small sample size, larger controlled trials are needed to confirm the effectiveness of their algorithm. In general, the treatment of PG involves both topical and systemic approaches. Topical therapy involves the use of dressings to control exudation, improve auto-debridement, protect the surrounding skin, and provide analgesia.Citation14 Deep wounds may benefit from vacuum sealing as it improves granulation.Citation15 The topical use of antimicrobials is not recommended as they may irritate the ulcers and surrounding skin. Topical tacrolimus has been shown to be effective in the treatment of less severe PG.Citation16
Systemic therapy is the mainstay of treatment for rapidly progressive PG. Corticosteroids such as prednisolone are initially used to prevent progression and arrest the inflammatory process. Combinations of steroid and cytotoxic drugs such as azathioprine, cyclophosphamide, cyclosporin, or mycophenolate mofetil are used in patients with steroid-resistant disease or as a steroid-sparing measure.Citation14 In the presence of secondary infections, corticosteroids should be combined with antibiotics.Citation4 Recently, tumor necrosis factor (TNF)-α blockers and other biologics have been used with some success.Citation17,Citation18 Infliximab, a monoclonal antibody against TNF-α, has been particularly effective in treating PG associated with inflammatory bowel disease.Citation13 Surgical debridement is contraindicated due to pathergy.Citation19 Surgical treatments such as skin grafts may be worthwhile but can only be performed with concomitant immunosuppression and in patients with stable disease or partial remission.Citation15
Despite the advances in medical therapy, the outlook of PG is unpredictable. Delayed diagnosis or misdiagnosis of PG will certainly lead to exacerbation and progression of the disease. In our case, misdiagnosis of the patient’s condition resulted in multiple debridements of her ulcers and a delay in instituting appropriate treatment. The outcome was extensive ulcerations and prolonged hospital recovery. Our patient still requires long-term follow-up to monitor for recurrence of PG and further evaluation to exclude genetic mutations associated with the spectrum of autoinflammatory syndromes.
Conclusion
PG is a rare occurrence and often proves to be a diagnostic challenge. The presence of a rapidly progressing, necrotizing ulcer and a history suggestive of pathergy are key features of PG. It is also important to reconsider a diagnosis when the disease process worsens or does not respond to treatment. A dermatology opinion should be obtained if there is any uncertainty regarding the diagnosis.
Disclosure
The authors report no conflicts of interest in this work.
References
- SuWPDDavisMDPWeenigRHPyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteriaInt J Dermatol2004431179080015533059
- GreensteinAJJanowitzHDSacharDBThe extraintestinal complications of Crohn’s disease and ulcerative colitis: a study of 700 patientsMedicine (Baltimore)197655401412957999
- StolmanLPRosenthalDYaworskyRPyoderma gangrenosum and rheumatoid arthritisArch Dermatol197511110201023786171
- PerryHOWinkelmannRKBullous pyoderma gangrenosum and leukemiaArch Dermatol19721069019054508849
- Braun-FalcoMKovnerystyyOLohsePRuzickaTPyoderma gangrenosum, acne, and suppuratives hidradenitis (PASH) – a new autoinflammatory syndrome distint from PAPA syndromeJ Am Acad Dermatol201266408415
- WiseCAGillumJDSeidmanCEMutations in CD2BP1 disrupt binding to PTP PEST and are responsible for PAPA syndrome, an autoinflammatory disorderHum Mol Genet20021196196911971877
- BrocqLA new contribution to the study of geometric phagedenismAnn Dermatol Syphiligr19169139
- O’LoughlinSPerryHOA diffuse pustular eruption associated with ulcerative colitisArch Dermatol197811410611064686728
- Wilson-JonesEWinkelmannRKSuperficial granulomatous pyoderma: a localized vegetative form of pyoderma gangrenosumJ Am Acad Dermatol1988185115213351014
- BennettCRBrageMEMassDPPyoderma gangrenosum mimicking postoperative infection in the extremities. A report of two casesJ Bone Joint Surg Am1999811013101810428135
- GreidanusTGHonlBAMore than a hand infection. Pyoderma gangrenosumPostgrad Med200211210710912510450
- Barańska-RybakWKakolMNaesstromMKomorowskaOSokołowska-WojdyłoMRoszkiewiczJA retrospective study of 12 cases of pyoderma gangrenosum: why we should avoid surgical intervention and what therapy to applyAm Surg201177121644164922273223
- MarzanoAVTrevisanVLazzariRCrostiCPyoderma gangrenosum: study of 21 patients and proposal of a ‘clinicotherapeutic classification’J Dermatolog Treat201122525426020666672
- WollinaUClinical management of pyoderma gangrenosumAm J Clin Dermatol20023314915811978136
- FulbrightRKWolfJETschenJAPyoderma gangrenosum at surgery sitesJ Dermatol Surg Oncol1985118838863876359
- MarzanoAVTrevisanVLazzariRCrostiCTopical tacrolimus for the treatment of localized, idiopathic, newly diagnosed pyoderma gangrenosumJ Dermatolog Treat201021314014319903010
- RegueiroMValentineJPlevySFleisherMRLichtensteinGRInfliximab for treatment of pyoderma gangrenosum associated with inflammatory bowel diseaseAm J Gastroenterol20039881821182612907338
- HubbardVGFriedmannACGoldsmithPSystemic pyoderma gangrenosum responding to infliximab and adalimumabBr J Dermatol20051521059106115888172
- BennettMLJacksonJMJorizzoJLPyoderma gangrenosum. A comparison of typical and atypical forms with an emphasis on time to remission. Case review of 86 patients from 2 institutionsMedicine (Baltimore)200079374610670408