Abstract
Acne vulgaris, an epidemic inflammatory skin disease of adolescence, is closely related to Western diet. Three major food classes that promote acne are: 1) hyperglycemic carbohydrates, 2) milk and dairy products, 3) saturated fats including trans-fats and deficient ω-3 polyunsaturated fatty acids (PUFAs). Diet-induced insulin/insulin-like growth factor (IGF-1)-signaling is superimposed on elevated IGF-1 levels during puberty, thereby unmasking the impact of aberrant nutrigenomics on sebaceous gland homeostasis. Western diet provides abundant branched-chain amino acids (BCAAs), glutamine, and palmitic acid. Insulin and IGF-1 suppress the activity of the metabolic transcription factor forkhead box O1 (FoxO1). Insulin, IGF-1, BCAAs, glutamine, and palmitate activate the nutrient-sensitive kinase mechanistic target of rapamycin complex 1 (mTORC1), the key regulator of anabolism and lipogenesis. FoxO1 is a negative coregulator of androgen receptor, peroxisome proliferator-activated receptor-γ (PPARγ), liver X receptor-α, and sterol response element binding protein-1c (SREBP-1c), crucial transcription factors of sebaceous lipogenesis. mTORC1 stimulates the expression of PPARγ and SREBP-1c, promoting sebum production. SREBP-1c upregulates stearoyl-CoA- and Δ6-desaturase, enhancing the proportion of monounsaturated fatty acids in sebum triglycerides. Diet-mediated aberrations in sebum quantity (hyperseborrhea) and composition (dysseborrhea) promote Propionibacterium acnes overgrowth and biofilm formation with overexpression of the virulence factor triglyceride lipase increasing follicular levels of free palmitate and oleate. Free palmitate functions as a “danger signal,” stimulating toll-like receptor-2-mediated inflammasome activation with interleukin-1β release, Th17 differentiation, and interleukin-17-mediated keratinocyte proliferation. Oleate stimulates P. acnes adhesion, keratinocyte proliferation, and comedogenesis via interleukin-1α release. Thus, diet-induced metabolomic alterations promote the visible sebofollicular inflammasomopathy acne vulgaris. Nutrition therapy of acne has to increase FoxO1 and to attenuate mTORC1/SREBP-1c signaling. Patients should balance total calorie uptake and restrict refined carbohydrates, milk, dairy protein supplements, saturated fats, and trans-fats. A paleolithic-like diet enriched in vegetables and fish is recommended. Plant-derived mTORC1 inhibitors and ω-3-PUFAs are promising dietary supplements supporting nutrition therapy of acne vulgaris.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Based on accumulating indirect translational and in vitro evidence, this review presents an update of the dietary impact on acne metabolomics, follicular inflammation, and comedogenesis. The first part links Western diet to disturbed sebaceous lipogenesis promoted by systemic aberrations of endocrine signaling. To understand the role of nutrigenomics in the pathogenesis of acne, two central players will be highlighted: the role of the metabolic transcription factor forkhead box O1A (FoxO1),Citation1–Citation4 and the nutrient-sensitive kinase mechanistic target of rapamycin complex 1 (mTORC1).Citation5–Citation8 The second part explains the molecular link between disturbed sebofollicular metabolomics and inflammation. The reader will understand that Western diet is the major factor overstimulating sebum production, Propionibacterium acnes overgrowth, and biofilm formation. Biofilm-transformed P. acnes produce abundant exogenous lipase, a virulence factor that increases local levels of free palmitic acid, a recently recognized danger signal activating the NLRP3 inflammasome. Abundance of sebum-derived free palmitate together with P. acnes-derived danger-associated molecular patterns (DAMPs) stimulates innate immunity, inflammasome activation, and interleukin-1β (IL-1β)-signaling. IL-1β finally orchestrates follicular and perifollicular inflammation with Th17 cell differentiation and IL-17-mediated local keratinocyte hyperproliferation.
IGF-1: central player of acne
Most textbooks of dermatology still define acne as an androgen-dependent skin disease. There is no doubt that androgen excess promotes acne and seborrhea, whereas acne does not develop under conditions of androgen receptor (AR) loss of function leading to androgen insensitivity.Citation9 These facts clearly point to the involvement of AR-dependent signaling in the pathogenesis of acne. Yet there is still an unsolved contradiction: it is well established that androgen serum levels increase during puberty and stay at high levels for decades, whereas acne physiologically fades spontaneously after puberty. After the climax of puberty, serum levels of insulin-like growth factor 1 (IGF-1), the major growth hormone of puberty, decrease continuously.Citation10 Deplewski and RosenfieldCitation11 pointed out that not serum androgens but serum IGF-1 levels correlate with the clinical manifestation of acne. Evidence will be presented that not androgens but IGF-1 plays the primary role in acne pathogenesis. IGF-1 signaling is the central endocrine pathway of puberty and sexual maturation, and is the converging point of nutrient signaling in acne.
Which facts do prove this change of paradigms? There is a human experiment of nature supporting the primary role of IGF-1 signaling in acne pathogenesis, the Laron syndrome. Short-statured individuals with Laron syndrome exhibit a congenital IGF-1 deficiency due to growth hormone receptor (GHR) mutations.Citation12 Notably, Laron patients, who are not treated with recombinant IGF-1, never develop acne or other common diseases of Western civilization.Citation13,Citation14 However, high-dose IGF-1 administration induces acne and hyperandrogenism in these GHR-deficient patients.Citation15 The occurrence of hyperandrogenism in IGF-1-treated Laron patients already implies that IGF-1 enhances AR-dependent signal transduction.
IGF-1 inhibits FoxO1 signaling at multiple regulatory layers
IGF-1 promotes cell growth and cell proliferation by activating the IGF-1 receptor (IGF1R), resulting in upregulation of the phosphoinositol-3-kinase (PI3K)–protein kinase B (AKT) signaling cascade.Citation16 Pioneering autoradiographic studies of Plewig et alCitation17 showed that acne is a hyperproliferative disease of the sebaceous follicle. In acne, increased cell proliferation has been demonstrated in keratinocytes of the acroinfundibulum and ductus seboglandularis, and sebocytes of the sebaceous gland.Citation17 Thus, the question arose as to how IGF-1 increases local proliferation of acroinfundibular keratinocytes, epithelial cells of the ductus seboglandularis, and sebocytes. To understand the stimulatory effects of IGF-1 on sebofollicular androgen signaling, it is of critical importance to become familiar with the major regulatory mechanisms that enhance AR transcriptional activity.Citation18,Citation19
The AR is a nuclear transcription factor that stimulates the expression of genes that promote androgen-dependent growth and proliferation.Citation18,Citation19 AR activation requires two major stimuli: 1) binding of its hormone ligand (androgen), and 2) derepression of its inhibitory nuclear coregulator FoxO1. Ligand-mediated activation of AR depends on androgen binding affinity. Highest AR binding affinity exhibits dihydrotestosterone (DHT), which is ten times higher compared with testosterone. IGF-1 is a potent inducer of gonadal testosterone and adrenal dehydroepiandrosterone (DHEA) synthesis and promotes the intracutaneous conversion of testosterone to DHT by enhancing 5α-reductase activity.Citation20,Citation21 Thus, IGF-1 increases the total amount of gonadal and adrenal androgen synthesis,Citation22–Citation25 and enhances androgen bioactivity by increasing the cutaneous availability of DHT,Citation21 the most powerful physiological androgen. Conversely, the androgens induce IGF-1 in the hair follicle.Citation26 Thus, IGF-1 stimulates AR signal transduction by upregulating the amount and affinity of AR-activating ligands.
Most dermatologists are not aware of the second most important IGF-1-dependent mechanism that increases AR signaling that involves the metabolic transcription factor FoxO1. In the nucleus, FoxO1 functions as an AR cosuppressor.Citation18,Citation19,Citation27,Citation28 Nuclear FoxO1 levels are negatively regulated by insulin and IGF-1.Citation29 Both sister hormones activate the PI3K–AKT pathway.Citation20,Citation29 Activated AKT phosphorylates FoxO1 in the nucleus, which is the critical step promoting its translocation into the cytoplasm.Citation29 FoxO1 suppresses AR transactivation by binding to the transcription activation unit 5 (TAU5) located in the AR N-terminal domain (NTD).Citation30 The TAU5 motif is most important for androgen-independent activation of the AR,Citation31 is controlled by insulin/IGF-1-mediated activation of AKT, and is thus connected to the nutrient status.
Taken together, AR activation requires two different IGF-1-dependent pathways: 1) enhanced ligand potentiation and ligand binding to the AR ligand binding domain and 2) activation of AR transactivation by the nuclear extrusion of the AR suppressor FoxO1 from the NTD. Notably, the NTD contains a polyglutamine-enriched region encoded by CAG trinucleotide repeats.Citation32 Expansion of these CAG repeats in the AR reduces AR activation, whereas AR polymorphisms featuring shorter CAG repeats are associated with androgenetic alopecia, hirsutism, and acne.Citation32 Individuals featuring AR polymorphisms with shorter CAG repeats in comparison with individuals with normal CAG repeat length apparently exhibit easier AR hyperactivation by insulin/IGF-1 signaling. These insights also explain increased AR signaling in states of hyperinsulinemia and insulin resistance and conditions with increased IGF-1 serum levels such as puberty and nutrient signaling of Western diet.Citation33 Individuals with shorter CAG repeats may thus exhibit stronger acneigenic reactions by dietary exposure to a high glycemic load diet and milk consumption, which both enhance insulin/IGF-1 signaling.Citation20,Citation29 My hypothesis of aberrant IGF-1/FoxO1 signaling in the pathogenesis of acne has recently been confirmed experimentally in SZ95 sebocyte cultures.Citation34,Citation35 Prolonged IGF-1 exposure of SZ95 sebocytes induced nuclear translocation of FoxO1 into sebocyte’s cytoplasm.Citation35 Thus, the transcriptional coordinator of metabolism FoxO1 links insulin/IGF-1 signaling to transcriptional activation of AR-dependent target genes. Notably, the highest nuclear FoxO1 activity is observed during starvation, whereas nutrient excess leads to reduced nuclear levels of FoxO1.Citation3,Citation36,Citation37
Serum levels of DHEA, the major adrenal androgen that increases during adrenarche, correlate with the onset of acne vulgaris.Citation38 Notably, DHEA induces ERK1/2-mediated phosphorylation and translocation of FoxO1.Citation39 Thus, increased adrenal DHEA signaling, which begins prior to puberty, already suppresses FoxO1 activity, increasing AR transactivation. DHEA-induced inactivation of FoxO1 may also explain neonatal hyperseborrhea and acne due to excessive fetal DHEA production, a physiological mechanism ensuring the generation of the vernix caseosa, which is important for birth.Citation40
Nuclear FoxO1, which is upregulated by isotretinoin treatment,Citation41 controls endocrine signaling of the hypothalamus,Citation42,Citation43 pituitary,Citation44 liver,Citation45 adrenal,Citation46 and sebaceous gland.Citation34,Citation35,Citation47 FoxO1 was recently reported to be an inhibitor of follicle stimulating hormone and luteinizing hormone production.Citation48–Citation50 Notably, luteinizing hormone/human chorionic gonadotropin triggers androgen synthesis in theca-interstitial cells of the ovary by activating mTORC1 signaling.Citation51 Insulin and IGF-1 act as negative regulators of FoxO1 activity and enhance gonadotropin expression.Citation52 Increased insulin/IGF-1 signaling of Western diet thus promotes the synthesis of pituitary gonadotropins, which are pivotal stimuli for gonadal steroidogenesis.
FoxO1 is a negative regulator of GHR,Citation45 which plays the key role in hepatic IGF-1 synthesis.Citation12 Thus, insulin signaling via repression of hepatic FoxO1 stimulates hepatic IGF-1 synthesis, demonstrating an interactive hepatic network of metabolic and growth factor signaling. Inactivation of hepatic FoxO1 by insulin signaling is required to adapt nutrient homeostasis and endocrine growth regulation.Citation45 Notably, isotretinoin, the most powerful antiacne drug, reduced serum concentrations of gonadotropins, adrenocorticotropic hormone, and IGF-1.Citation53–Citation55 This can be well explained by isotretinoin-mediated upregulation of nuclear FoxO1 activity at various regulatory levels of the somatotropic axis.Citation41
Acne correlates with increased sebum production. GH, insulin, and IGF-1 increase sebaceous gland growth, differentiation, and sebaceous lipogenesis.Citation11,Citation56 Vora et alCitation57 observed a linear correlation between serum IGF-1 concentrations and facial sebum excretion rates of male acne patients. Remarkably, increased serum IGF-1 levels have been measured in women with post-adolescent acne.Citation58,Citation59 Recently, an association between IGF-1 gene polymorphism and acne has been reported.Citation60 Patients who observed an aggravation of their acne by food intake exhibited higher IGF-1 serum levels (mean =543.9 ng/mL) compared with those who observed no acne aggravation by food intake (mean IGF-1 =391.3 ng/mL).Citation61
IGF-1 plays a pivotal role in sebaceous lipogenesis.Citation62,Citation63 Downstream of IGF-1/PI3K/AKT signaling respond four key lipogenic transcription factors: the AR,Citation18,Citation19,Citation27,Citation28 peroxisome proliferator-activated receptor-γ (PPARγ),Citation64–Citation67 liver X receptor-α (LXRα),Citation68,Citation69 and sterol response element binding protein-1c (SREBP-1c),Citation62,Citation63,Citation70 which are all negatively regulated by FoxO1 ().Citation18,Citation19,Citation27,Citation28,Citation71–Citation77 IGF-1 stimulated SREBP-1 expression and induced lipogenesis in SEB-1 sebocytes via activation of the PI3K/AKT pathway.Citation63 Mirdamadi et alCitation35 confirmed that IGF-1 suppresses nuclear FoxO1 in SZ95 sebocytes associated with increased lipogenesis. Under conditions of nutrient excess and high-insulin/IGF-1 signaling, downregulated nuclear FoxO1 thus derepresses all master transcription factors of sebaceous lipogenesis such as AR, PPARγ, LXRα, and SREBP-1c. In fact, Kwon et alCitation78 observed decreased SREBP-1 expression in facial acne skin after 4 weeks of a low glycemic load diet. Notably, acne-free Kitavan islanders,Citation79 who are still exposed to a paleolithic diet (less-hyperglycemic carbohydrates, no milk and dairy products, but plenty of fish intake), exhibit low basal insulin serum levels that are only half of those of Europeans living under conditions of Western neolithic diet.Citation80 Incubation of epithelial cells with IGF-1-deficient serum of Laron patients exhibited increased nuclear FoxO1 activity and decreased expression of TOR.Citation14 Notably, excessive meat intake is another characteristic feature of Western diet. Recent epidemiological evidence underlines that low protein intake is associated with a major reduction in serum IGF-1 in the middle-aged population.Citation81
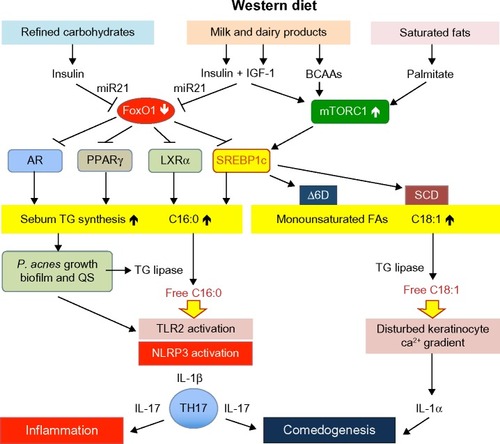
Figure 1 Acne vulgaris: a Western diet-induced sebofollicular inflammasomopathy.
FoxO1 interacts with TGFβ- and β-catenin signaling
McNairn et alCitation82 demonstrated that transforming growth factor-β (TGFβ) signaling is necessary and sufficient for maintaining sebocytes in an undifferentiated state. TGFβ receptor type 2 (TGFR2)–SMAD2 signaling decreased the expression of genes required for sebaceous lipogenesis and sebocyte differentiation such as Δ6-desaturase and PPARγ, thereby decreasing sebaceous lipid accumulation. A recent genome-wide association study identified three novel susceptibility loci of the TGFβ pathway for severe acne vulgaris, namely, transforming growth factor β2 (TGFB2), Ovo, Drosophila, homologue-like 1 (OVOL1), and follistatin (FST).Citation83 The authors noted a significant reduction in TGFB2 and OVOL1 transcript levels in lesional compared with non-lesional skin of acne patients.Citation83
Canonical TGFβ signaling starts after binding of TGFβ to TGFR2, which recruits and activates TGFR1. TGFR1 phosphorylates the receptor-bound transcription factors SMAD2 and SMAD3, which later associate with SMAD4. The activated SMAD2/3/4 complex translocates into the nucleus and executes its transcriptional functions.Citation84 Importantly, activated SMAD proteins associate with FoxO1, FoxO3, and FoxO4. In human keratinocytes, FoxO–SMAD synexpression plays a crucial role in the induction of the cyclin-dependent kinase inhibitors p15 and p21.Citation85,Citation86 Genes that require FoxO–SMAD synexpression in response to TGFβ coordinate cell cycle control via p15 and p21 and adaptive cell signaling responses such as OVOL1.Citation85–Citation87 Increased expression of p21 has been detected in sebocytes treated with isotretinoin,Citation88 the most potent antiacne drug that obviously functions as a FoxO1 inducer.Citation41,Citation89 Thus, it is conceivable that isotretinoin enhances SMAD–FoxO1-mediated expression of p21. Western diet with exaggerated insulin/IGF-1 signaling thus affects SMAD–FoxO1-regulated synexpression of important cell cycle checkpoints of keratinocytes and sebocytes. Furthermore, conditional deletion of TGFβ signaling resulted in PI3K/AKT activation,Citation90 the major FoxO1-controlled pathway promoting sebaceous lipogenesis.Citation34
FoxO1 interacts with Wingless (Wnt)/β-catenin signaling, which blocks differentiation toward the sebocyte phenotype, since inhibition of Wnt target genes promotes sebocyte development.Citation91–Citation93 β-catenin reduces c-Myc-stimulated sebocyte differentiation.Citation94,Citation95 Notably, β-catenin strongly binds FoxO1 and FoxO3a. This interaction enhances FoxO’s transcriptional activity.Citation96
FoxOs are negative regulators of the nutrient-sensitive kinase mTORC1
FoxO1 and FoxO3 are negative regulators of the nutrient-sensitive kinase mTORC1.Citation97,Citation98 mTORC1 has recently been recognized to play a major role in diet-induced acne.Citation47,Citation99,Citation100 FoxO1 activates the transcription of the eukaryotic initiation factor 4 binding protein-1 (4EBP-1), which is a major downstream substrate of mTORC1 and functions as a potent translational inhibitor and growth suppressor.Citation101,Citation102 Insulin and IGF-1 activate mTORC1, the cell’s master regulator orchestrating insulin and IGF-1 signaling, nutrient, glucose, energy, and amino acid availability.Citation103–Citation105 Insulin, IGF-1, and amino acids are required for full activation of mTORC1 signaling. The essential branched-chain amino acid (BCAA) leucine plays a primary role in mTORC1 activation.Citation105–Citation107 Glutamine, an abundant amino acid constituent of milk proteins, has recently also been demonstrated to have a supportive role in mTORC1 activation.Citation108 Leucine and glutamine stimulate mTORC1 by rag GTPase-dependent and independent mechanisms. In contrast to other amino acids, leucine promotes mTORC1 signaling also independent of lysosomal translocation of mTOR.Citation109
mTORC1 regulates anabolism,Citation110 nutrient-dependent cell cycle progression,Citation111 and activates lipogenesisCitation112 by inducing the expression and activation of SREBP-1c and PPARγ.Citation113–Citation116 Insulin/IGF-1-mediated activation of AKT results in mTORC1 activation. Importantly, mTORC1 phosphorylates and inactivates the negative SREBP-1 regulator lipin 1Citation114 and promotes gene expression of SREBP-1c.Citation116 mTORC1 via activation of the kinase S6K1 promotes SREBP-1c cleavage into its transcriptionally active form.Citation113 Thus, several converging mTORC1-dependent pathways enhance the activation of the lipogenic transcription factor SREBP-1c.
SREBP-1c promotes sebum fatty acid desaturation
It is of critical importance to consider that SREBP-1c is a key regulator of stearoyl-CoA desaturase and Δ6-desaturase gene expression. Insulin stimulates the expression of Δ6-desaturase.Citation117,Citation118 Stearoyl-CoA desaturase catalyzes the conversion of stearic acid (18:0) to oleic acid (18:1), a major fatty acid of sebum triglycerides. Δ6-Desaturase and Δ5-desaturase are key enzymes for the synthesis of highly unsaturated fatty acids such as arachidonic acid, which is the precursor of proinflammatory eicosanoids such as leu-kotriene B4 (LTB4) and prostaglandin E2 (PGE2) involved in inflammatory responses of sebaceous glands.Citation119 Sebocyte Δ6-desaturase converts palmitic acid (16:0) to sapienic acid (16:1),Citation120 which functions as a natural antimicrobial agent involved in epidermal host defenses.Citation121,Citation122 Thus, sebocyte SREBP-1c activity not only controls the total amount of synthesized sebum triglycerides but, via SREBP-1c-mediated gene expression of Δ6-desaturase and stearoyl-CoA desaturase, increases sebum triglyceride levels of monounsaturated fatty acids. In fact, an association between the synthesis of total sebum triglycerides with increased triglyceride levels of sapienic acid (16:1) and decreased stearic acid (18:0) – due to its conversion to oleic acid (18:1) – has been observed ().Citation123
FoxO1 is critically involved in the regulation of SREPB-1c activity via GHR-mediated hepatic IGF-1 synthesis,Citation45 FoxO1-regulated expression of IGF binding protein 1,Citation45 FoxO1-mediated suppression of LXRα, and FoxO1-regulated expression of SREBP-1c.Citation75–Citation77 FoxO-mediated inhibition of mTORC1 also controls mTORC1-dependent SREBP-1c expression and its final nuclear activation.Citation113–Citation116
Western diet drives acne metabolomics
High-acne prevalence rates of over 90% during adolescence, and increasing persistence of acne into the second and third decades of life in around 64% and 43% of individuals respectively, clearly point to the predominance of environmental and epigenetic factors.Citation124,Citation125 Populations exposed to paleolithic dietary conditions (low glycemic load, no milk and dairy consumption) such as the Kitavan islanders of Papua New Guinea, the Ache hunters in Paraguay, the Inuit, and adolescents of rural areas of Brazil are examples of acne-free populations. An increase in acne prevalence has been reported for Inuits, Okinawa islanders, and Chinese after transition from their traditional diets to Westernized nutrition. Accumulating epidemiological, clinical, and translational evidence underlines the impact of nutritional factors in the pathogenesis of common acne vulgaris. Especially nutrients that increase insulin/IGF-1 signaling and thus reduce nuclear FoxO1 levels but enhance mTORC1 have been identified as the most critical inducers of epidemic acne. According to Burris et al,Citation126 acne severity in a cohort of New York young adults was associated with: 1) increased intake of sugar (high glycemic load), 2) number of milk servings per day, and 3) amount of saturated fat and trans-fatty acid (TFA) intake. The nutrigenomic impact of these acneigenic food classes will now be discussed in more detail.
Hyperglycemic carbohydrates
There is a general consensus that a high intake of refined carbohydrates plays a pivotal role in acne pathogenesis.Citation127 The effect of high glycemic load diets on the induction and aggravation of acne has been confirmed by several placebo-and case-controlled studies.Citation126,Citation128–Citation132 A low glycemic load diet increased IGF binding protein 1 (IGFBP1) and IGFBP3, whereas a high glycemic load diet decreased sex hormone binding globulin (SHBG).Citation131 Thus, the amount of hyperglycemic carbohydrates modulates the bioactivity of free serum IGF-1 and free serum androgens. Importantly, Kwon et alCitation78 observed a decrease of sebaceous gland size and reduced SREBP-1 expression in facial acne skin after 10 weeks of a low glycemic load diet. This metabolic reaction pattern is explained by attenuated AKT–mTORC1 signaling due to carbohydrate reduction with attenuated insulin signaling. Resulting increases of nuclear FoxO1 and decreased mTORC1 activity are in accordance with reduced cutaneous expression of SREBP-1. Decreased cutaneous SREBP-1 expression should not only reduce total sebum production but should also decrease the rate of sebum triglyceride fatty acid desaturation. In fact, a low glycemic load diet increased the ratio of saturated to monounsaturated fatty acids in skin surface triglycerides.Citation133 In contrast, increased sebum outflow was associated with an increase in the proportion of monounsaturated fatty acids, thus reflecting SREBP-1-driven total lipogenesis as well as increased SREBP-1c-dependent stimulation of desaturase activity (). Thus, a high glycemic load changes the composition of sebum fatty acids, a most critical proinflammatory and comedogenic mechanism that will be discussed later.
There is recent evidence that diet also modifies the expression of microRNAs that play an important role in posttranscriptional regulation of metabolism.Citation134 High glucose concentration upregulates microRNA-21 in macrophages.Citation135 MicroRNA-21 is a central regulator of cell proliferation and inflammation.Citation136 MicroRNA-21 promotes macrophage polarization toward proinflammatory M1 macrophages secreting IL-1β, and stimulates Th17 cell differentiation.Citation137,Citation138
Milk
In 1885, BulkleyCitation139 reported on acne-aggravating effects of milk consumption in his extensive dietary studies involving 1,500 patients with acne. Harvard epidemiologists Adebamowo et alCitation140–Citation142 provided the first epidemiological evidence for the association between milk consumption and acne by evaluating data of the retrospective Nurses’ Health Study II and the prospective Growing-up Today Study. Further controlled clinical studies corroborated the milk–acne connection.Citation61,Citation129,Citation143 A recent semantic connectivity map approach of 563 subjects showed that moderate-to-severe adolescent acne was closely associated with high consumption of milk, in particular, skim milk, cheese/yogurt, sweets/cakes, chocolate, and a low consumption of fish, and limited intake of fruits/vegetables,Citation144 which is the opposite food pattern of paleolithic nutrition.
Milk is a very special functional food designed by evolution to promote anabolism and growth of newborn mammals. To understand milk’s impact on acne, it is important to realize that milk promotes anabolic mTORC1 signaling.Citation145 To fulfill its growth-promoting function, this secretory product of mammary glands transfers a hardware consisting of amino acids that promote insulin/IGF-1/mTORC1 signaling, and a software delivering exosomal microRNAs, including microRNA-21 that enhances AKT–mTORC1 signal transduction ().Citation145
Daily consumption of 710 mL ultra-heat-treated (UHT) milk in prepubertal Mongolian children not used to milk consumption over 4 weeks substantially increased serum GH and IGF-1 levels.Citation146 Notably, IGF-1 serum levels increased by 23% from pretreatment concentrations. These data clearly show that milk consumption switches the somatotropic axis. It is important to realize that it is not the IGF-1 content of cow’s milk that exaggerates serum IGF-1 levels of the milk consumer, but the milk-driven hepatic production of IGF-1 by the transfer of amino acids that promotes IGF-1 synthesis in the liver of the milk recipient.Citation145 Notably, the major whey protein α-lactalbumin has the highest tryptophan content among all other protein food sources.Citation147 Tryptophan availability is of critical importance for hepatic IGF-1 synthesis.Citation148 Milk’s essential BCAAs (leucine, isoleucine, and valine) induce pancreatic insulin secretion and explain the high insulinemic index of whole milk and skim milk.Citation149,Citation150
Thus, milk intake enhances insulin/IGF-1 signaling. Furthermore, milk proteins transfer high amounts of the insulinotropic amino acid leucine, which promotes mTORC1 activation.Citation145 Whey proteins contain the highest amount of leucine (14%) compared with all other animal proteins such as beef (8%).Citation151 In comparison with beef protein (4.74 g glutamine/100 g), milk protein (8.09 g glutamine/100 g) contains about twice as much glutamine.Citation152 Glutamine not only promotes cellular leucine uptake,Citation153 but is the precursor of the glutaminolysis pathway that is critically involved in mTORC1 activation.Citation108,Citation109,Citation154 Remarkably, the glutaminolysis pathway plays a special role in sebaceous lipogenesis and sebocyte proliferation.Citation155 In freshly isolated human chest sebaceous glands, glutamine deprivation reduced cell proliferation and lipogenesis by 41% and 37%, respectively.Citation155 These data indicate that milk is the ideal fuel for FoxO1/mTORC1/SREBP-1c-regulated sebaceous gland hyperplasia and sebaceous lipogenesis. Increased IGF-1 production by milk protein intake is thus superimposed on exaggerated IGF-1 signaling of puberty, which explains the earlier onset of puberty and the persistence of acne in the third decade of life in milk-consuming populations.
Analogously to androgen abuse in the bodybuilding environment, excessive milk protein intake has to be considered as a form of doping.Citation156 It is of critical concern that milk protein (whey and casein) abuse in the fitness and bodybuilding scenario is associated with the onset and aggravation of acne.Citation157–Citation160
The recent prediction of Melnik et alCitation145,Citation149 that milk transfers a gene-regulatory metabolically active software consisting of exosomal bioactive microRNAs has recently been confirmed experimentally for cow’s milk.Citation161–Citation163 Binding of microRNAs through partial sequence homology to the 3′-untranslated region of target mRNAs causes translational block or degradation of target mRNAs.Citation164 MicroRNAs, enclosed by membranous microvesicles (exosomes), allow intercellular transfer of microRNAs over long distances.Citation165,Citation166 Milk is apparently the exosomal signaling system of mammals that allows maternal–neonatal communication.Citation145,Citation167 It is of critical concern that the 245 microRNAs of pasteurized cow’s milk are absorbed by humans in biologically meaningful amounts, reach the systemic circulation, and affect the expression of more than the estimated 11,000 genes of the human milk consumer.Citation161 In fact, it has been shown that exosomal milk-derived microRNAs are taken up by human cells and modify gene expression.Citation161,Citation163 Intriguingly, bovine microRNA-21, a predominant microRNA constituent of cow’s milk, is identical to human microRNA-21.Citation168 MicroRNA-21 inhibits mRNA expression of phosphatase and tensin homologue (PTEN).Citation169,Citation170 PTEN is a dual protein/lipid phosphatase. Its main substrate, phosphatidyl-inositol 3,4,5, triphosphate, is the product of PI3K. MicroRNA-21-mediated suppression of PTEN mRNA thus promotes PI3K/AKT signaling, which downregulates nuclear FoxO1. Furthermore, there is recent evidence that microRNA-21 directly targets FoxO1 mRNA.Citation171,Citation172 Another recently identified target of microRNA-21 is IGFBP3,Citation173 which reduces the bioavailability of IGF-1. The recent observation that exosomal microRNA-21 downregulates the expression of TGFβR2Citation174 is of critical importance for acne-prone individuals with a genetic weakness of TGFβ signaling.Citation83 Thus, milk-derived microRNA-21 inhibits FoxO1- as well as TGFβ-signaling at various layers of posttranscriptional regulation.
DanbyCitation175 emphasized that 75%–90% of marketed commercial milk and milk products in the US are derived from pregnant cows. The milk of these animals contains DHT precursors. During pregnancy, the bovine adrenal gland produces substantial amounts of DHEA, which can be converted to androstenedione via the enzyme 3β-hydroxysteroid dehydrogenase. Androstenedione levels increase in cow’s plasma and milk during pregnancy.Citation176 Raw milk of pregnant versus nonpregnant cows contains 3.4 times more androstenedione (mean =36.7 versus 10.9 ng/dL), 1.2 times more DHEA (mean =10.5 versus 8.7 ng/dL), and 1.3 times more testosterone (mean =10.3 versus 8.0 ng/dL), respectively.Citation177 Activation of estrogen receptor beta and AR by the DHEA metabolites androst-5-ene-3,17-dione, androst-5-ene-3β,17β-diol, DHT, and 5α-androstane-3β,17β-diol increased microRNA-21 transcription in HepG2 human hepatoma cells, increasing cell proliferation.Citation178 Thus, both milk-derived exosomal microRNA-21 and milk androgen precursor-mediated expression of microRNA-21 may enhance PI3K–AKT-signaling, decreasing FoxO1’s nuclear activity. Intriguingly, there has recently been interest in the role of microRNAs as natural ligands of toll-like receptors (TLRs).Citation179 MicroRNA-21 and microRNA-29a, both components of cow’s milk, can directly bind to TLR8.Citation180 TLR8 stimulation activates the inflammasome and upregulates IL-1β secretion.Citation181,Citation182
Saturated and trans-fats
Recently, Yasuda et alCitation183 provided evidence that the major saturated fatty acid palmitate activates mTORC1 and enhances its lysosomal translocation, whereas the ω3-fatty acid eicosapentaenoic acid (EPA), a major fatty acid of fish oil, inhibited mTORC1 activation. It is thus conceivable that sebum-derived free palmitate may activate cell proliferation of acroinfundibular keratinocytes by palmitate-driven mTORC1 signaling, thereby promoting comedogenesis. Notably, palmitate is a major fatty acid, constituting 32% of milk triglycerides.Citation184,Citation185 Burris et alCitation126 and Jung et alCitation61 observed an aggravation of acne with increased intake of saturated fat, whereas a higher intake of fish, a nutrient source enriched in ω3-fatty acids, exhibited an acne-protective effect.Citation61,Citation143,Citation144
Industrially produced TFAs, which structurally resemble palmitate, are major components of fast food and have been found to aggravate acne.Citation61,Citation126 Their mTORC1-activating effect is predictable, but has not yet been studied. These partially hydrogenated fats have displaced natural solid fats and liquid oils in many areas, the most notable ones being in fast food, snack food, fried food, and baked goods that have all been associated with diet-induced acne.Citation61,Citation126 In a comparative study of the TFA content of Swedish bakery products in 2007, 3 of 41 products had TFA levels above 2% of total fatty acids.Citation186 However, TFA intakes of Canadian children aged 5–6 years have decreased since 2004 to a 95% intake of 1.28% energy.Citation187 TFA intake during pregnancy and lactation of rats increased the expression of TNF receptor-associated factor 6 (TRAF6) in the rat offspring.Citation188 Remarkably, TRAF6 mediates IL-1 signaling.Citation189 Toll/IL-1 receptor (TIR) domain-containing adaptor protein (TIRAP) is involved in bridging MyD88 to the receptor complex for TLR2 and TLR4 signaling in response to bacterial infection.Citation190 Verstak et alCitation190 characterized a novel role for TIRAP in facilitating the direct recruitment of TRAF6 to the plasma membrane, which is necessary for TLR2- and TLR4-induced transactivation of NF-κB and induction of subsequent proinflammatory responses. Thus, Western diet-derived TFA intake via TRAF6-mediated stimulation of proinflammatory TLR2/TLR4 signaling may contribute nutrient-mediated inflammatory responses of pilosebaceous follicles.
Western diet promotes NRLP3 inflammasome activation
It has long been known that “sebum is the oil of the acne flame.” P. acnes flourishes when sebum production increases. Regional variations in density of P. acnes are correlated with sebum secretion.Citation191 P. acnes strain 266, which belongs to the IA (I-1a/ST18) phylotype, is associated with moderate to severe acne and possesses particular virulence potential.Citation192 The gehA gene (PPA2105) encoding the secreted triacylglycerol lipase is a virulence factor that is upregulated in P. acnes strain 266 during exponential growth phases.Citation193 Recently, P. acnes biofilm formation has been confirmed in sebaceous follicles of acne patients.Citation194 Bacteria undergo behavioral and transcriptional changes based on the surrounding bacterial population, a process called quorum sensing (QS).Citation195 QS inhibitors appear to play an important role in the inhibition of biofilm formation.Citation196 Biofilm formation substantially increases P. acnes virulence associated with enhanced expression of exogenous P. acnes triglyceride lipase that increases sebum concentrations of free palmitate and oleate ().Citation197,Citation198 Zouboulis et alCitation199 recently emphasized that not only the total amount of sebum but, predominantly, alterations of sebum lipid composition are main players in the induction of inflammatory acne. Notably, free oleic acid generated by SREBP-1c-dependent stearoyl desaturase and subsequent triacylglycerol lipase-mediated hydrolysis increases P. acnes adherence and growth.Citation200,Citation201 Thus, P. acnes lipase may aid colonization and biofilm formation within the pilosebaceous follicle, by promoting oleate-dependent cell adherence.Citation200
Innate immunity is activated in acne. Incubation of human keratinocytes with P. acnes fractions induced the expression of TLR2 and TLR4.Citation202 Positive TLR2 expression in epidermis, pilosebaceous units, and dermal inflammatory infiltrates has been demonstrated immunohistochemically in acne-involved skin.Citation203 Notably, excess saturated fatty acids appear to function as danger signals (DAMPs),Citation204 which activate TLR2/TLR4-driven inflammatory signaling.Citation195 Snodgrass et alCitation205 recently demonstrated that human monocyte TLR2 activation and inflammasome-mediated secretion of IL-1β are modulated by dietary fatty acids. Remarkably, palmitic acid directly activates TLR2 by inducing heterodimerization with TLR1, whereas docosahexaenoic acid (DHA), a major ω3-fatty acid of fish oil, inhibited TLR2/TLR1 dimerization.Citation205 TLR2/TLR1 dimerization is thus a most critical palmitate-dependent regulatory mechanism in inflammasome activation resulting in subsequent IL-1β secretion. This molecular mechanism apparently links enhanced levels of free sebum palmitate to TLR2-driven inflammasome activation of the pilosebaceous follicle in acne. There is recent evidence that inflammatory TLR2–NF-κB signaling in macrophages is well enhanced by palmitate.Citation206 Sebum free saturated fatty acids apparently promote a TLR-mediated danger response of the sebaceous follicle associated with upregulated β-defensin-2 expression of human sebocytes.Citation207 Palmitate has been recognized as a crucial stimulator of the NLRP3 inflammasome and plays an important role in lipotoxic inflammasome activation of macrophages.Citation208,Citation209 In human monocyte/macrophages, both palmitate and stearate triggered IL-1β secretion in a caspase-1/ASC/NLRP3-dependent pathway.Citation210 In chondrocytes as well, palmitate synergized with IL-1β in stimulating proinflammatory cellular responses.Citation211 Thus, excessive production and release of sebum-derived free palmitic acid appears to be a lipotoxic danger signal of the sebaceous follicle that drives inflammation.
The NLRP3 inflammasome is regarded as a sensor of metabolic danger signals activated by lysosomal rupture, potassium efflux, and reactive oxygen species production.Citation212 Kistowska et alCitation213 demonstrated that lysosomal rupture is required for IL-1β secretion in response to P. acnes. Notably, palmitate is known to destabilize lysosomes, leading to NLRP3 inflammasome activation.Citation208 Thus, excess saturated fatty acids stimulate and augment a danger response via TLR2 activation and lysosomal destabilization finally processed by the NLRP3 inflammasome that mediates IL-1β signaling ().Citation208,Citation214 In addition to palmitate, P. acnes itself triggers NLRP3 inflammasome activation of monocyte–macrophages and human sebocytes, increasing IL-1β secretion.Citation213,Citation215,Citation216
IL-1β release stimulates the Th17 response
IL-1β activates IL-17A positive T cells (Th17 cells) and CD83 dendritic cells in acne lesions, resulting in the activation of Th17-related cytokines.Citation217 In addition to IL-17A, both Th1 and Th17 effector cytokines, transcription factors, and chemokine receptors are strongly upregulated in acne lesions.Citation218 IL-17A and IL-17F are key cytokines for the recruitment and activation of neutrophils and can target keratinocytes, endothelial cells, monocytes, and fibroblasts to produce proinflammatory mediators such as IL-6, TNFα, IL-1β, PGE2, nitric oxide, matrix metalloproteinases, and various chemokines.Citation219 IL-17-related antimicrobial peptide and CXCL chemokine production with neutrophil attraction in acne lesions are thus important factors triggering the inflammatory infiltrate. There is substantial support for the hypothesis of Lwin et al,Citation195 who suggest that P. acnes sends no signals or only “safety signals” when present in controlled quantities under commensal conditions, but becomes pathogenic and sends “danger signals” via QS in the form of excessive free fatty acid production, which stimulates TLR2 and TLR4 as the bacterial population and its virulence increases ().
Sebum free fatty acids promote comedogenesis
Abnormal follicular keratinization is important for comedo formation in acne. Diet-induced changes in sebum quantity and composition may not only induce the inflammation of acne but may also drive the process of comedogenesis. Increased release of the danger signal “free palmitate” activates TLR2/IL-1β signaling of dendritic cells that promote Th17 cell differentiation with increased secretion of IL-17A.Citation220 In fact, increased local levels of IL-1β and IL-17A have been detected in lesional acne skin ().Citation217 IL-17 is a key cytokine that stimulates keratinocyte proliferation via IL-6/STAT3 signaling.Citation221 IL-17 contributes to keratinocyte hyperproliferation and attenuates keratinocyte differentiation.Citation222 Thus, IL-17 disturbs follicular keratinocyte homeostasis in acne, a comparable mechanism driving keratinocyte hyperproliferation in psoriasis.Citation223
Choi et alCitation224 reported that oleic acid applied on the inner surface of the ear of New Zealand White rabbits induced comedones. Permeability barrier disruption in oleic-acid-applied follicular keratinocytes may disrupt the keratinocyte intracellular calcium gradient, leading to keratinocyte proliferation and follicular hyperkeratosis.Citation224 In fact, application of oleic acid and palmitoleic acid induced scaly skin, abnormal keratinization, and epidermal hyperplasia.Citation225 Furthermore, application of unsaturated fatty acids increased the intracellular calcium concentration of the keratinocytes. Notably, intracellular calcium increase of keratinocytes stimulated by exposure to free oleic acid increased the production of IL-1α (),Citation226 which has been implicated in comedogenesis.Citation227–Citation231
Taken together, there is compelling evidence that the nutrigenomic changes promoted by Western diet increase the local availability of sebum free palmitic and oleic acid, driving IL-1β- and IL-1α-mediated comedogenesis. Both cytokines not only play an important role in early- and late-inflammatory responses in acne,Citation232 but apparently represent key mediators of comedo formation.
Nutrition therapy of acne
In 2005, CordainCitation233,Citation234 emphasized the beneficial effects of a paleolithic diet (no hyperglycemic carbohydrates, no milk and dairy products) for the treatment of acne. Today, his dietary recommendations can be interpreted on the basis of nutrigenomic disturbances induced by Western diet. Apparently, dietary and pharmacological treatment of acne have a common mode of action: the increase of nuclear FoxO1 and the attenuation of mTORC1 signaling ().Citation235 Natural dietary compounds that either increase FoxO1 or inhibit mTORC1 as well as inflammasome activation are promising agents for the dietary cure of acne.Citation236 The acne-preventive effect of fish consumption is well explained by the anti-inflammatory effects of ω3-fatty acids. A preliminary case study showed an overall improvement of acne severity by 12-week daily supplementation of 3 g fish oil (930 mg EPA).Citation237 Dietary supplementation of acne patients with either 2 g EPA and DHA or borage oil containing 400 mg γ-linoleic acid significantly decreased inflammatory and noninflammatory acne lesions.Citation235 DHA has been demonstrated to inhibit TLR2/TLR1 dimerization, TLR2 signaling, and thus inflammasome activation.Citation205 In fact, DHA reduced macrophage IL-1β production by limiting inflammasome activation.Citation238 This inhibition required DHA binding to free fatty acid receptor 4, also known as GPR120/40, which recruits the adapter protein β-arrestin 1/2.Citation239 ω3- and ω6-PUFAs (polyunsaturated fatty acids) are both natural ligands of GPR120/40.Citation240 After receptor binding ω3-fatty acids inhibited the NLRP3 inflammasome.Citation208,Citation239 Remarkably, both the NLRP3 inflammasome and mTORC1 are activated by palmitic acid and inhibited by DHA, respectively.Citation183,Citation208,Citation239 Furthermore, PUFAs counteract the activation of SREBP-1c by increasing SREBP-1c proteolytic cleavage and decreasing its mRNA abundance ().Citation241,Citation242
Table 1 Acneigenic food components of Western diet
Table 2 Paleolithic-type diet for the nutrition therapy of acne
mTORC1 activity is also attenuated by plant-derived natural compounds such as the major green tea polyphenol epigallocatechin-3-gallate (EGCG) and the stilbenol resveratrol.Citation236 EGCG suppressed IGF-1-induced lipogenesis, reduced the activation of AKT and mTOR, and attenuated the expression of IL-1, IL-6, and IL-8 in SZ95 sebocytes.Citation243 EGCG is a dual PI3K/mTOR inhibitor, and it enhances nuclear FoxO1 and attenuates mTORC1 signaling,Citation244 explaining the improvement of acne by topical EGCG treatment.Citation245 EGCG has been shown to inhibit SREBP-1 in SEB-1 sebocytes, and improved acne in an 8-week randomized clinical trial with EGCG.Citation244 EGCG-mediated activation of AMP-activated kinase is another inhibitory mechanism attenuating mTORC1–SREBP-1 signaling, which explains EGCG-mediated suppression of sebaceous lipogenesis.Citation245 These data are in accordance with reduced sebum production of healthy volunteers topically treated with a 3% green tea emulsion.Citation246 Notably, a preliminary case study reported improvement of acne with daily oral intake of 1 g EPA and 200 mg EGCG ().Citation247
Resveratrol, the polyphenolic flavonoid from grapes and red wine, downregulates PI3K/AKT/mTORC1 signaling.Citation248–Citation252 Furthermore, it inhibits the growth of P. acnes,Citation253 directly inhibits PI3K,Citation254 upregulates FoxO1, and downregulates PPARγ mRNA expression.Citation254 Importantly, resveratrol inhibited SZ95 sebocyte growth through inactivation of the PI3K/AKT pathway.Citation255 Resveratrol via stimulation of FoxO1 signaling apparently inhibits SREBP-1c.Citation254,Citation256–Citation258 In fact, topical treatment of facial acne vulgaris in 20 patients with a resveratrol-containing gel (0.01% wt/vol) significantly reduced the number of microcomedones, papules, and pustules compared with vehicle control.Citation259 Furthermore, resveratrol eradicated P. acnes biofilm formation ().Citation260
Conclusion
Food is a conditioning environment that shapes the activity of the human genome.Citation261 Acne is obviously the visible outcome of imbalanced nutrigenomics induced by Western diet, the maximized form of neolithic nutrition, that exaggerates insulin/IGF-1 signaling.Citation33 Suppression of FoxO1 by Western diet increases the activity of most important transcription factors involved in sebaceous lipogenesis (). Upregulated SREBP-1c not only enhances total sebum production but modifies sebum triglyceride fatty acid composition by generating a proinflammatory and comedogenic fatty acid pattern. These metabolomic changes are of critical importance for P. acnes overgrowth and biofilm formation and subsequent P. acnes-driven inflammation. Oleic acid promotes P. acnes adherence, which favors biofilm formation with QS that enhances P. acnes virulence by increasing the synthesis of exogenous lipase that releases free palmitic and oleic acid. Free palmitic acid functions as a danger signal that stimulates TLR2-mediated activation of the NLRP3 inflammasome providing proinflammatory IL-1β. IL-1β with subsequent Th17 activation and IL-17 signaling promotes comedogenesis and inflammation.
There is good reason to assume that genetic predispositions to acne increase the acneigenic responsiveness to Western diet. Individuals with persistent insulin resistance, hyperinsulinemia, and hyperandrogenism, such as women with polycystic ovary syndrome (PCOS), will exhibit increased responsiveness to the acneigenic signals of Western diet.Citation262 Notably, PCOS responds favorably to metformin,Citation263 a recently characterized mTORC1 inhibitor.Citation264 Exaggerated mTORC1–S6K1 signaling links acne to increased BMI and insulin resistance.Citation265
Androgen abuse has synergistic acneigenic effects with Western diet-driven nutrient signaling, because androgens activate mTORC2 that activates AKT and thus reduces nuclear levels of FoxO1.Citation266,Citation267
Nutrient signaling induced by Western diet synergizes with IGF-1 polymorphism associated with increased serum IGF-1 levels,Citation60,Citation268 fibroblast growth factor receptor-2 (FGFR2) gain-of-function mutation (Apert syndrome) with increased activation of AKT,Citation269,Citation270 CAG repeat polymorphism with enhanced AR transcriptional activity,Citation32 P450 polymorphisms with accelerated retinoic acid catabolism decreasing nuclear levels of FoxO1,Citation271 disturbed TFGβ signaling impairing FoxO–SMAD-dependent gene synexpression,Citation83–Citation86 IL-1α polymorphism with increased IL-1α signaling,Citation272 and, finally, the IL-1β-producing PAPA (pyogenic arthritis, pyoderma gangrenosum, and acne) syndrome.Citation273–Citation275
Epidemic acne vulgaris is an mTORC1-driven systemic disease of Western civilization such as obesity, diabetes, and cancer.Citation47,Citation99,Citation276–Citation278 Acne patients should control their total calorie uptake and restrict sugar and refined carbohydrates, milk, whey, and casein protein supplements, saturated fats, and trans-fats. Acne patients should avoid pasteurized fresh milk intake that transfers bioactive microRNA-21, a most critical microRNA that downregulates FoxO1 and promotes inflammation.Citation136,Citation161,Citation162,Citation171,Citation172
The ideal “antiacne diet” will be a paleolithic-like nutrition with accentuated intake of vegetables and fruits with low glycemic index and sea fish enriched in anti-inflammatory ω3-fatty acids.Citation279–Citation281 Beneficial and acne-preventive nutrients should contain plant-derived natural mTORC1 inhibitors such as green tea (EGCG), resveratrol, curcumin, genistein, and silymarin ().Citation236,Citation282–Citation284
Western diet obviously induces an IGF-1/mTORC1-driven pilosebaceous inflammasomopathy of adolescence, unmasking a visible metabolic danger signal, which should alert the medical community. Comparable NLRP3-driven reaction patterns have been realized as major pathogenic factors of serious diseases of civilization.Citation212 The advice of Kapahi et alCitation285 “with TOR less is more” apparently applies for the treatment and prevention of the most common diet-induced inflammatory skin disease. Future acne research should determine in vivo mTOR expression and mTORC1-dependent phosphorylation states of S6K1 and 4E-binding protein 1 in acne skin, which could explain the disturbed diet-induced metabolomics in acne skin and their corrections by dietary intervention such as the decreased expression of SREBP and IL-8 in lesional skin of acne patients during a low glycemic load diet.Citation78
Acknowledgments
No sources of funding were used to assist in the preparation of this paper.
Disclosure
The author reports no conflict of interest in this work.
References
- PuigOTjianRNutrient availability and growth: regulation of insulin signaling by dFOXO/FOXO1Cell Cycle20065550350516552183
- KousteniSFoxO1, the transcriptional chief of staff of energy metabolismBone201250243744321816244
- GrossDNWanMBirnbaumMJThe role of FOXO in the regulation of metabolismCurr Diab Rep20099320821419490822
- Lettieri BarbatoDAquilanoKCirioloMRFoxO1 at the nexus between fat catabolism and longevity pathwaysBiochim Biophys Acta20141841101555156025135341
- GulatiPThomasGNutrient sensing in the mTOR/S6K1 signalling pathwayBiochem Soc Trans200735Pt 223623817371247
- DibbleCCManningBDSignal integration by mTORC1 coordinates nutrient input with biosynthetic outputNat Cell Biol201315655556423728461
- HowellJJRicoultSJBen-SahraIManningBDA growing role for mTOR in promoting anabolic metabolismBiochem Soc Trans201341490691223863154
- CuyàsECorominas-FajaBJovenJMenendezJACell cycle regulation by the nutrient-sensing mammalian target of rapamycin (mTOR) pathwayMethods Mol Biol2014117011314424906312
- Imperato-McGinleyJGautierTCaiLQYeeBEpsteinJPochiPThe androgen control of sebum production. Studies of subjects with dihydrotestosterone deficiency and complete androgen insensitivityJ Clin Endocrinol Metab19937625245288381804
- JuulABangPHertelNTSerum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size, and body mass indexJ Clin Endocrinol Metab19947837447528126152
- DeplewskiDRosenfieldRLGrowth hormone and insulin-like growth factors have different effects on sebaceous cell growth and differentiationEndocrinology199914094089409410465280
- LaronZKowaldo-SilbergeldAEshetRPertzelnAGrowth hormone resistanceAnn Clin Res19801252692776263171
- Ben-AmitaiDLaronZEffect of insulin-like growth factor-1 deficiency or administration on the occurrence of acneJ Eur Acad Dermatol Venereol201125895095421054577
- Guevara-AguirreJBalasubramanianPGuevara-AguirreMGrowth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humansSci Transl Med201137070ra13
- KlingerBAninSSilbergeldAEshetRLaronZDevelopment of hyperandrogenism during treatment with insulin-like growth factor-I (IGF-I) in female patients with Laron syndromeClin Endocrinol (Oxf)199848181879509072
- DenleyACosgroveLJBookerGWMolecular interactions of the IGF systemCytokine Growth Factor Rev2005164–542143915936977
- PlewigGFultonJEKligmanAMCellular dynamics of comedo formation in acneArch Dermatol Forsch1971242112294258128
- LaiJJChangPLaiKPChenLChangCThe role of androgen and androgen receptor in the skin-related disordersArch Derm Res2012304749951022829074
- LiJAl-AzzawiFMechanism of androgen receptor actionMaturitas200963214214819372015
- MelnikBCSchmitzGRole of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgarisExp Dermatol2009181083384119709092
- HortonRPasupulettiVAntonipillaiIAndrogen induction of steroid 5 alpha-reductase may be mediated via insulin-like growth factor-IEndocrinology199313324474518344190
- PetersMAMolJAvan WolferenMEOosterlaken-DijksterhuisMATeerdsKJvan SluijsFJExpression of the insulin-like growth factor (IGF) system and steroidogenic enzymes in canine testis tumorsReprod Biol Endocrinol200312212646054
- WangGMO’ShaughnessyPJChubbCRobaireBHardyMPEffects of insulin-like growth factor I on steroidogenic enzyme expression levels in mouse leydig cellsEndocrinology2003144115058506412959969
- BerenszteinEBBaquedanoMSPepeCMRole of IGFs and insulin in the human testis during postnatal activation: differentiation of steroidogenic cellsPediatr Res200863666266618520331
- DennerLBodenburgYHJiangJPagèsGUrbanRJInsulin-like growth factor-I activates extracellularly regulated kinase to regulate the p450 side-chain cleavage insulin-like response element in granulosa cellsEndocrinology201015162819282520371701
- InuiSItamiSAndrogen actions on the human hair follicle: perspectivesExp Dermatol201322316817123016593
- FanWYanaseTMorinagaHInsulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptorJ Biol Chem2007282107329733817202144
- MaQFuWLiPFoxO1 mediates PTEN suppression of androgen receptor N- and C-terminal interactions and coactivator recruitmentMol Endocrinol200923221322519074551
- Van der HeideLPHoekmanMFSmidtMPThe ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulationBiochem J2004380Pt 229730915005655
- ZhaoYTindallDJHuangHModulation of androgen receptor by FOXA1 and FOXO1 factors in prostate cancerInt J Biol Sci201410661461924948874
- DehmSMReganKMSchmidtLJTindallDJSelective role of an NH2-terminal WxxLF motif for aberrant androgen receptor activation in androgen depletion independent prostate cancer cellsCancer Res20076720100671007717942941
- SawayaMEShalitaARAndrogen receptor polymorphisms (CAG repeat lengths) in androgenetic alopecia, hirsutism, and acneJ Cutan Med Surg1998319159677254
- MelnikBCJohnSMSchmitzGOver-stimulation of insulin/IGF-1 signaling by Western diet may promote diseases of civilization: lessons learnt from Laron syndromeNutr Metab (Lond)201184121699736
- MelnikBCFoxO1 – the key for the pathogenesis and therapy of acne?J Dtsch Dermatol Ges20108210511120151947
- MirdamadiYSThielitzAWiedeAIGF-1 induces nuclear up-regulation of p-Akt and controls expression of nuclear transcription factor FoxO1 levels in SZ95 sebocytes. 41th Annual Meeting of the Arbeitsgemeinschaft Dermatologische Forschung (ADF), P098, e17Exp Dermatol2014233E18
- NemotoSFergussonMMFinkelTNutrient availability regulates SIRT1 through a forkhead-dependent pathwayScience200430657042105210815604409
- KramerJMDavidgeJTLockyerJMStaveleyBEExpression of Drosophila FOXO regulates growth and can phenocopy starvationBMC Dev Biol20033512844367
- LuckyAWBiroFMHusterGALeachADMorrisonJARattermanJAcne vulgaris in premenarchal girls. An early sign of puberty associated with rising levels of dehydroepiandrosteroneArch Dermatol199413033083148129408
- LindschauCKirschTKlingeUKolkhofPPetersIFiebelerADehydroepiandrosterone-induced phosphorylation and translocation of FoxO1 depend on the mineralocorticoid receptorHypertension201158347147821747041
- ZouboulisCCBaronJMBöhmMFrontiers in sebaceous gland biology and pathologyExp Dermatol200817654255118474083
- MelnikBCIsotretinoin and FoxO1: a scientific hypothesisDermatoendocrinol20113314116522110774
- SasakiTKitamuraTRoles of FoxO1 and Sirt1 in the central regulation of food intakeEndocr J2010571193994621048357
- YangGLimCYLiCFoxO1 inhibits leptin regulation of pro-opiomelanocortin promoter activity by blocking STAT3 interaction with specificity protein 1J Biol Chem200928463719372719049975
- ThackrayVGFox tales: regulation of gonadotropin gene expression by forkhead transcription factorsMol Cell Endocrinol20143851–2627024099863
- DongXCCoppsKDGuoSInactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulationCell Metab200881657618590693
- BurnsKHOwensGEOgbonnaSCNilsonJHMatzukMMExpression profiling analyses of gonadotropin responses and tumor development in the absence of inhibinsEndocrinology2003144104492450712959983
- MelnikBCZouboulisCCPotential role of FoxO1 and mTORC1 in the pathogenesis of Western diet-induced acneExp Dermatol201322531131523614736
- ArriolaDJMayoSLSkarraDVBensonCAThackrayVGFOXO1 transcription factor inhibits luteinizing hormone β gene expression in pituitary gonadotrope cellsJ Biol Chem201228740334243343522865884
- ChoiYSLeeHJKuCRFoxO1 is a negative regulator of FSHβ gene expression in basal and GnRH-stimulated conditions in femaleEndocrinology201415562277228624437485
- SkarraDVArriolaDJBensonCAThackrayVGForkhead box O1 is a repressor of basal and GnRH-induced Fshb transcription in gonadotropesMol Endocrinol201327111825183924065703
- PalaniappanMMenonKMLuteinizing hormone/human chorionic gonadotropin-mediated activation of mTORC1 signaling is required for androgen synthesis by theca-interstitial cellsMol Endocrinol201226101732174222827930
- SkarraDVThackrayVGFOXO1 is regulated by insulin and IGF1 in pituitary gonadotropesMol Cell Endocrinol2015405142425676570
- KaradagASErtugrulDTTutalEAkinKOIsotretinoin influences pituitary hormone levels in acne patientsActa Derm Venereol2011911313421103844
- KaradagASTakciZErtugrulDTBilgiliSGBalahorogluRTakirMThe effect of different doses of isotretinoin on pituitary hormonesDermatology2015230435435925721216
- KaradagASErtugrulDTTutalEAkinKOShort-term isotretinoin treatment decreases insulin-like growth factor-1 and insulin-like growth factor binding protein-3 levels: does isotretinoin affect growth hormone physiology?Br J Dermatol2010162479880220128787
- DeplewskiDRosenfieldRLRole of hormones in pilosebaceous unit developmentEndocr Rev200021436339210950157
- VoraSOvhalAJerajaniHNairNChakraborttyACorrelation of facial sebum to serum insulin-like growth factor-1 in patients with acneBr J Dermatol2008159499099118652583
- AizawaHNiimuraMElevated serum insulin-like growth factor-1 (IGF-1) levels in women with postadolescent acneJ Dermatol19952242492527608381
- CappelMMaugerDThiboutotDCorrelation between serum levels of insulin-like growth factor 1, dehydroepiandrosterone sulfate, and dihydrotestosterone and acne lesion counts in adult womenArch Dermatol2005141333333815781674
- TasilLTurgutSKacarNInsulin-like growth factor-I gene polymorphism in acne vulgarisJ Eur Acad Dermatol Venereol201327225425723457723
- JungJYYoonMYHongJSChoiYSSuhDHThe influence of dietary patterns on acne vulgaris in KoreansEur J Dermatol201020615
- SmithTMCongZGillilandKLClawsonGAThiboutotDMInsulin-like growth factor-1 induces lipid production in human SEB-1 sebocytes via sterol response element-binding protein-1J Invest Dermatol200612661226123216575389
- SmithTMGillilandKClawsonGAThiboutotDIGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathwayJ Invest Dermatol200812851286129317989724
- RosenfieldRLDeplewskiDKentsisACilettiNMechanisms of androgen induction of sebocyte differentiationDermatology1998196143469557223
- DownieMMSandersDAMaierLMStockDMKealeyTPeroxisome proliferator-activated receptor and farnesoid X receptor ligands differentially regulate sebaceous differentiation in human sebaceous gland organ cultures in vitroBr J Dermatol2004151476677515491415
- DozsaADezsoBTothBIPPARγ-mediated and arachidonic acid-dependent signaling is involved in differentiation and lipid production of human sebocytesJ Invest Dermatol2014134491092024129064
- TrivediNRCongZNelsonAMPeroxisome proliferator-activated receptors increase human sebum productionJ Invest Dermatol200612692002200916675962
- RussellLEHarrisonWJBahtaAWZouboulisCCBurrinJMPhilpottMPCharacterization of liver X receptor expression and function in human skin and the pilosebaceous unitExp Dermatol2007161084485217845217
- HongILeeMHNaTYZouboulisCCLeeMOLXRalpha enhances lipid synthesis in SZ95 sebocytesJ Invest Dermatol200812851266127217960176
- HarrisonWJBullJJSeltmannHZouboulisCCPhilpottMPExpression of lipogenic factors galectin-12, resistin, SREBP-1, and SCD in human sebaceous glands and cultured sebocytesJ Invest Dermatol200712761309131717363919
- DowellPOttoTCAdiSLaneMDConvergence of peroxisome proliferator-activated receptor gamma and Foxo1 signaling pathwaysJ Biol Chem200327846454854549112966085
- FanWImamuraTSonodaNFOXO1 transrepresses peroxisome proliferator-activated receptor gamma transactivation, coordinating an insulin-induced feedorward response in adipocytesJ Biol Chem200928418121881219719246449
- ArmoniMHarelCKarniSFOXO1 represses peroxisome proliferator-activated receptor-gamma1 and -gamma2 gene promoters in primary adipocytes. A novel paradigm to increase insulin sensitivityJ Biol Chem200628129198811989116670091
- QuSSuDAltomonteJPPARα mediates the hypolipidemic action of fibrates by antagonizing FoxO1Am J Physiol Endocrinol Metab20072922E421E43416985262
- KameiYMiuraSSuganamiTRegulation of SREBP1c gene expression in skeletal muscle: role of retinoid X receptor/liver X receptor and forkhead-O1 transcription factorEndocrinology200814952293230518202130
- LiuXQiaoAKeYFoxO1 represses LXRα-mediated transcriptional activity of SREBP-1c promoter in HepG2 cellsFEBS Lett2010584204330433420868688
- DengXZhangWO-SullivanIFoxO1 inhibits sterol regulatory element-binding protein-1c (SREBP-1c) gene expression via transcription factors Sp1 and SREBP-1cJ Biol Chem201228724201322014322511764
- KwonHHYoonJYHongJSJungJYParkMSSuhDHClinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trialActa Derm Venereol201292324124622678562
- CordainLLindebergSHurtadoMHillKEatonSBBrand-MillerJAcne vulgaris: a disease of Western civilizationArch Dermatol2002138121584159012472346
- LindebergSEliassonMLindahlBAhrénBLow serum insulin in traditional Pacific Islanders – the Kitava StudyMetabolism199948101216121910535381
- LevineMESuarezJABrandhorstSLow protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older populationCell Metab201419340741724606898
- McNairnAJDoucetYDemaudeJTGFβ signaling regulates lipogenesis in human sebaceous glands cellsBMC Dermatol201313223343495
- NavariniAASimpsonMAWealeMGenome-wide association study identifies three novel susceptibility loci for severe acne vulgarisNat Commun20145402024927181
- MassaguéJGomisRRThe logic of TGFbeta signalingFEBS Lett2006580122811282016678165
- ArdenKCFoxO: linking new signaling pathwaysMol Cell200414441641815149589
- GomisRRAlarcónCHeWA FoxO-Smad synexpression group in human keratinocytesProc Natl Acad Sci U S A200610334127471275216908841
- SeoaneJLeHVShenLAndersonSAMassaguéJIntegration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferationCell2004117221122315084259
- NelsonAMGillilandKLCongZThiboutotDM13-cis retinoic acid induces apoptosis and cell cycle arrest in human SEB-1 sebocytesJ Invest Dermatol2006126102178218916575387
- MelnikBCThe role of transcription factor FoxO1 in the pathogenesis of acne vulgaris and the mode of isotretinoin actionG Ital Dermatol Venereol2010145555957120930691
- BianYTerseADuJProgressive tumor formation in mice with conditional deletion of TGF-beta signaling in head and neck epithelia is associated with activation of the PI3K/Akt pathwayCancer Res200969145918592619584284
- MerrillBJGatUDasGuptaRFuchsETcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skinGenes Dev200115131688170511445543
- NiemannCOwensDMHulskenJBirchmeierWWattFMExpression of DeltaNLef1 in mouse epidermis results in differentiation of hair follicles into squamous epidermal cysts and formation of skin tumoursDevelopment200212919510911782404
- HanGLiAGLiangYYSmad7-induced beta-catenin degradation alters epidermal appendage developmentDev Cell200611330131216950122
- NiemannCDifferentiation of the sebaceous glandDermatoendocrinol200912646720224685
- Lo CelsoCBertaMABraunKMCharacterization of bipotent epidermal progenitors derived from human sebaceous gland: Contrasting roles of c-myc and β-cateninStem Cells20082651241125218308950
- EssersMAde Vries-SmitsLMBarkerNPoldermanPEBurgeringBMKorswagenHCFunctional interaction of beta-catenin and FOXO in oxidative stress signalingScience200530857251181118415905404
- ChenCCJeonSMBhaskarPTNogueiraVFoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and RictorDev Cell201018459260420412774
- HayNInterplay between FOXO, TOR, and AktBiochim Biophys Acta20111813111965197021440577
- MelnikBDietary intervention in acne: attenuation of increased mTORC1 signaling promoted by Western dietDermatoendocrinol201241203222870349
- MelnikBCThe role of mTORC1 in acne pathogenesis and treatmentExp Rev Dermatol201386617622
- JüngerMARintelenFStockerHDrosophila forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signalingJ Biol2003232012908874
- PuigOMarrMTRuhfMLTjianRControl of cell number by Drosophila FOXO: downstream and feedback regulation of the insulin receptor pathwayGenes Dev200317162006202012893776
- FosterKGFingarDCMammalian target of rapamycin (mTOR): conducting the cellular signalling symphonyJ Biol Chem201028519140711407720231296
- KimSGBuelGRBlenisJNutrient regulation of the mTOR complex 1 signaling pathwayMol Cells201335646347323694989
- Bar-PeledLSabatiniDMRegulation of mTORC1 by amino acidsTrends Cell Biol201424740040624698685
- AvruchJLongXOrtiz-VegaSRapleyJPapageorgiouADaiNAmino acid regulation of TOR complex 1Am J Physiol Endocrinol Metab20092964E592E60218765678
- DoddKMTeeARLeucine and mTORC1: a complex relationshipAm J Physiol Endocrinol Metab201230211E1329E134222354780
- JewellJLKimYCRussellRCMetabolism. Differential regulation of mTORC1 by leucine and glutamineScience2015347621819419825567907
- AverousJLambert-LanglaisSCarraroVRequirement for lysosomal localization of mTOR for its activation differs between leucine and other amino acidsCell Signal20142691918192724793303
- ChantranupongLWolfsonRLSabatiniDMNutrient-sensing mechanisms across evolutionCell20151611678325815986
- WangXProudCGNutrient control of TORC1, a cell-cycle regulatorCell2009196260267
- RicoultSJManningBDThe multifaceted role of mTORC1 in the control of lipid metabolismEMBO Rep201314324225123399656
- QuinnWJ3rdBirnbaumMJDistinct mTORC1 pathways for transcription and cleavage of SREBP-1cProc Natl Acad Sci U S A201210940159741597523012450
- PorstmannTSantosCRLewisCGriffithsBSchulzeAA new player in the orchestra of cell growth: SREBP activity is regulated by mTORC1 and contributes to the regulation of cell and organ sizeBiochem Soc Trans200937Pt 127828319143646
- LewisCAGriffithsBSantosCRPendeMSchulzeARegulation of the SREBP transcription factors by mTORC1Biochem Soc Trans201139249549921428927
- LaplanteMSabatiniDMRegulation of mTORC1 and its impact on gene expression at a glanceJ Cell Sci2013126Pt 81713171923641065
- NakamuraMTNaraTYGene regulation of mammalian desaturasesBiochem Soc Trans200230Pt 61076107912440976
- ArboIHalleCMalikDBrattbakkHRJohansenBInsulin induces fatty acid desaturase expression in human monocytesScand J Clin Lab Invest201171433033921413848
- AlestasTGancevicieneRFimmelSMüller-DeckerKZouboulisCCEnzymes involved in the biosynthesis of leukotriene B4 and prostaglandin E2 are active in sebaceous glandsJ Mol Med (Berl)2006841758716388388
- GeLGordonJSHsuanCStennKProutySMIdentification of the delta-6 desaturase of human sebaceous glands: expression and enzyme activityJ Invest Dermatol2003120570771412713571
- DrakeDRBrogdenKADawsonDVWertzPWThematic review series: skin lipids. Antimicrobial lipids at the skin surfaceJ Lipid Res200849141117906220
- FischerCLBlanchetteDRBrogdenKAThe roles of cutaneous lipids in host defenseBiochim Biophys Acta20141841331932223994607
- AkazaNAkamatsuHNumataSFatty acid compositions of triglycerides and free fatty acids in sebum depend on amount of triglycerides, and do not differ in presence or absence of acne vulgarisJ Dermatol201441121069107625388081
- GhodsiSZOrawaHZouboulisCCPrevalence, severity, and severity risk factors of acne in high school pupils: a community-based studyJ Invest Dermatol200912992136214119282841
- BhateKWilliamsHCEpidemiology of acne vulgarisBr J Dermatol2013168347448523210645
- BurrisJRietkerkWWoolfKRelationships of self-reported dietary factors and perceived acne severity in a cohort of New York young adultsJ Acad Nutr Diet2014114338439224412232
- MahmoodSNBoweWPDiet and acne update. Carbohydrates emerge as the main culpritJ Drugs Dermatol201413442843524719062
- BurrisJRietkerkWWoolfKAcne: the role of medical nutrition therapyJ Acad Nutr Diet2013113341643023438493
- IsmailNHManafZAAzizanNZHigh glycemic load diet, milk and ice cream consumption are related to acne vulgaris in Malaysian young adults: a case control studyBMC Dermatol2012121322898209
- SmithRNMannNJBraueAMäkeläinenHVarigosGAA low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trialAm J Clin Nutr200786110711517616769
- SmithRMannNMäkeläinenHRoperJBraueAVarigosGA pilot study to determine the short-term effects of a low glycemic load diet on hormonal markers of acne: a nonrandomized, parallel, controlled feeding trialMol Nutr Food Res200852671872618496812
- WolkensteinPMiseryLAmiciJMSmoking and dietary factors associated with moderate-to-severe acne in French adolescents and young adults: results of a survey using a representative sampleDermatology20152301343925413494
- SmithRNBraueAVarigosGAMannNJThe effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglyceridesJ Dermatol Sci2008501415218178063
- FlowersEWonGYFukuokaYMicroRNAs associated with exercise and diet: a systematic reviewPhysiol Genomics201547111125465031
- ShangYYFangNNWangFMicroRNA-21, induced by high glucose, modulates macrophage apoptosis via programmed cell death 4Mol Med Rep201512146346925738901
- SheedyFJTurning 21: induction of miR-21 as a key switch in the inflammatory responseFront Immunol201529;619
- WangZBrandtSMedeirosAMicroRNA 21 is a homeostatic regulator of macrophage polarization and prevents prostaglandin E2-mediated M2 generationPLoS One2015102e011585525706647
- MurugaiyanGda CunhaAPAjayAKMicroRNA-21 promotes Th17 differentiation and mediates experimental autoimmune encephalomyelitisJ Clin Invest201512531069108025642768
- BulkleyLDAcne, its Etiology, Pathology and TreatmentNew York, NYGP Putnam’s Sons1885
- AdebamowoCASpiegelmanDDanbyFWFrazierALWillettWCHolmesMDHigh school dietary dairy intake and teenage acneJ Am Acad Dermatol200552220721415692464
- AdebamowoCASpiegelmanDBerkeyCSMilk consumption and acne in adolescent girlsDermatol Online J2006124117083856
- AdebamowoCASpiegelmanDBerkeyCSMilk consumption and acne in teenaged boysJ Am Acad Dermatol200858578779318194824
- Di LandroACazzanigaSParazziniFFamily history, body mass index, selected dietary factors, menstrual history, and risk of moderate to severe acne in adolescents and young adultsJ Am Acad Dermatol20126761129113522386050
- GrossiECazzanigaSCrottiSThe constellation of dietary factors in adolescent acne: a semantic connectivity map approachJ Eur Acad Dermatol Venereol Epub1222014
- MelnikBCJohnSMSchmitzGMilk is not just food but most likely a genetic transfection system activating mTORC1 signaling for postnatal growthNutr J20131210323883112
- Rich-EdwardsJWGanmaaDPollakMNMilk consumption and the prepubertal somatotropic axisNutr J200762817900364
- HeineWRadkeMWutzkeKDPetersEKundtGAlpha-lactalbumin enriched low-protein infant formulas: a comparison to breast milk feedingActa Paediatr1996859102410288888911
- HarpJBGoldsteinSPhillipsLSNutrition and somatomedin. XXIII. Molecular regulation of IGF-I by amino acid availability in cultured hepatocytesDiabetes1991401951011901809
- MelnikBCThe pathogenic role of persistent milk signaling in mTORC1- and milk-microRNA-driven type 2 diabetes mellitusCurr Diabetes Rev2015111466225587719
- HoytGHickeyMSCordainLDissociation of the glycaemic and insulinaemic responses to whole and skimmed milkBr J Nutr200593217517715788109
- MillwardDJLaymanDKToméDSchaafsmaGProtein quality assessment: impact of expanding understanding of protein and amino acid needs for optimal healthAm J Clin Nutr20088751576S1581S18469291
- LendersCMLiuSWilmoreDWEvaluation of a novel food composition database that includes glutamine and other amino acids derived from gene sequencing dataEur J Clin Nutr200963121433143919756030
- NicklinPBergmanPZhangBBidirectional transport of amino acids regulates mTOR and autophagyCell2009136352153419203585
- DuranRVOppligerWRobitailleAMGlutaminolysis activates Rag-mTORC1 signalingMol Cell201247334935822749528
- DownieMMKealeyTHuman sebaceous glands engage in aerobic gly-colysis and glutaminolysisBr J Dermatol2004151232032715327538
- MelnikBCAndrogen abuse in the communityCurr Opin Endocrinol Diabetes Obes200916321822319373082
- MelnikBCEvidence for acne-promoting effects of milk and other insulinotropic dairy productsNestle Nutr Workshop Ser Pediatr Program201167131145
- SimonartTAcne and whey protein supplementation among bodybuildersDermatology2012225325625823257731
- SilverbergNBWhey protein precipitating moderate to severe acne flares in 5 teenaged athletesCutis2012902707222988649
- Pontes TdeCFernandes FilhoGMTrindade AdeSSobral FilhoJFIncidence of acne vulgaris in young adult users of protein-calorie supplements in the city of João Pessoa – PBAn Bras Dermatol201388690791224474098
- BaierSRNguyenCXieFWoodJRZempleniJMicroRNAs are absorbed in biologically meaningful amounts from nutritionally relevant doses of cow milk and affect gene expression in peripheral blood mononuclear cells, HEK-293 kidney cell cultures, and mouse liversJ Nutr2014144101495150025122645
- HowardKMJati KusumaRBaierSRLoss of miRNAs during processing and storage of cow’s (Bos taurus) milkJ Agric Food Chem201563258859225565082
- IzumiHTsudaMSatoYBovine milk exosomes contain microRNA and mRNA and are taken up by human macrophagesJ Dairy Sci20159852920293325726110
- AmbrosVThe functions of animal microRNAsNature2004431700635035515372042
- LudwigAKGiebelBExosomes: small vesicles participating in inter-cellular communicationInt J Biochem Cell Biol2012441111522024155
- ChenXLiangHZhangJZenKZhangCYHorizontal transfer of microRNAs: molecular mechanism and clinical applicationsProtein Cell201231283722314808
- IgazIIgazPPossible role for microRNAs as inter-species mediators of epigenetic information in disease pathogenesis: is the non-coding dark matter of the genome responsible for epigenetic interindividual or interspecies communication?Med Hypotheses201584215015425535106
- ChenXGaoCLiHIdentification and characterization of microRNAs in raw milk during different periods of lactation, commercial fluid, and powdered milk productsCell Res201020101128113720548333
- MengFHensonRWehbe-JanekHGhoshalKJacobSTPatelTMicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancerGastroenterology2007133264765817681183
- HanMLiuMWangYAntagonism of miR-21 reverses epithelial-mesenchymal transition and cancer stem cell phenotype through AKT/ERK1/2 incactivation by targeting PTENPLoS One201276e3952022761812
- LeiBXLiuZHLiZJLiCDengYFmiR-21 induces cell proliferation and suppresses the chemosensitivity in glioblastoma cells via down-regulation of FOXO1Int J Clin Exp Med2014782060206625232387
- SongWWangLWangLLiQInterplay of miR-21 and FoxO1 modulates growth of pancreatic ductal adenocarcinomaTumour Biol2015 Epub1272015
- YangCHYueJPfefferSRMicroRNA-21 promotes glioblastoma tumorigenesis by down-regulating insulin-like growth factor-binding protein-3 (IGFBP3)J Biol Chem201428936250792508725059666
- TianTZhuYLZhouYYExosomal uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 deliveryJ Biol Chem201428932222582226724951588
- DanbyFWAcne and milk, the diet myth, and beyondJ Am Acad Dermatol200552236036215692488
- GaianiRChiesaFMattioliMNannettiGGaleatiGAndrostenedione and testosterone concentrations in plasma and milk of the cow throughout pregnancyJ Reprod Fertil198470155596694152
- RegalPCepedaAFenteCDevelopment of an LC-MS/MS method to quantify sex hormones in bovine milk and influence of pregnancy in their levelsFood Addit Contam Part A Chem Anal Control Expo Risk Assess201229577077922332636
- TengYLitchfieldLMIvanovaMMProughRAClarkBJKlingeCMDehydroepiandrosterone-induces miR-21 transcription in HepG2 cells through estrogen receptor β and androgen receptorMol Cell Endocrinol20143921–2233624845419
- ChenXLiangHZhangJZenKZhangCYmicroRNAs are ligands of Toll-like receptorsRNA201319673773923554231
- FabbriMPaoneACaloreFMicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory responseProc Natl Acad Sci U S A201210931E2110E211622753494
- SchwarzHPosseltGWurmPUlbingMDuschlAHorejs-HoeckJTLR8 and NOD signaling synergistically induce the production of IL-1β and IL-23 in monocyte-derived DCs and enhance the expression of the feedback inhibitor SOCS2Immunobiology2013218453354222795647
- GuoHGaoJTaxmanDJTingJPSuLHIV-1 infection induces interleukin-1β production via TLR8 protein-dependent and NLRP3 inflammasome mechanisms in human monocytesJ Biol Chem201428931217162172624939850
- YasudaMTanakaYKumeSFatty acids are novel nutrient factors to regulate mTORC1 lysosomal localization and apoptosis in podocytesBiochim Biophys Acta2014184271097110824726883
- JensenRGFerrisAMLammi-KeefeCJThe composition of milk fatJ Dairy Sci1991749322832431779072
- BitmanJWoodDLChanges in milk fat phospholipids during lactationJ Dairy Sci1990735120812162365882
- TrattnerSBeckerWWretlingSÖhrvikVMattissonIFatty acid composition of Swedish bakery products, with emphasis on trans-fatty acidsFood Chem201517542343025577101
- MulderKAFerdinandsARRichardsonKJInnisSMSources of trans and saturated fatty acids in the diets of Vancouver childrenCan J Diet Pract Res201374171323449208
- de OliveiraJLOyamaLMHachulACHydrogenated fat intake during pregnancy and lactation caused increase in TRAF-6 and reduced AdipoR1 in white adipose tissue, but not in muscle of 21 days old offspring ratsLipids Health Dis2011102221266050
- CaoZXiongJTakeuchiMKuramaTGoeddelDVTRAF6 is a signal transducer for interleukin-1Nature199638365994434468837778
- VerstakBNagpalKBottomleySPGolenbockDTHertzogPJMansellAMyD88 adapter-like (Mal)/TIRAP interaction with TRAF6 is critical for TLR2- and TLR4-mediated NF-kappaB proinflammatory responsesJ Biol Chem200928436241922420319592497
- McGinleyKJWebsterGFRuggieriMRLeydenJJRegional variations in density of cutaneous propionibacteria: correlation of Propionibacterium acnes populations with sebaceous secretionJ Clin Microbiol19801256726757276142
- LomholtHBKilianMPopulation genetic analysis of Propionibacterium acnes identifies a subpopulation and epidemic clones associated with acnePLoS One201058e1227720808860
- BrzuszkiewiczEWeinerJWollherrAComparative genomics and transcriptomics of Propionibacterium acnesPLoS One201166e2158121738717
- JahnsACLundskogBGancevicieneRAn increased incidence of Propionibacterium acnes biofilms in acne vulgaris: a case-control studyBr J Dermatol20121671505822356121
- LwinSMKimberIMcFaddenJPAcne, quorum sensing and dangerClin Exp Dermatol201439216216724524558
- BrackmanGCoenyeTQuorum sensing inhibitors as anti-biofilm agentsCurr Pharm Des201521151125189863
- CoenyeTPeetersENelisHJBiofilm formation by Propionibacterium acnes is associated with increased resistance to antimicrobial agents and increased production of putative virulence factorsRes Microbiol2007158438639217399956
- MiskinJEFarrellAMCunliffeWJHollandKTPropionibacterium acnes, a resident of lipid-rich human skin, produces a 33 kDa extracellular lipase encoded by gehAMicrobiology1997143Pt 5174517559168624
- ZouboulisCCJourdanEPicardoMAcne is an inflammatory disease and alterations of sebum composition initiate acne lesionsJ Eur Acad Dermatol Venereol201428552753224134468
- GribbonEMCunliffeWJHollandKTInteraction of Propionibacterium acnes with skin lipids in vitroJ Gen Microbiol19931398174517518409917
- UshijimaTTakahashiMOzakiYAcetic, propionic, and oleic acid as the possible factors influencing the predominant residence of some species of Propionibacterium and coagulase-negative Staphylococcus on normal human skinCan J Microbiol19843056476526744125
- JugeauSTenaudIKnolACInduction of toll-like receptors by Propionibacterium acnesBr J Dermatol200515361105111316307644
- BakryOASamakaRMSebikaHSeleitIToll-like receptor 2 and P. acnes: do they trigger initial acne vulgaris lesions?Anal Quant Cytopathol Histpathol201436210011024902362
- CsakTGanzMPespisaJKodysKDolganiucASzaboGFatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cellsHepatology201154113314421488066
- SnodgrassRGHuangSChoiIWRutledgeJCHwangDHInflammasome-mediated secretion of IL-1β in human monocytes through TLR2 activation; modulation by dietary fatty acidsJ Immunol201319184337434724043885
- HuangSRutkowskyJMSnodgrassRGSaturated fatty acids activate TLR-mediated proinflammatory signaling pathwaysJ Lipid Res20125392002201322766885
- NakatsujiTKaoMCZhangLZouboulisCCGalloRLHuangCMSebum free fatty acids enhance the innate immune defense of human sebocytes by upregulating beta-defensin-2 expressionJ Invest Dermatol2010130498599420032992
- Legrand-PoelsSEsserNL’hommeLFree fatty acids as modulators of the NLRP3 inflammasome in obesity/type 2 diabetesBiochem Pharmacol201492113114125175736
- WeberKSchillingJDLysosomes integrate metabolic-inflammatory cross-talk in primary macrophage inflammasome activationJ Biol Chem20142891391589157124532802
- L’hommeLEsserNRivaLUnsaturated fatty acids prevent activation of NLRP3 inflammasome in human monocytes/macrophagesJ Lipid Res201354112998300824006511
- Alvarez-GarciaORogersNHSmithRGLotzMKPalmitate has proapoptotic and proinflammatory effects on articular cartilage and synergizes with interleukin-1Arthritis Rheumatol20146671779178824591481
- SchroderKZhouRTschoppJThe NLRP3 inflammasome: a sensor for metabolic danger?Science2010327596329630020075245
- KistowskaMGehrkeSJankovicDIL-1β drives inflammatory responses to Propionibacterium acnes in vitro and in vivoJ Invest Dermatol2014134367768524157462
- WenHGrisDLeiYFatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signalingNat Immunol201112540841521478880
- QinMPirouzAKimMHKrutzikSRGarbánHJKimJPropionibacterium acnes induces IL-1β secretion via the NLRP3 inflammasome in human monocytesJ Invest Dermatol2014134238138823884315
- LiZJChoiDKSohnKCPropionibacterium acnes activates the NLRP3 inflammasome in human sebocytesJ Invest Dermatol2014134112747275624820890
- KelhäläHLPalatsiRFyhrquistNIL-17/Th17 pathway is activated in acne lesionsPLoS One201498e10523825153527
- KistowskaMMeierBProustTPropionibacterium acnes promotes Th17 and Th17/Th1 responses in acne patientsJ Invest Dermatol2015135111011825010142
- MaddurMSMiossecPKaveriSVBayryJTh17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategiesAm J Pathol2012181181822640807
- HuangGWangYChiHRegulation of Th17 cell differentiation by innate immune signalsCell Mol Imunnol20129287295
- DuFNakamuraYTanTLExpression of snail in epidermal keratinocytes promotes cutaneous inflammation and hyper-plasia conducive to tumor formationCancer Res20107024100801008921159631
- HaHLWangHPisitkunPIL-17 drives psoriatic inflammation via distinct, target cell-specific mechanismsProc Nat Acad Sci U S A201411133E3422E3431
- MartinDATowneJEKricorianGThe emerging role of IL-17 in the pathogenesis of psoriasis: preclinical and clinical findingsJ Invest Dermatol20131331172622673731
- ChoiEHAhnSKLeeSHThe changes of stratum corneum interstices and calcium distribution of follicular epithelium of experimentally induced comedones (EIC) by oleic acidExp Dermatol19976129359067704
- KatsutaYIidaTInomataSDendaMUnsaturated fatty acids induce calcium influx into keratinocytes and cause abnormal differentiation of epidermisJ Invest Dermatol200512451008101315854043
- KatsutaYIidaTHasegawaKInomataSDendaMFunction of oleic acid on epidermal barrier and calcium influx into keratinocytes is associated with N-methyl D-aspartate-type glutamate receptorsBr J Dermatol20091601697418808414
- HammerbergCBata-CsorgoZVoorheesJJCooperKDIL-1 and IL-1 receptor antagonist regulation during keratinocyte cell cycle and differentiation in normal and psoriatic epidermisArch Dermatol Res199829073673749749991
- EadyEAGoodwinCECoveJHInghamECunliffeWJInflammatory levels of interleukin 1 alpha are present in the majority of open come-dones in acne vulgarisArch Dermatol19911278123812391830734
- InghamEEadyEAGoodwinCECoveJHCunliffeWJPro-inflammatory levels of interleukin-1 alpha-like bioactivity are present in the majority of open comedones in acne vulgarisJ Invest Dermatol19929868959011534342
- GuyRGreenMRKealeyTModeling acne in vitroJ Invest Dermatol199610611761828592071
- SelwayJLKurczabTKealeyTLanglandsKToll-like receptor 2 activation and comedogenesis: implications for the pathogenesis of acneBMC Dermatol2013131024011352
- ThiboutotDMInflammasome activation by Propionibacterium acnes: the story of IL-1 in acne continues to unfoldJ Invest Dermatol2014134359559724518111
- CordainLImplications for the role of diet in acneSemin Cutan Med Surg2005242849116092796
- CordainLThe Dietary Cure for AcneFort Collins, COPaleo Diet Enterprises2006
- MelnikBCSchmitzGAre therapeutic effects of antiacne agents mediated by activation of FoxO1 and inhibition of mTORC1?Exp Dermatol201322750250423800068
- MelnikBCWestern-diet mediated mTORC1 signaling in acne, psoriasis, atopic dermatitis, and related disorders of civilization: therapeutic role of plant-derived natural mTORC1 inhibitorsWatsonRRZibadiSBiocative Dietary Factors and Plant Extracts in DermatologyNew York, NYSpringer Science + Business Media2013397419
- KhayefGYoungJBurns-WhitmoreBSpaldingTEffects of fish oil supplementation on inflammatory acneLipids Health Dis20121116523206895
- JungJYKwonHHHongJSEffect of dietary supplementation with omega-3 fatty acid and gamma-linolenic acid on acne vulgaris: a randomised, double-blind, controlled trialActa Derm Venereol201494552152524553997
- Williams-BeyYBoularanCVuralAOmega-3 free fatty acids suppress macrophage inflammasome activation by inhibiting NF-κB activation and enhancing autophagyPLoS One201496e9795724911523
- MobratenKHaugTMKleivelandCRLeaTOmega-3 and omega-6 PUFAs induce the same GPR120-mediated signalling events, but with different kinetics and intensity in Caco-2 cellsLipids Health Dis20131210123849180
- XuJChoHO’MalleySParkJHClarkeSDDietary polyunsaturated fats regulate rat liver sterol regulatory element binding proteins-1 and -2 in three distinct stages and by different mechanismsJ Nutr2002132113333333912421847
- KimHJTakahashiMEzakiOFish oil feeding decreases mature sterol regulatory element-binding protein 1 (SREBP-1) by down-regulation of SREBP-1c mRNA in mouse liver. A possible mechanism for down-regulation of lipogenic enzyme mRNAsJ Biol Chem199927436258922589810464332
- ImMKimSYSohnKCEpigallocatechin-3-gallate suppresses IGF-I-induced lipogenesis and cytokine expression in SZ95 sebocytesJ Invest Dermatol2012132122700270822763784
- Van AllerGSCarsonJDTangWEpigallocatechin gallate (EGCG), a major component of green tea, is a dual phosphoinositide-3-kinase/mTOR inhibitorBiochem Biophys Res Commun2011406219419921300025
- YoonJYKwonHHMinSUThiboutotDMSuhDHEpigallocatechin-3-gallate improves acne in humans by modulating intracellular molecular targets and inhibiting P. acnesJ Invest Dermatol2013133242944023096708
- MahmoodTAkhtarNKhanBAKhanHMSaeedTOutcomes of 3% green tea emulsion on skin sebum production in male volunteersBosn J Basic Med Sci201010326026420846135
- RubinMGKimKLoganACAcne vulgaris, mental health and omega-3 fatty acids: a report of casesLipids Health Dis200873618851733
- JiangHShangXWuHResveratrol downregulates PI3K/Akt/mTOR signaling pathways in human U251 glioma cellsJ Exp Ther Oncol200981253319827268
- BritoPMDevillardRNegre-SalvayreResveratrol inhibits the mTOR mitogenic signaling evoked by oxidized LDL in smooth muscle cellsAtherosclerosis2009205112613419108833
- LinJNLinVCRauKMResveratrol modulates tumor cell proliferation and protein translation via SIRT1-dependent AMPK activationJ Agric Food Chem20105831584159219928762
- FröjdöSCozzoneDVidalHPirolaLResveratrol is a class IA phosphoinositide 3-kinase inhibitorBiochem J2007406351151817550345
- Villa-CuestaEBoylanJMTatarMGrupposoPAResveratrol inhibits protein translation in hepatic cellsPLoS One2011612e2951322242130
- DochertyJJMcEwenHASweetTJBaileyEBoothTDResveratrol inhibition of Propionibacterium acnesJ Antimicrob Chemother20075961182118417449884
- Costa CdosSRohdenFHammesTOResveratrol upregulated SIRT1, FOXO1, and adiponectin and downregulated PPARγ1-3 mRNA expression in human visceral adipocytesObes Surg201121335636120872255
- KimSYHyunMYGoKCZouboulisCCKimBJResveratrol exerts growth inhibitory effects on human SZ95 sebocytes through the inactivation of the PI3-K/Akt pathwayInt J Mol Med20153541042105025672876
- WuLZhangYMaXZhangNQinGThe effect of resveratrol on FoxO1 expression in kidneys of diabetic nephropathy ratsMol Biol Rep20123999085909322733486
- GanjamGKDimovaEYUntermanTGKietzmannTFoxO1 and HNF-4 are involved in regulation of hepatic glucokinase gene expression by resveratrolJ Biol Chem200928445307833079719740748
- WangGLFuYCXuWCFengYQFangSRZhouXHResveratrol inhibits the expression of SREBP1 in cell model of steatosis via Sirt1-FOXO1 signaling pathwayBiochem Biophys Res Commun2009380364464919285015
- FabbrociniGStaibanoSDe RosaResveratrol-containing gel for the treatment of acne vulgaris: a single-blind, vehicle-controlled, pilot studyAm J Clin Dermatol201112213314121348544
- CoenyeTBrackmanGRigolePEradication of Propioni-bacterium acnes biofilms by plant extracts and putative identification of icariin, resveratrol and salidroside as active compoundsPhytomedicine201219540941222305279
- LandeckerHFood as exposure: nutritional epigenetics and the new metabolismBiosocieties20116216719423227106
- MayerSBEvansWSNestlerJEPolycystic ovary syndrome and insulin: our understanding in the past, present and futureWomens Health (Lond Engl)201511213714925776288
- JohnsonNPMetformin use in women with polycystic ovary syndromeAnn Transl Med2014265625333031
- MelnikBCSchmitzGMetformin: an inhibitor of mTORC1 signalingJ Endocrinol Diabetes Obes2014221029
- MelnikBCJohnSMPlewigGAcne: risk indicator for increased body mass index and insulin resistanceActa Derm Venereol201393664464923975508
- FangZZhangTDizeyiNAndrogen receptor enhances p27 degradation in prostate cancer cells through rapid and selective TORC2 activationJ Biol Chem201228732090209822139837
- HeemersHVVerhoevenGSwinnenJVAndrogen activation of the sterol regulatory element-binding protein pathway: current insightsMol Endocrinol200620102265227716455816
- GuFSchumacherFRCanzianFEighteen insulin-like growth factor pathway genes, circulating levels of IGF-I and its binding protein, and risk of prostate and breast cancerCancer Epidemiol Biomarkers Prev201019112877288720810604
- MelnikBCRole of FGFR2-signaling in the pathogenesis of acneDermatoendocrinol20091314115620436882
- ChenWObermayer-PietschBHongJBAcne-associated syndromes: models for better understanding of acne pathogenesisJ Eur Acad Dermatol Venereol201125663764621198949
- MelnikBCThe P450 system and mTORC1 signalling in acneExp Dermatol201423531831924588627
- SzabóKTaxGKisKInterleukin-1A +4845(G> T) polymorphism is a factor predisposing to acne vulgarisTissue Antigens201076541141520630038
- SmithEJAllantazFBennettLClinical, molecular, and genetic characteristics of PAPA syndrome: a reviewCurr Genomics201011751952721532836
- MastersSLSimonAAksentijevichIKastnerDLHorror autoinflammaticus: the molecular pathophysiology of autoinflammatory diseaseAnnu Rev Immunol20092762166819302049
- BrennerMRuzickaTPlewigGThomasPHerzerPTargeted treatment of pyoderma gangrnosum in PAPA (pyogenic arthritis, pyoderma gangrenosum and acne) syndrome with the recombinant human interleukin-1 receptor antagonsit anakinraBr J Dermatol200916151199120119673875
- ZoncuREfeyanASabatiniDMmTOR: from growth signal integration to cancer, diabetes and ageingNat Rev Mol Cell Biol2011121213521157483
- SutcliffeSGiovannucciEIsaacsWBWillettWCPlatzEAAcne and risk of prostate cancerInt J Cancer2007121122688269217724724
- ZhangMQureshiAAFortnerRTTeenage acne and cancer risk in US women: a prospective cohort studyCancer2015121101681168725572604
- FrassettoLASchloetterMMietus-SnyderMMorrisRCJrSebastianAMetabolic and physiologic improvements from consuming a paleolithic, hunter-gatherer type dietEur J Clin Nutr200963894795519209185
- Carrera-BastosPFontes-VillalbaMO’KeefeJHLinderbergSCordainLThe western diet and lifestyle and diseases of civilizationRes Report Clin Cardiol201121535
- KuipersRSJoordensJCMuskietFAA multidisciplinary reconstruction of Palaeolithic nutrition that holds promise for the prevention and treatment of diseases of civilisationNutr Res Rev20122519612922894943
- GharagozlooMJavidENRezaeiAMousavizadehKSilymarin inhibits cell cycle progression and mTOR activity in activated human T cells: therapeutic implications for autoimmune diseasesBasic Clin Pharmacol Toxicol2013112425125623121838
- XiangLNakamuraYLimYMTetrahydrocurcumin extends life span and inhibits the oxidative stress response by regulating the FOXO forkhead transcription factorAging (Albany NY)20113111098110922156377
- LiZCZhangLMWangHBMaJXSunJZCurcumin inhibits lung cancer progression and metastasis through induction of FOXO1Tumour Biol201435111111623888319
- KapahiPChenDRogersANWith TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in agingCell Metab201011645346520519118
