Abstract
Inadequate hygiene, aggressive cleansing, and chafing skin folds, as well as urine, feces, and sweat may trigger irritative contact dermatitis in the anogenital area. Serious recommendations for protection of the skin toward irritants include hygienic aspects and the use of appropriate skin care. Furthermore, preventing an accumulation of irritants on unprotected skin is mandatory. An intraindividual comparison study with 30 participants (17 female, 13 male; age: 44.2±8.3 years) was performed to evaluate the properties of a newly developed water-in-oil (W/O) balm on artificial sodium dodecyl sulfate-damaged epidermal barrier. The balm was applied 14 days twice daily, and transepidermal water loss and erythema were investigated. A significant improvement of both parameters after 12 days and even after 21 days could be confirmed. Two major clinical trials were performed to evaluate the safety and efficacy regarding protective and regenerative properties of the W/O balm on irritated skin in the anogenital area. Therefore, 29 children were enrolled (14 male, 15 female, age: 15.5±7.8 months) in an open-labeled 4-week clinical study. The balm was used in the area under disposable diapers at least after diaper change or if required. Furthermore, in a second open, multicenter study, 43 women (mean age: 46.2±16.9) with predisposition to skin irritation in the outer anogenital region were included. The product was applied for 4 weeks 1–2 times daily. In both studies, skin tolerability, applicability, scent, spreadability, and removability of the balm were evaluated by participants and practitioners predominantly as good or even very good, also skin hydration, protection, and regeneration were judged positively. The studies confirmed that the newly developed W/O balm exhibits excellent tolerability and is easy to remove. At the same time, excellent properties with respect to efficacy regarding regeneration and protection could be observed, without any undesired effects at any time.
Introduction
In general, irritant contact dermatitis directly affects the barrier properties of the epidermis.Citation1 This results in an inflammatory nonimmunologic cutaneous reaction.Citation2 The clinical spectrum ranges from erythema, slight scaling, edema, and erosions to eczematous conditions.Citation3
The resulting imbalances of immune regulators cause painful skin erosion, which is the basis for skin infections.Citation4,Citation5 The initial trigger factors are physical irritants such as sweat, inadequate hygiene, aggressive cleansing, and chafing skin folds, as well as chemical irritants such as urine, feces, and sweat, which remain in skin folds. Indeed, irritant contact dermatitis is common for the early childhood,Citation6–Citation8 but it also occurs in the elderly because of an impaired skin barrier, increased skin folds, and frequent incontinence accompanied with accumulation of urine and feces.Citation9,Citation10 Furthermore, high physical activity, for example, biking, horse riding, and marathon running, may also predispose for skin irritation, mainly in the anogenital area.Citation11,Citation12 Other typical areas prone to skin irritation are regions of the body where skin folds chafe on each other like the regions under the breasts and the umbilicus but also the axillae and the skin between the digits.Citation13
Recommendations for protection against development of an irritant contact dermatitis in the anogenital area aim not only on reducing contact with the irritants, but also hygienic aspects such as keeping the folds dry and using appropriate skin care.Citation14 Skin care products should be designed as sufficient water-in-oil (W/O) formulations.Citation15 W/O emulsions protect the skin toward outside irritants but without occlusion to allow for evaporation of water from inside.Citation16 Waterfree, mineral oil-based products should be avoided because they may damage the epidermal barrier by creating occlusive conditions and may even promote secondary infections or foster skin irritation.Citation17–Citation20 Unfortunately, traditional protective lipophilic creams, for example, for baby care or the intimate area, are mostly based on mineral oils and often contain zinc oxide,Citation21 which generates a hard to remove occlusive film and covers the skin tightly.Citation22
Taking these recommendations into account, a skin protecting only slightly occlusive W/O balm was developed and clinically evaluated, which is suitable for the anogenital area and all intertriginous regions of women and men and for daily care of the nappy areas of babies as well.
Materials and methods
The clinical studies were performed according to the requirements of the Declaration of Helsinki and were approved by the relevant ethical committees (013/1133, 013/1238, 013/1084) and registered in the german register for clinical trials (Deutsche Register Klinischer Studien) with following IDs: DRKS00009497, DRKS00009503 and DRKS00009504. According to that, all subjects or parents/legal guardians have given consent to the studies in written form.
Cosmetical formulation
The cosmetic product Linola® protective balm was used. The balm is a W/O emulsion consisting of caprylic/capric triglyceride, cocoglycerides, aqua, octyldodecyl myristate, oleyl erucate, Acacia decurrens/jojoba/sunflower seed cera/polyglyceryl-3 esters, glyceryl stearate, hydrogenated castor oil, glyceryl dibehenate, glyceryl behenate, tribehenin, benzyl alcohol, butylene glycol, pentylene glycol, octenidine HCl, hydroxyphenyl propamidobenzoic acid, Zingiber officinale root extract, bisabolol, and ascorbyl palmitate.
Clinical study to assess protection and regeneration after SDS-induced skin irritation in healthy volunteers
Study design
An artificial irritation of the skin by sodium dodecyl sulfate (SDS) was performed to mimic epidermal barrier damage and to evaluate the regenerative and protective properties of Linola® protective balm. Thirty participants (17 female, 13 male; age: 44.2±8.3 years) were included in this intraindividual comparison study. The product was applied on the inner forearm for 14 days twice daily. Testing parameters were skin hydration (Corneometer MPA 5 CPU), transepidermal water loss (TEWL) (Tewameter TM 210), skin redness (Chromameter SN C 8202118), and skin roughness (PRI-MOS compact high-res S/N 108-00042). Untreated skin areas served as control.
Study design
During days 1–7, the test area had to be washed once daily with 5% SDS solution to induce an artificial irritation of the skin. At day 8, the test parameters were measured. During the second phase (regeneration phase) from days 9 to 14, the test product was applied twice daily and regenerative product properties were measured 12–14 hours after the application on days 10, 12, and 15. From days 15 to 21 (protection phase), the protective product properties were evaluated. For this purpose, the participants were asked to continue the product application procedure twice daily, but to additionally wash the testing area with 5% SDS solution 1 hour after each product application. Measurements were taken 5–6 hours after product application and washing in the morning on days 17, 19, and 21.
Clinical study to assess protection from diaper rash in babies and toddlers
Study design
A total of 29 children were enrolled (14 male, 15 female; age: 15.5±7.8 months) in this open-labeled clinical study. The test product was used in the area under disposable diapers at least after each diaper change and even more often if required, according to the respective needs and normal using habits. The duration of treatment was 4 weeks. At the beginning, as well as after 4 weeks of treatment, erythema, dryness, scaling, fissures, papules, pustules, edema, vesicles, and weeping were assessed as objective parameters by a pediatrician. Subjective assessment of product properties was performed by subject questionnaire. Active skin diseases at the test area, such as acute diaper dermatitis, by not using the diaper during the day and night due to potty training or other reasons were excluded.
Study execution
A dermatological examination of the skin in the test area was performed by a pediatrician prior to start of the trial. The parents/legal guardians of the children were instructed to use the test product at least after each diaper change and even more often if required, according to the babies’ needs and their normal using habits. The parents/legal guardians continued to use the normally used cleansing products for their participating children. The test product was applied for 4 weeks. After 28 days, the parents/legal guardians were asked to complete a questionnaire. Again, the pediatrician performed a dermatological examination of the skin in the test area. The objective test parameters were erythema, dryness, scaling, fissures, papules, pustules, edema, vesicles, weeping, other, which were evaluated on a numerical scale between 0 and 3 (0= no result, 1= slight, 2= moderate, 3= severe). Adverse effects were noted.
Clinical study to assess efficacy and tolerability in the anogenital area of women suffering from skin irritation
Study design
In an open, multicenter study in 2013, 43 women (mean age: 46.2±16.9) with predisposition to skin irritation in the outer anogenital region were included. The product was applied to the outer anogenital area for 28 days one to two times daily, at least after every cleansing. Testing parameters monitored during the study were skin tolerability and efficacy (skin care and protection). Product properties of Linola® protective balm were assessed subjectively by the volunteers according to a questionnaire. Women with skin infection, skin erosion, neoplasia, skin diseases, pregnancy, or hypersensitivity against one of the ingredients as well as women in or after radiotherapy or simultaneous application of another skin protection product were excluded from the study.
Study execution
Before starting the study (visit 1), a physician took demographic data, diseases, and “risk factors” for skin irritations, such as excessive sports and excessive sweating, overwashing of the intimate area, urine and/or fecal incontinence, or tight clothing. In addition, the date of beginning of the irritation of each participant was documented. Women were asked to answer a questionnaire concerning the severity of their complaints such as excoriation, burning, stinging and itching, roughness, and swelling and to evaluate their subjective symptoms. The symptoms were graded by a 4-scale score (0= not existing, 1= slight, 2= moderate, 3= severe). Moreover, an objective assessment of their symptoms was performed by a physician. Therefore, skin glow, itching, and hyperkeratosis were graded using a 4-scale score (0= not existing, 1= slight, 2= moderate, 3= severe).
During the study, the women were asked to document frequency of application and risk factors. Furthermore, on a weekly basis they graded their subjective complaints and noted undesired effects according to cosmetic regulation 1223/2009/EG and other products they had applied in this time. At the end of the study, a final objective assessment of the symptoms was performed (visit 2).
Results
Clinical study to assess protection and regeneration after SDS-induced skin irritation in healthy volunteers
After 8 days of skin irritation with 5% SDS solution, TEWL increased to 5.6±3.7, 6±3.5 g/h m2, respectively (). After the regeneration phase from days 8 to 15, the untreated irritated area exhibited a TEWL of 1.5±1.7 g/h m2, whereas the treated area exhibited a TEWL of 0.7±1.7 g/h m2 at day 8 (P<0.05). After the protection phase on day 21, TEWL increased again due to SDS irritation in the untreated area to 5.6±3.2 g/h m2, whereas in the treated area, a TEWL of 3.37±2.5 g/h m2 was measured (P<0.05) ().
Figure 1 Regenerative and protective properties of Linola® protective balm.
Abbreviations: SDS, sodium dodecyl sulfate; TEWL, transepidermal water loss; SD, standard deviation.
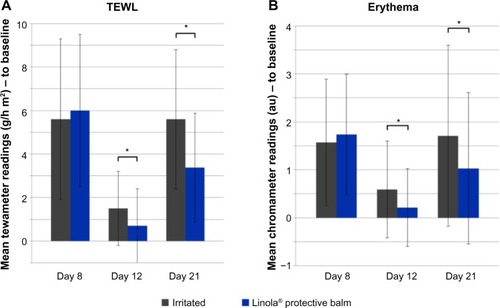
Erythema increased up to day 8 to 1.57±1.32 au and 1.74±1.25 au, respectively. During the regeneration phase, erythema decreased in the untreated irritated area (0.59±1.01 au) and in the treated area (0.21±0.81 au). The difference was statistically significant (P<0.05). After the protection phase, erythema increased again (1.71±1.89 au in untreated and 1.03±1.53 au in treated area). The increase in the treated area was statistically significantly lower (P<0.05) ().
Clinical study to assess protection from diaper rash in babies and toddlers
In this study, parents/legal guardians applied the balm 3–4 times daily in 58.6% and even >4 times daily in 41.4% of all cases. Dermatological evaluation at baseline revealed findings in 62.5% of the babies: very mild papules in 25%, very mild erythema in 31%, and a mild erythema in 3% of the babies. After 4 weeks of application, only 12.5% of the babies showed findings upon dermatological evaluation: very mild erythema in only 9% and moderate papules in only 3% of the babies ().
Figure 2 Results of an application trial in the diaper area.
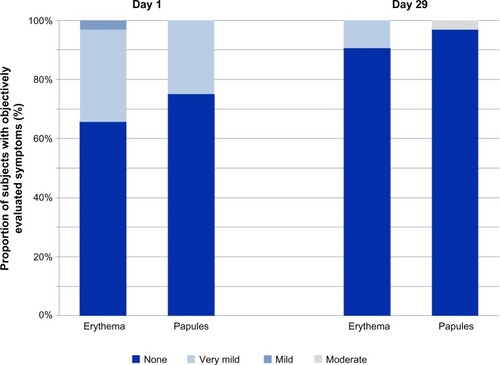
The subjective evaluation by the parents/legal guardians showed in 69% a very good spreadability of the balm. Furthermore, 69% documented a better removability of the balm compared to the previously used products. Overall, the majority of the parents/legal guardians were satisfied with the balm. Approximately 75.9% of the parents/legal guardians confirmed that they would like to continue the usage of the test product for the diaper area of their babies.
One adverse reaction occurred with possible relation to the test product in terms of papules with moderate severity in the diaper area.
Clinical study to assess efficacy and tolerability in the anogenital area of women suffering from skin irritation
In this study, 73.8% of the women suffered from symptoms >6 months with a permanent manifestation in 69% of all cases.
The sum score of the single complaints (excoriation, burning, stinging and itching, roughness, swelling, and itching) was 11.4±2.7 at the beginning. During the application of Linola® protective balm, a statistically significant decline was observed (week 1: 8.0±4.0; week 2: 6.1±4.3; week 3: 4.7±4.3; week 4: 4.4±4.8) (P<0.0001) (). After the first week, already 81% of the test persons felt an improvement of their complaints, further increasing up to 90.5% after week 2 and 92.9% after weeks 3 and 4. A more detailed view on the results of the evaluation of single complaints (excoriation, burning, stinging and itching, roughness, swelling, and itching) revealed an ongoing decline of all complaints. The improvement toward baseline was statistically significant for all complaints (P<0.05), except stinging after week 1 (). Furthermore, the proportion of subjects in which objective symptoms, evaluated by a gynecologist, were no longer present decreased from visit 1 to visit 2 by at least 14%. The proportion of women without erythema increased from 28.6% to 42.9%, without skin glow from 45.2% to 66.7%, and without swelling from 50% to 73.8%. Overall, a gynecologist evaluated the global tolerability for 61.9% of the subjects as very good and good for 35.7%, and the efficacy was evaluated as good or even very good for 80.9% of the subjects, which is statistically significant compared to baseline.
Figure 3 Results of an application trial to test the efficacy and skin tolerability of Linola®.
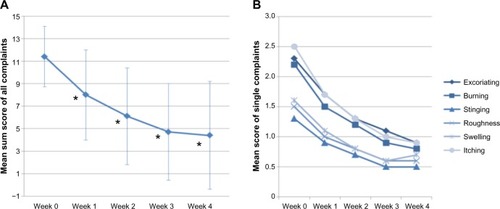
Moreover, the number of women without any symptom increased with increasing length of the study. Comparing visit 1 (at the beginning of the study) and visit 2 (at the end of the study), the proportion of women without itching increased from 2.4% up to 47.6%, without excoriations from 4.8% to 45.2%, without burning from 7.1% up to 52.4%, without feeling of roughness from 23.8% to 64.3%, without swelling from 26.2% to 59.5%, and without stinging from 31% to 64.3%.
The Protective Balm was assessed as good (gynecologist: 52.4%; women: 47.6%) or very good (gynecologist: 35.7%; women: 19.0%) for all enquired items. During the 4 weeks application study, no severe adverse events were observed at any time.
Discussion
Unfortunately, no general recommendations regarding skin care for the regeneration of and protection of the intimate area from irritant contact dermatitis and intertriginous skin lesions are available, and studies are still lacking. Only guidelines for skin care related to urinary and fecal incontinence are published.Citation19,Citation23 Since these are trigger factors for irritant contact dermatitis in the anogenital area, as sports or over-washing are likewise, the recommendations can be extended for other irritants and generalized – with some limitations – for all ages (eg, babies and elderly). Since all physical and chemical irritants damage the epidermal barrier,Citation1 the maintenance of this barrier should be the main goal of appropriate skin care. Appropriate W/O emulsions are preferred because of minor occlusive but at the same time protecting properties. In contrast, waterfree, mineral oil-based products should be avoided because they may damage the epidermal barrier, create occlusive conditions, and may even promote secondary infections or foster skin irritation.Citation16,Citation18–Citation20 Unfortunately, still today, most intimate care products are mineral oil based, and even in daily baby care, the use of occlusive, hard to remove pastes or ointments is still common. On the one hand, the formulations have to protect against moisture, on the other hand, the skin care product has to permit water to evaporate because the skin should not be covered too tightly.Citation16 Therefore, the new W/O balm was developed with minor occlusive properties and compared to a common mineral oil-based intimate care product. A simple experiment was performed and exhibited minor occlusive effect for the balm compared to the mineral oil-based product (data not shown).
In general, the development of intimate care products should focus on the composition of the formulationCitation24 since intravaginal traces of the applied products cannot be excluded. Just recently, in multivariable analysis, women reporting the intravaginal use of petroleum jelly are significantly more likely to test positive for bacterial vaginosis.Citation25 Therefore, the use of petroleum jelly (vaseline, petrolatum) containing products in the anogenital area should be reconsidered. Utilizing plant-derived lipids to formulate a W/O emulsion with mild occlusive properties, the balm was developed to protect against moisture and other irritative influences and being suitable for long-term use because intimate care products should not only be used to prevent friction from skin folds or diapers, but also to protect the skin from contact with moisture and other chemical irritants in the long term.Citation26 In general, mild emulsifiers and humectants used as ingredients in the balm lead to a transparent, water repellent, and long-lasting film on the skin, which not only protects the skin against irritants but also simplifies their removal. Furthermore, appropriate clinical tests with respect to excellent tolerability are important,Citation27,Citation28 especially incontinence-associated dermatitis (IAD) is a preventable condition, whereby persistent skin contact with urine, feces, and sweat leads to skin irritation. IAD has a complex pathogenesis and is a form of moisture-associated dermatitis, aggravated by occlusion and maceration that produces erythema, inflammation, and eventual loss of skin integrity.Citation29,Citation30 Therefore, initially an artificial irritation of the skin by SDS was performed to mimic mentioned characteristics of IAD. The properties of the balm were investigated by monitoring the improvement of TEWL and erythema. After 4 days of application, the balm improved TEWL and erythema significantly and provided first indications of the efficacy and tolerability of the balm.
The objective dermatological evaluation of the diaper area of the babies revealed a reduction of number of babies with complaints after 4 weeks of application of the balm. Only three babies suffered from erythema with very mild symptoms, whereas the number of subjects showing papules was reduced to one. However, all of the studies showed a very good skin tolerability of the balm. No serious adverse events were observed after application in the conducted clinical studies. The removability of the newly developed product was addressed in a study on babies and toddlers during 4 weeks of application. It was assessed in 69% of the parents/legal guardians as even better compared to previously used mostly zinc oxide-based products.
The objective assessment of the symptoms and abnormal findings, so-called secondary morphology,Citation31 assessed by physicians provided specific evidence of the regenerative and protective properties of the balm in the main multicenter study with women predisposed to skin irritation in the outer anogenital region. The severity of erythema, skin glow, swelling, and hyperkeratosis declined significantly during 4 weeks. The subjective evaluation in the study by the women revealed also high efficacy for the newly developed balm. The severity of the subjective complaints decreased permanently for each single item such as excoriation, burning, stinging and itching, roughness, swelling, and itching.
Conclusion
In summary, the balm was developed as a nonirritating, water-repellent W/O emulsion with low occlusive properties, without mineral oil, silicones, or zinc oxide, based on plant-derived lipids with anti-irritative compounds. The balm is suitable for the daily care in the intimate and anogential area and protects the skin from excessive moisture, which may cause irritative contact dermatitis. It regenerates the epidermal barrier damaged by irritative substances and is easy to apply and to remove. The efficacy regarding regenerative and protective properties, skin tolerability, and cosmetic acceptance of the balm was evaluated positively on babies and toddlers, as well as in women with predisposition to skin irritation in the outer intimate region.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
The tested products were formulated according to the requirements by Dr August Wolff GmbH & Co KG Arzneimittel. The study was designed by Dr August Wolff GmbH & Co KG Arzneimittel and performed by Pharmalog, Derma Consult Concept GmbH, and proDERM.
Disclosure
CA and MK are employees of Dr August Wolff Arzneimittel GmbH & Co KG (sponsor of the studies). VK was the investigator of one clinical study. The authors report no other conflicts of interest in this work.
References
- MathiasCGMaibachHIDermatotoxicology monographs I. Cutaneous irritation: factors influencing the response to irritantsClin Toxicol1978133333346369770
- TanCHRasoolSJohnstonGAContact dermatitis: allergic and irritantClin Dermatol20143211612424314385
- AleISMaibachHIIrritant contact dermatitisRev Environ Health201429319520625274939
- YosipovitchGTurECohenORuseckiYSkin surface pH in intertriginous areas in NIDDM patients. Possible correlation to candidal intertrigoDiabetes Care1993165605638462378
- SibbaldRGKelleyJKennedy-EvansKLLabrecqueCWatersNPractical Approach to the Prevention and Management of Intertrigo, or Moisture-associated Skin Damage, Due to Perspiration: Expert Consensus on Best Practice11CanadaWound Care Canada2013
- Braun-FalcoODermatologyBerlin HeidelbergSpringer-Verlag1996466
- HonigPJFriedenIJKimHJYanACStreptococcal intertrigo: an under recognized condition in childrenPediatrics20031126 pt 11427142914654624
- LauKHögerPHDermatologische Probleme bei Kindern mit AdipositasBundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz20135653954223529600
- FarageMAMillerKWBerardescaEMaibachHIIncontinence in the aged: contact dermatitis and other cutaneous consequencesContact Dermatitis200757421121717868212
- KötherIAltenpflege Professionell. Thiemes AltenpflegeStuttgartGeorg Thieme Verlag Auflage2007
- DeryaAElgenEMetinECharacteristics of sports-related dermatoses for different types of sports: a cross-sectional studyJ Dermatol20053262062516334860
- AnejaSTaylorJSSkin disorders in athletes: professional and recreational sportsAnejaSTaylorJSKanerva’s Occupational DermatologyNew YorkSpringer201216611678
- LinJYShihYLHoHCFoot bacterial intertrigo mimicking interdigital tinea pedisChang Gung Med J2011341444921392473
- MistiaenPvan Halm-WaltersMPrevention and treatment of intertrigo in large skin folds of adults: a systematic reviewBMC Nurs201091220626853
- DanielsRKnieUGalenik der Dermatika – Grundlagen, Eigenschaften, FreisetzungJDDG20075536738317451381
- SchäferPBewick-SonntagCBerardescaEPhysiological changes in skin barrier function in relation to occlusion level, exposure time and climatic conditionsSkin Pharmacol Physiol2002151719
- FluhrJWLazzeriniSDistanteFGloorMBerardescaEEffects of prolonged occlusion on stratum corneum barrier function and water holding capacitySkin Pharmacol Appl Skin Physiol19991219319810420139
- FluhrJElsnerPBerardescaEMaibachHIBioengineering of the Skin Water and the Stratum Corneum Dermatology: Clinical and Basics Science Series2nd edFloridaCRC Press2005
- NashanDSchumannHMeisJKlieschSHarninkontinenz: Übersicht zur Klinik, Prävention und Therapie dermatologischer Folgeerkrankungen Available at: http://www.dkvg.de/download/inkontinenz.pdfAustraliaHandbuch Geriatrie, Deutsche Krankenhaus-Verlagsgesellschaft2005 German
- Van der ValkPGMMaibachHIPost-application occlusion substantially increases the irritant response of the skin to repeated short-term sodium lauryl sulfate (SLS) exposureContact Dermatitis19892153353382620512
- StamatasGNTierneyNKDiaper dermatitis: etiology, manifestations, prevention, and managementPediatric Dermatology20143111724224482
- WilliamsDFSchmittWHChemistry and Technology of the Cosmetics and Toiletries Industry2nd edLondonChapman and Hall1996
- GillibrandWFaecal incontinence in the elderly: issues and interventions in the homeBr J Community Nurs201217836436822875210
- FarageMASchefflerHAssessing the dermal safety of products intended for genital mucosal exposureCurr Probl Dermatol20114011612421325846
- BrownJMHessKLBrownSMurphyCWaldmanALHezarehMIntravaginal practices and risk of bacterial vaginosis and candidiasis infection among a cohort of women in the United StatesObstet Gynecol2013121477378023635677
- BryantRAcute and Chronic Wounds: Nursing ManagementSt LouisMosby1992
- AdamRSkin care of the diaper areaPediatr Dermatol200825442743318789081
- WolfGHögerPHHypoallergenic and non-toxic emollient therapies for childrenJ Dtsch Dermatol Ges200971506019138296
- LarnerJMatarHGoldmanVSChilcotRPDevelopment of a cumulative irritation model for incontinence-associated dermatitisArch Dermatol Res2015307394825416151
- GrayMBlackJMBaharestaniMMMoisture-associated skin damage: overview and pathophysiologyJ Wound Ostomy Continence Nurs201138323324121490547
- BornsteinJSideriMTattiSWalkerPPrendivilleWHaefnerHKNomenclature Committee of International Federation for Cervical Pathology and Colposcopy2011 terminology of the vulva of the international federation for cervical pathology and colposcopyJ Low Genit Tract Dis20121629029522659778
