Abstract
Atopic dermatitis (AD) or atopic eczema is the common inflammatory skin disorder, the prevalence of which has considerably increased during the last 30 years. It affects 15%–30% of children and 2%–10% of adults. AD characteristically alternates between periods of exacerbation or flares and periods of remission, which may be therapeutically induced or spontaneous. Current knowledge about AD includes abnormalities of the skin barrier (physical and chemical), the immune barrier, and more recently, the microbial barrier or microbiota. There is growing evidence for a tight relationship between them. To obtain satisfactory control of this condition, the clinical strategy to manage AD involves prescribing both anti-inflammatory medications and dermocosmetic products. The role of the physician is therefore to advise the patient with regard to hygiene measures aimed to help to improve these three barriers or to prevent any further deterioration.
Introduction
Atopic dermatitis (AD) or atopic eczema is the common inflammatory skin disorder with a substantial socioeconomic burden and impact on quality of life.Citation1 AD is considered as the first step of the so-called atopic march.Citation2 The three main pillars of the management of AD, which are the basis of most guidelines include 1) an allergological work-up and identification of provocation factors, 2) a severity-adapted prescription of anti-inflammatory medications, and 3) a basic therapy, ie, an ideally individually adapted skin care aimed to improve the impaired barrier function.Citation3,Citation4 AD was initially thought to be an immunological disease, where the chronic inflammation of the skin causes and/or exacerbates the skin barrier impairment. The pathophysiology of AD is as complexCitation5 as the clinical phenotype, and recent studies revealed the genetic origin of skin barrier function abnormalities and the role of filaggrin mutations in 10%–30% of patients with AD in Caucasian populations.Citation5,Citation6 They also revealed the involvement of other stratum corneum proteins in an innate deficiency of the physical and chemical skin barriers.Citation7,Citation8 These abnormalities enable different environmental factors to penetrate the epidermis, including chemical, allergic, or infectious agents. This results in an inappropriate immune response with chronic inflammation and the presence of allergens or pathogenic agents, which could stimulate a T-helper 2/T-helper 22-type reaction. This inflammatory reaction can itself impact on the integrity of the barrier function. Finally, a kind of selective antimicrobial peptide deficiency leads to a loss of microbial diversity with an overabundance of the Staphylococcus bacterial genus, witnessing the role of the microbiome in the pathogenesis of AD.Citation9 This abnormal microbial colonization justifies AD being regarded as a state of dysbiosis.
The overall therapeutic strategy used in mild-to-moderate AD management aims to rapidly reduce the inflammatory response and/or regulate the immune response with either topical corticosteroids or calcineurin inhibitorsCitation10 and to improve the skin barrier function using emollients to rebuild the chemical and/or physical lipid barrier.Citation11 Basic therapy improving the barrier function is also an established part of the management of severe forms where systemic treatments have to be considered.Citation12–Citation14 Moreover, direct manipulation of the cutaneous microbiome to treat the dysbiosis and reestablish the microbial diversity will represent a new treatment approach to be considered in combination with current strategies. Advice provided by the physician is essential. In daily practice, the practitioner’s attitude is to concomitantly prescribe medications and skin care products belonging to dermocosmetics. This approach forms part of a genuine care protocol where each product has its place, from daily hygiene to medical treatments, to ensure a complete and personalized approach. The skin is an organ, which must be treated in its entirety in order to obtain both a therapeutic result and the patient’s psychological and esthetic satisfaction.
Restoring the skin barrier in AD using emollients
Emollients are prescribed to improve the skin barrier function. They restore stratum corneum lipids and may have anti-inflammatory properties, which are demonstrated in in vitro or in vivo clinical trials.Citation15 As it is vital for atopic patients to limit moisture loss and restore the hydrolipidic film, emollients are an essential part of their daily skin care regimen. “To moisturize” does not only mean providing moisture, it also means preventing moisture evaporation from the skin.
To meet these needs emollients can be formulated with the following ingredients:
Emollient agents make the skin softer and more pliable by filling the space between corneocytes and restoring the physical barrier function. Raw materials with this property include 1) hydrogenated or nonhydrogenated vegetable oils, 2) mineral oils (eg, paraffin and petroleum jelly), 3) vegetable butters (eg, shea and cocoa), 4) alcohols, fatty acids, and esters, 5) triglycerides, and 6) ceramides.
Humectant or moisturizing agents are water-soluble substances, which help the stratum corneum capture water from the outside and rebalance the cutaneous hydrolipidic film. Glycerol (and glycerol derivatives), urea, lactic acid, and α- and β-hydoxyacids are the most commonly used humectant ingredients.
Occlusive agents create a sealed barrier and prevent moisture evaporation from the surface of the epidermis. Petroleum jelly is undoubtedly the most effective occlusive substance but agents with film-forming properties also exist such as lipophilic (silicones and silicone elastomers) and hydrophilic film-forming agents. The latter consist of very large molecules capable of “capturing” water and forming a film on the surface of the skin stopping water evaporation. They include 1) proteins (collagen), 2) complex carbohydrates (glycosaminoglycan: hyaluronic acid and sulfated sugars), 3) synthetic polymers (carbomer, 2-acrylamido-2-methylpropane sulfonic acid [AMPS®], and 4) waxes. However, the disadvantage of these occlusive agents is their sticky and/or greasy consistency making them less pleasant to use.
The quality of the emollient is best when a perfect balance is obtained from combining 1) efficacy, 2) cosmetic quality, and 3) patient tolerance. This is the art of formulation. The patient’s compliance is highly dependent on the formulation.Citation16 The formulation must ideally meet dermatologist expectations as well as patient needs and must be effective. Different formulations have different objectives; some ingredients have moisturizing efficacy (eg, glycerin and pyroglutamic acid), whereas others promote penetration of active ingredients into the skin, ensuring their bioavailability. Therefore, emollients can contribute to the success of dermatological treatments by ensuring optimal hygiene and relieving the irritation and dryness caused either by the condition or by the medical treatments. Some emollients can also provide real clinical benefit, alone or in combination with medical products.Citation17
Numerous formulations can be used; however for a condition like AD, the most appropriate pharmaceutical formula contains raw materials that provide a therapeutic effect, with few side effects, especially with no sensitization risk. Raw materials are chosen based on the following criteria: 1) safety, 2) purity, and 3) cosmetovigilance data. Any known allergens are avoided, and the packaging is designed to limit the use of preservatives (eg, fine nozzle tubes that can reduce microbiological contamination, or single doses, which ensure a sterile cosmetic product). Water content provides an environment suited for bacterial growth, so nowadays, formulation chemists tend to combine raw materials with low free water content, thus allowing to reduce the preservative concentration as much as possible.
Manipulation of the cutaneous microbiota in AD by emollients
For decades, microbiologists throughout the world have attempted to improve bacterial culture media in laboratory. From a bacterial point of view, skin is a culture medium. On average, skin houses one million bacteria per centimeter square. This specific ecosystem is poorly understood. Cosmetic and dermatological treatments provide the skin with external components thus modifying its nutritional characteristics and acting on its microbiome. This million bacteria per centimeter square is represented by several hundred different microbes that cohabit, fight, and collaborate the image of life in a human community.Citation18,Citation19
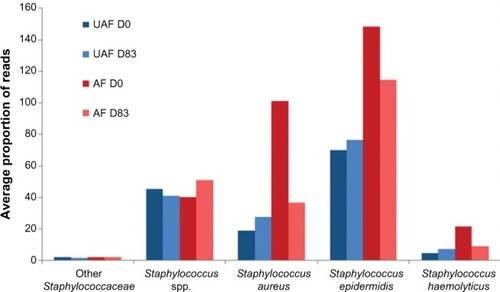
For a long time, it has been known that Staphylococcus aureus is present on both healthy and affected skin of >90% of patients with AD. The presence of S. aureus is at least partially explained by an antimicrobial peptide deficiency and by the poor quality of the skin barrier, which allows easy access to complex substrates.Citation20,Citation21 The arrival of metagenomics enabled scientists to study the cutaneous microbiome more precisely, particularly in skin affected by AD.Citation9,Citation22 These studies showed that in AD there is a loss of microbial diversity. During flares, this loss of diversity and the predominance of Staphylococci worsen in correlation with flare severity. Bacterial diversity is greater when treatment is proactive compared to no treatment. This suggests that there is a relationship between the recovery of bacterial diversity and remission.Citation9 Additionally, the presence of Staphylococcus epidermidis also increases during flares, which could correspond to a compensatory and antagonistic mechanism in response to the increase of S. aureus.Citation23
Given that each individual has a unique microbiome linked with his/her genetic make-up, diet, lifestyle, and surrounding environment and that the majority of studies performed in this field compare healthy to atopic individuals, it is important to validate the observations made in these studies by comparing intrapatient skin areas.Citation24 Recent studies have confirmed that AD lesions are dominated by Staphylococci and have a lower microbial diversity than that of the unaffected adjacent skin.Citation9,Citation23 An emollient can equalize the composition of an affected or lesional area (AF) to a state similar to that of uninvolved skin (nonlesional or unaffected UAF area) ( and ).Citation23 Also keratolytic bacteria (ie, Xanthomonadaceae family), naturally present on the skin, have access to keratin, which is favorable to their growth.Citation23,Citation25 These results reveal new insight into the cutaneous microbiome, the targeted manipulation of which is a new approach for AD treatment.
Figure 1 Bacterial landscape of the 30 main bacterial genera before (D0 – A) and after (D83 – B) 83 days of daily treatment with emollient on the affected (lesional) zones (AF) and the unaffected (nonlesional) adjacent zones of healthy appearance (UAF) in atopic patients (N=49).
Abbreviations: UAF, unaffected; AF, affected; D, day.
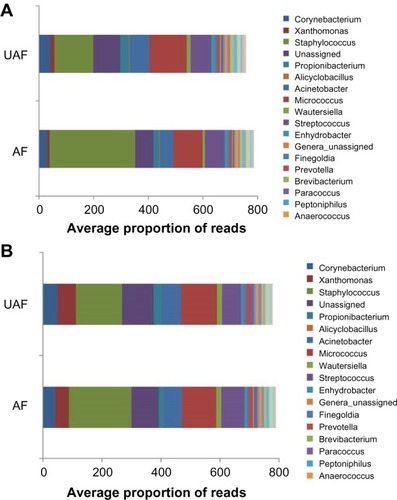
Figure 2 Different species of Staphylococci present on the affected zones (AF – in red) and the unaffected zones (UAF – in blue) of atopic patients (N=49) before (D0, darker) and after (D83, lighter) 83 days of daily treatment with an emollient.
Abbreviations: UAF, unaffected; AF, affected; D, day.
Other than in cases of superinfection, combating S. aureus has never been definitely proven to be of therapeutic benefit. Topical antiseptic or antibiotic treatments, for example, the use of diluted bleach baths, are still sometimes recommended, despite this lack of demonstrated efficacy on the microbiome, their potentially irritant effect, the risk of bacterial resistance induction, and the action on bacteria, which could control S. aureus such as S. epidermidis.Citation26
Numerous clinical trials have been conducted with the objective of demonstrating a preventive action of probiotic supplementation (living nonpathogenic bacteria) on the initial development and/or flares and severity of AD. Lastly, several meta-analyses support the conclusion that prebiotics or probiotics could have a preventive effect on the development of AD but do not appear to have any direct therapeutic benefit.Citation27–Citation29 Nevertheless, some recent studies would disagree with this conclusion.Citation30–Citation32
Similarly, applying dead extracts from nonpathogenic bacteria onto the skin could be a new therapeutic approach to modulate or balance the immune system and would enable manipulation of the cutaneous microbiome. This hypothesis is based on results obtained from the following four key studies: 1) a significant improvement in the severity of AD in a randomized trial using a cream containing a lysate from a Gram-negative bacterium, Vitreoscilla filiformis, in the treatment of AD;Citation33 2) a mouse model of AD revealing that the V. filiformis lysate is able to reduce the clinical inflammatory manifestations and the inflammatory reaction to an allergen when it was applied, suggesting a significant modulation of the immune response;Citation34 3) an in vitro model on a reconstructed epidermis revealed that, surprisingly, the V. filiformis lysate stimulated the β-defensin production and other innate immune defense mechanisms through activation of toll-like receptor 2;Citation35 and finally 4) a randomized trial showing the reduction in the probability of relapse of AD concomitant to a substantial impact on the composition of the microbiome.Citation36 As concluded in a recent review on this topic, after identification of diminished bacterial diversity in the skin microbiome of atopic individuals with consecutive loss of anti-inflammatory and tolerogenic interleukin 10, substitution of these tolerance promoting innate immune signals using microbes or microbial components is a new and promising therapeutic strategy.Citation37
Conclusion
To control AD is the goal of its management, which involves the concomitant prescription of medical products and der-mocosmetic products. The role of the dermatologist is to prescribe and inform the patient about appropriate hygiene measures that restore and/or prevent worsening of the skin barrier alteration while strengthening the immune barrier and regulating the skin microbiome. A well-balanced emollient with effective ingredients that restore skin barrier function, acceptable cosmetic quality, and excellent dermatological tolerance, supplemented with ingredients able to revive natural cutaneous defenses by providing nutritional supplements to regulate the cutaneous microbiota, offers the best option for AD. Finally, an impaired skin barrier function has been suspected to be instrumental in the emergence of immunoglobulin E-mediated sensitization and the atopic march. There is first evidence that an adapted basic therapy could reduce and/or delay the appearance of AD in newborns at high risk to develop this condition.Citation38–Citation40 Thus, emollients able to impact on the microbiotic dysbiosis could be part of the future early intervention strategies aimed to prevent AD and possibly the atopic march.
Acknowledgments
The authors would like to thank N Cleren and G Sore for information provided about the formulation and dosage forms of emollients and R Martin for his microbiological expertise. The authors also acknowledge the editing support of Charlotte Wright (Speak the Speech Consulting).
Disclosure
S Seité is an employee of La Roche-Posay but has no financial interest in the company. T Bieber has been a lecturer for La Roche-Posay. The authors report no other conflicts of interest in this work.
References
- BieberTAtopic dermatitisN Engl J Med2008358141483149418385500
- DharmageSCLoweAJMathesonMCBurgessJAAllenKJAbramsonMJAtopic dermatitis and the atopic march revisitedAllergy201369172724117677
- RingJAlomarABieberTGuidelines for treatment of atopic eczema (atopic dermatitis) part IJ Eur Acad Dermatol Venereol20122681045106022805051
- EichenfieldLFTomWLChamlinSLGuidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitisJ Am Acad Dermatol201470233835124290431
- EyerichKNovakNImmunology of atopic eczema: overcoming the Th1/Th2 paradigmAllergy201368897498223889510
- SmithFJIrvineADTerron-KwiatkowskiALoss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgarisNat Genet200638333734216444271
- BoguniewiczMLeungDYAtopic dermatitis: a disease of altered skin barrier and immune dysregulationImmunol Rev2011242123324621682749
- KuoIHYoshidaTDe BenedettoABeckLAThe cutaneous innate immune response in patients with atopic dermatitisJ Allergy Clin Immunol2013131226627823374259
- KongHHOhJDemingCTemporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitisGenome Res201222585085922310478
- SchmittJvon KobyletzkiLSvenssonAApfelbacherCEfficacy and tolerability of proactive treatment with topical corticosteroids and calcineurin inhibitors for atopic eczema: systematic review and meta-analysis of randomized controlled trialsBr J Dermatol2011164241542820819086
- RingJAlomarABieberTGuidelines for treatment of atopic eczema (atopic dermatitis) Part IIJ Eur Acad Dermatol Venereol20122691176119322813359
- SimonDBieberTSystemic therapy for atopic dermatitisAllergy2014691465524354911
- BieberTStraeterBOff-label prescriptions for atopic dermatitis in EuropeAllergy201570161125074686
- HowellMDParkerMLMustelinTRanadeKPast, present, and future for biologic intervention in atopic dermatitisAllergy201570888789625879391
- LaneMEHadgraftJOliveiraGVieiraRMohammedDHirataKRational formulation designInt J Cosmet Sci201234649650122882873
- HonKLChingGKLeungTFChoiCYLeeKKNgPCEstimating emollient usage in patients with eczemaClin Exp Dermatol2010351222619489850
- DrenoBAraviiskaiaEBerardescaEThe science of dermocosmetics and its role in dermatologyJ Eur Acad Dermatol Venereol201428111409141724684296
- GriceEAKongHHConlanSTopographical and temporal diversity of the human skin microbiomeScience200932459311190119219478181
- GriceEASegreJAThe human microbiome: our second genomeAnnu Rev Genomics Hum Genet20121315117022703178
- OngPYOhtakeTBrandtCEndogenous antimicrobial peptides and skin infections in atopic dermatitisN Engl J Med2002347151151116012374875
- NakatsujiTGalloRLAntimicrobial peptides: old molecules with new ideasJ Invest Dermatol20121323 pt 288789522158560
- GriceEAThe skin microbiome: potential for novel diagnostic and therapeutic approaches to cutaneous diseaseSemin Cutan Med Surg20143329810325085669
- SeiteSFloresGEHenleyJBMicrobiome of affected and unaffected skin of patients with atopic dermatitis before and after emollient treatmentJ Drugs Dermatol201413111365137225607704
- GriceEASegreJAThe skin microbiomeNat Rev Microbiol20119424425321407241
- YamamuraSMoritaYHasanQYokoyamaKTamiyaEKeratin degradation: a cooperative action of two enzymes from Stenotrophomonas spBiochem Biophys Res Commun2002294511381143 Erratum in: Biochem Biophys Res Commun. 2002;295(4):103412074595
- HuangJTAbramsMTlouganBRademakerAPallerASTreatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severityPediatrics20091235e808e81419403473
- PanduruMPanduruNMSalavastruCMTiplicaGSProbiotics and primary prevention of atopic dermatitis: a meta-analysis of randomized controlled studiesJ Eur Acad Dermatol Venereol201529223224224698503
- MuraroAHalkenSArshadSHEAACI food allergy and anaphylaxis guidelines. Primary prevention of food allergyAllergy201469559060124697491
- KimSOAhYMYuYMChoiKHShinWGLeeJYEffects of probiotics for the treatment of atopic dermatitis: a meta-analysis of randomized controlled trialsAnn Allergy Asthma Immunol2014113221722624954372
- WangIJWangJYChildren with atopic dermatitis show clinical improvement after Lactobacillus exposureClin Exp Allergy201545477978725600169
- DragoLDe VecchiEToscanoMVassenaCAltomareGPigattoPTreatment of atopic dermatitis eczema with a high concentration of Lactobacillus salivarius LS01 associated with an innovative gelling complex: a pilot study on adultsJ Clin Gastroenterol201448Suppl 1S47S5125291127
- NiccoliAAArtesiALCandioFPreliminary results on clinical effects of probiotic Lactobacillus salivarius LS01 in children affected by atopic dermatitisJ Clin Gastroenterol201448Suppl 1S34S3625291124
- GuenicheAKnaudtBSchuckEEffects of nonpathogenic gram-negative bacterium Vitreoscilla filiformis lysate on atopic dermatitis: a prospective, randomized, double-blind, placebo-controlled clinical studyBr J Dermatol200815961357136318795916
- VolzTSkabytskaYGuenovaENonpathogenic bacteria alleviating atopic dermatitis inflammation induce IL-10-producing dendritic cells and regulatory Tr1 cellsJ Invest Dermatol201413419610423812300
- MaheYFPerezMJTacheauCA new Vitreoscilla filiformis extract grown on spa water-enriched medium activates endogenous cutaneous antioxidant and antimicrobial defenses through a potential Toll-like receptor 2/protein kinase C, zeta transduction pathwayClin Cosmet Investig Dermatol20136191196
- SeitéSZelenkovaHMartinRFiererNUsing a Specific Emollient to Manage Skin Microbiome DysbiosisVancouver, CanadaWorld Congress of Dermatology2015
- BiedermannTSkabytskaYKaeslerSVolzTRegulation of T cell immunity in atopic dermatitis by microbes: the Yin and Yang of cutaneous inflammationFront Immunol2015635326217343
- FlohrCMannJNew approaches to the prevention of childhood atopic dermatitisAllergy2014691566124372089
- SimpsonELChalmersJRHanifinJMEmollient enhancement of the skin barrier from birth offers effective atopic dermatitis preventionJ Allergy Clin Immunol2014134481882325282563
- FukuieTNomuraIHorimukaiKProactive treatment appears to decrease serum immunoglobulin-E levels in patients with severe atopic dermatitisBr J Dermatol201016351127112920545693
