Abstract
Purpose
The aim of this study was to investigate the priority of periodontal plaque as a risk factor compared to other risk factors, namely hypertension and diabetes mellitus type II, regarding the initiation and severity of peri-implant mucositis, eventually reinforcing the importance of plaque control, periodic maintenance and supportive periodontic treatment after implant placement in order to prevent peri-implant diseases.
Patients and Methods
A total of 58 patients (84 implants) were enrolled; each individual implant was considered as a separate sample first, then sampling by patient was also applied, implants were divided into group A: systemically healthy patients and B: patients with hypertension and diabetes mellitus type II, the status of peri-implant tissue was followed after the healing abutment placement, with regard to implant mucosal index (IMI), probing pocket depth (PPD) and bleeding on probing (BOP); when sampling was done by patient, the mean of scores of all examined implants in each patient was taken to represent one sample.
Results
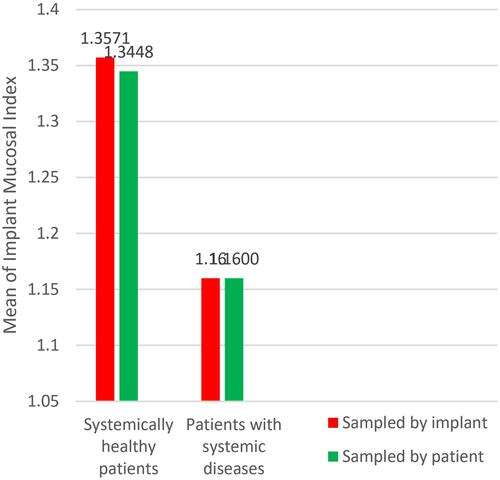
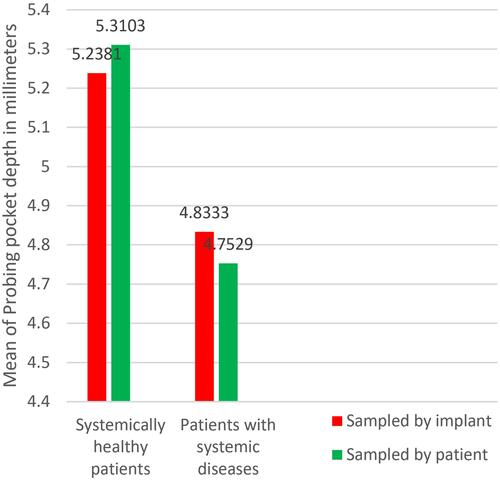
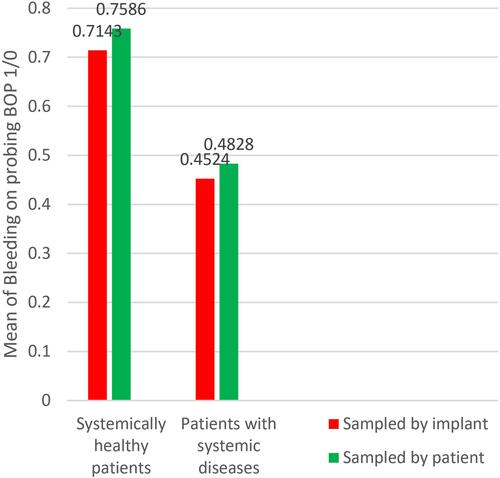
Group A implants showed higher mean scores of PPD (5.2 mm) than group B (4.2 mm) with significance (P = 0.014), and higher mean scores of BOP, group A = 0.71, group B = 0.45 with (P = 0.015); there was no statistical difference with regard to IMI, group A = 1.35, group B = 1.16 with (P = 0.172). Similar results were obtained when the sampling was calculated by patient; PPD: group A (5.31 mm), group B (4.75 mm) and P = 0.008, IMI: group A (1.34), group B (1.16) and P = 0.131, BOP: group A (0.75), group B (0.48) and P = 0.03.
Conclusion
In the absence of proper plaque control, systemic diseases showed no impact on the initiation and severity of peri-implant mucositis when compared to systemically healthy patients.
Graphical abstract

Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Dental implants are globally well accepted as the best choice for replacement of missing teeth,Citation1 because they do not involve any other teeth preparation, have better psychological acceptance by patients,Citation2 better load distributionCitation3 and a better preservation of periodontal tissue (alveolar bone and gingiva.)Citation4
The developments in the dental implantology field concerning a fixture’s design, material composition, accessories, etc.,Citation5–Citation7 raised the challenge from functional survival of the implant to aesthetic survival of the implant, many dental implants can survive with reduced bone level functionally, but aesthetically that is not sufficient.Citation8
The aesthetic aspect of implants cannot be met without maintaining healthy soft tissue supported by a satisfactory height of alveolar bone level,Citation9 preventing the exposure of unwanted titanium fixture or its metallic shadow in the cervical region especially in the anterior region.Citation10
That being said, it does not underestimate the danger of serious deterioration and eventual loss of the implant and the surrounding bone due to true peri-implantitis, despite the advancement in implantology mentioned above, all these fearsome scenarios start with a reversible disease, namely the peri-implant mucositis,Citation11 that develops mainly due to lack of proper plaque control in addition to other systemic risk factors; mostly diabetes mellitus and behavioral risk factors such as smoking.Citation12,Citation13
The mirroring similarity between periodontium and peri-implant tissue extends to the diseases too, the 2017 world workshop set some clear conditions for peri-implant mucositis by the presence of cardinal inflammatory signs like: bleeding on probing (BOP), increased probing level compared to previous readings (PPD) but with the absence of radiological bone loss (RBL),Citation14 although in 55% of peri-implant diseased sites (Staphylococcus spp., enterics and Candida spp.) were found exclusively and 19 different species, among which Porphyromonas Gingivalis, Prevotella Intermedia and Tannerella forsythus appear at higher rates, still the overall microbiological picture is highly similar.Citation15 This disease affects nearly 50% of implants and if left untreated, develops into the more serious condition ie, peri-implantitis.Citation16
It is beyond debate now that, with the exception of congenital and auto-immune problems, the plaque pathogens like P. Gingivalis, Aggregatibacter Actinomycetemcomitans, etc. are the key factor behind periodontal problems,Citation17,Citation18 this is true for peri-implant diseases tooCitation19 starting from simple plaque related gingivitis until more serious conditions such as loss of attachment related periodontitis in its most known form (previously named chronic and acute periodontitis) recently changed into only periodontitis.Citation20
It is believed too that systemic overall condition also plays a role in the prognosis of dental implant survivalCitation15,Citation21 and that some dentists refuse to operate on a patient with uncontrolled diabetic patients (which is the right thing to do). The contraindication is related to delayed wound healing due to impairment of microvasculature and activation of inflammatory pathways that may affect a cell's apoptosis.Citation22–Citation24
The American College of Cardiology and American Heart Association set new guidelines for hypertension in 2017, lowering the reading that is considered hypertensive to 130/80 mmHg less than the previous guideline (140/90 mmHg).Citation25
Singh et al found that 20.8% of 832 implants failed due to hypertension, several studies and meta-analysis showed a positive relationship between hypertension and periodontal problems.Citation26 Fernandes et al found Prevotella intermedia, a known periodontal pathogenic bacteria, in 92.8% and traces of other periodontal pathogens in atherosclerotic plaque samples taken from patients with vascular disease.Citation27
Despite the interlinking between several risk factors and behavior-related effects, plaque control measures are still the most decisive factor for periodontal health.Citation28,Citation29
The condition in question (peri-implant mucositis) actually can be stopped and reversed back to normal if correct maintenance is performed, the removal of plaque will stop further deterioration, in three weeks, tissue can be restored to its normal condition, thus plaque control should be the main factor to focus on, as Salvi et al found.Citation30,Citation31 In order to do that, we need good compliance by patients; yet, several limitations arise, since patients cannot notice periodontal problems (as well as peri-implant problems) as it is mostly painless, other limitations might be dentist-related, as it is sometimes not easy to tell the difference between peri-implant mucositis and peri-implantitis on clinical bases.Citation32 This can also be seen in the wide range of reported peri-implant mucositis prevalence in literature, ranging from 8–46% and peri-implantitis up to 25%.Citation33
Patients who are committed to regular supportive recall visits, professional and home plaque control measures lost (0.7) tooth per subject over 30 years, Matarese et al found.Citation33
This article investigates the role of plaque accumulation in peri-implant diseases initiation and progression as a risk factor as opposed to systemic diseases, aiming to raise the attention to the supportive periodontal therapy (SPT) that includes total examination of peri-implant tissue, plaque control and careful periodic observation following the prosthetic part placement.
The null hypothesis states that there is no difference between patients with systemic diseases and systemically healthy patients regarding the implant mucosal index, probing pocket depth, and bleeding on probing.
Patients and Methods
Principles of the Declaration of Helsinki were considered and met, all patients were informed about the idea of the research and signed a letter of consent, the research was also approved by the ethical committee of the College of Dentistry/University of URUK (no. 23 in 2020).
Study Design
This is a cross-sectional non-interventional study, the comparison took place between two groups.
Group A: patients that are systemically healthy.
Group B: patients suffering from hypertension and diabetes mellitus type II.
Both of them failed to follow proper plaque control measures, and had a mean plaque index ≥1, they all received equal treatment protocols, oral hygiene instructions and motivation.
Patients’ History Taking and Selection
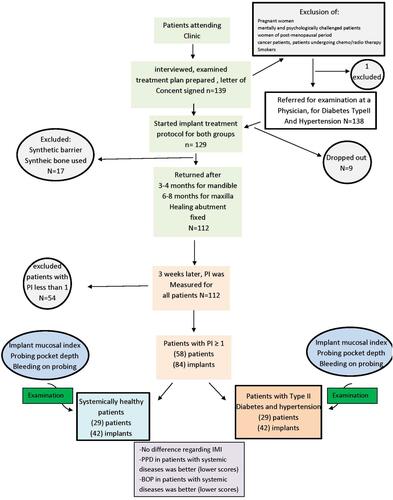
Before final filtering, 139 patients (male and female) were interviewed aged between 18–50-years-old, before the start of the treatment, medical, dental and familial history was recorded in case sheets, then patients were followed through all stages of the implant procedure ranging from 3–8 months. Fractions of patients were excluded due to exclusion criteria at the interview (1), others dropped out due to certain surgical considerations (17), and some dropped out during the research for personal reasons (9).
Exclusion criteria were:
Pregnant women.
Mentally and psychologically challenged patients.
Women of post-menopausal period.
Cancer patients, patients undergoing chemo/radiotherapy.
Patients with synthetic bone/barrier implant procedure.
Smokers.
For this article, final filtration kept only fifty-eight patients (eighty-four implants) who failed to follow proper plaque control, and whose plaque index is more than 1 after three weeks of healing abutment placement, see .
Patient Systemic Diseases Diagnosis
All patients were sent to a physician to obtain a signed diagnosis paper with their blood pressure measurement, HbA1c percentage and fasting blood sugar level, once after the interview and again on the operation day.
Readings higher than 130/80 mmHg were considered hypertensive.Citation25
Readings higher than 6.5% HbA1c were considered diabetic.Citation22
Surgical Procedure
Samples included were subjected to conventional dental implantology protocol, Dentium implants, no sinus lift, no synthetic bone and/or membrane, Delayed exposure and delayed loading protocol, exposure was scheduled as:
Maxillary implants: 6–8 months.Citation34
Mandibular implants: 3–4 months.Citation34
Before exposure, osteointegration was examined using periapical x-ray, and that delayed the exposure by 1–2 months in some cases of mandibular arch.
Patients did not receive any antibiotic treatment during exposure procedure and healing abutment placement until the examination time.
Selection of Implants
When patients who failed to follow proper home plaque control measures, returned to complete the implant therapy (prosthetic part), three weeks after healing abutment insertion, which is enough to develop signs of peri-implant mucositis around the healing abutment,Citation30 the implants were carefully examined using peri-apical x-rays, OPG’s and clinically with a periodontal probe (CPITN Probe) and mirror, the selected implants were fully integrated within the jaw bone, showing no signs of radiolucency, mobility, and the healing abutment was a mounted fit without any looseness ().
Table 1 Descriptive Numbers of Patients and Implants Characteristics That Participated in the Research
Samples Grouping
Samples were divided into two groups () ().
Group A: healthy patients.
Group B: patients with systemic diseases (hypertension and diabetes mellitus type II).
Sampling by Implant
Each individual implant was considered as an individual sample, divided between implants belonging to systemically healthy patients and implants belonging to patients with hypertension and diabetes mellitus Type II ( and ).
Table 2 Descriptive Statistics of Plaque Index, Implant Mucosal Index, Probing Pocket Depth and Bleeding on Probing for Two Groups When Each Implant Was Considered a Sample
Sampling by Patient
The mean of scores of implants within the same patient was taken to represents one sample (as per patient), here each patient represented an individual sample ( and ).
Measurement of plaque, implant mucosal index, probing pocket depth.
Table 3 Descriptive Statistics of Plaque Index, Implant Mucosal Index, Probing Pocket Depth and Bleeding on Probing for the Two Groups When Patients are Considered as Samples
Plaque Index (PI)
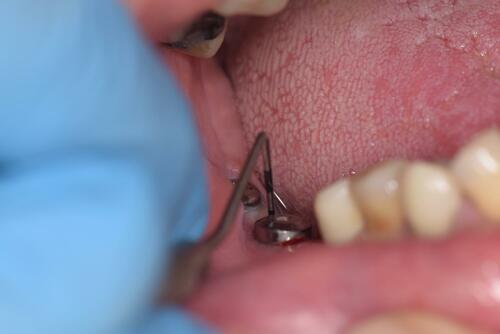
After the healing abutment was attached to the implant, three weeks period of waiting elapsed before each peri-implant tissue was measured using a periodontal probe () PD-CPITN, model: PI-1304 New York, USA. Plaque index of Löe 1967 was used for evaluation of plaque accumulation and thus the overall oral hygiene status of the patient, its scores are as follows:
Score 0: absence of plaque by both vision and gentle probing.Citation35
Score 1: absence of plaque by vision but presence on probing.Citation35
Score 2: visible plaque accumulation.Citation35
Score 3: abundant amount of plaque exceeding the cervical third of the healing abutment.Citation35
Each one of the 4 surfaces (mesial, facial, lingua/palatal, distal) of healing abutment was measured by moving the probe gently on the surface in a sweeping motion, after dryness of the surrounding field, a mean of them was considered to represent the reading of the individual implant.
Implant Mucosal Index (IMI)
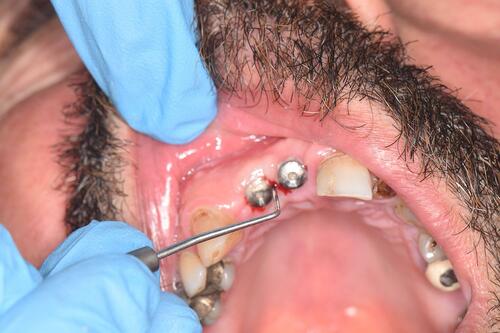
After good dryness of field, the same type of periodontal probe was inserted gently in sulcus, no more than 1 mm, then moved in an encircling movement with a little pressure directed towards the soft tissue (), after a few seconds, scores were observed as:
Score 0: no bleeding.Citation36
Score 1: single point bleeding.Citation36
Score 2: moderate multi points bleeding.Citation36
Score 3: profuse bleeding in multiple points on probing.Citation36
Score 4: suppuration.Citation36
All the four surfaces (lingual, distal, mesial and labial) of the healing abutment were measured and a mean of them was considered to represent the reading of the individual implant.
Bleeding on Probing (BOP)
With the periodontal probe, we encircle the sulcus around the implant gently with minimum pressure and then observe. Scoring the following:
Score 0: no bleeding.Citation37
Score 1: presence of bleeding in one or more surface(s).Citation37
If a single surface showed bleeding, the sample was considered as (1) since it’s a 1/0 measurement.
Probing Pocket Depth (PPD)
Using the same probe, insertion with minimal pressure into the interface between healing abutment and gingiva then the depth of pocket was recorded till the tissue stops the probe without further pressure, the deepest side was chosen to represent the implantCitation38 ().
Calibration/Inter-Observer Reliability
Examination was run by the authors, one examiner was the lead, the other one was for intercalibration purpose, for plaque index the procedure was slightly complicated, since the plaque removed by the first examiner cannot be observed by the following examiner, thus we invited a third examiner (a periodontist) to observe the procedure while the lead examiner and co-examiner do the work.
The scores of both examiners were tested using Cohen’s Kappa test and the results were:
For all parameters (plaque index, implant mucosal index (IMI), bleeding on probing and probing pocket depth) the significance of Cohen’s Kappa test was (P = 0.000).
PI kappa’s value = 0.692 (good inter-rater reliability).
IMI Kappa’s value = 0.929 (excellent inter-rater reliability).
BOP Kappa’s value = 0.828 (excellent inter-rater reliability).
PPD Kappa’s value = 0.715 (good inter-rater reliability).
Statistical Analysis
Statistical analysis was performed using SPSS® version 26 by IBM®. Null hypothesis proposed that there is no statistical difference between group A and group B with regard to implant mucosal index, probing pocket depth and bleeding on probing.
Cohen’s Kappa test was used to test the inter-rater reliability between the scores of the two clinicians who did the measurements.
Normality test: Kolmogorov–Smirnov normality test’s result was highly significant (P = 0.00) and data were considered as abnormally distributed.
Mann–Whitney test was used to test the null hypothesis during the comparison between IMI and PPD of group A and group B.
Chi-square test was used to test the null hypothesis during the comparison between BOP of group A and group B.
Results
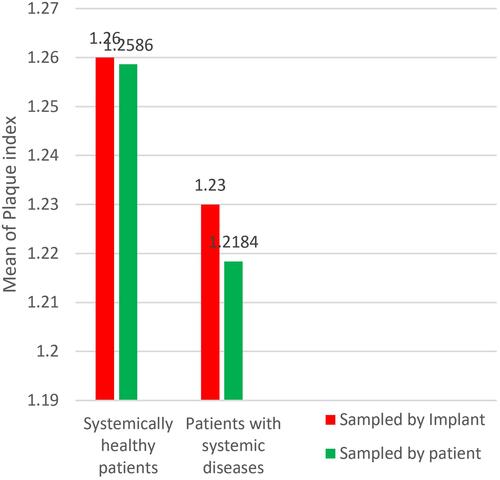
When implants were considered as individual samples, the mean of PI of group A was = 1.26 (it was = 1.25 when patients were considered as individual samples) higher than mean of PI of group B = 1.23 (1.21 when patients were considered as individual samples) (), when tested using the Mann–Whitney test, the result was non-significant P = 0.802 (P = 0.84 when patients were considered as individual samples) and thus the null hypothesis was accepted as there is no difference in the mean of PI between the two groups.
When comparing means of IMI of the 84 implants (58 patients) ( and ), group A = 1.35 (when patients were considered as individual samples group A = 1.34) and B = 1.16 (when patients were considered as individual samples group B = 1.16), although means of IMI in group A was higher than that of group B (), non-significance test result was obtained as P = 0.172 (P = 0.131 when patients were considered as individual samples), accepting the null hypothesis that there is no statistical evidence of a difference between group A and group B ( ).
Table 4 Mann–Whitney Test for Means of Plaque Index (PI), Implant Mucosal Index (IMI) and Probing Pocket Depth (PPD) Comparison Between Group A and Group B (Each Sample Represents an Implant)
Table 5 Mann–Whitney Test for Means of Plaque Index (PI), Implant Mucosal Index (IMI) and Probing Pocket Depth (PPD) Comparison Between Group A and Group B (Each Sample Represents a Patient)
When comparing the probing pocket depth ( and ), the results showed a higher mean of probing pocket depth in group A = 5.2 mm (5.31 mm when patients were considered as individual samples), as compared to group B = 4.5 mm (4.75 mm when patients were considered as individual samples) (), upon testing the results using the Mann–Whitney test, we obtained a statistical significance P = 0.014 (P = 0.008 when patients were considered as individual samples) proving an existent difference between groups A and B ( and ).
Regarding the bleeding on probing (BOP) ( and ), descriptive statistics showed a higher tendency for bleeding on probing in healthy patients = 0.71 (0.75 when patients were considered as individual samples) than patients with hypertension and DM type II = 0.45 (0.48 when patients were considered as individual samples) (); chi-square test showed a significant relationship of P = 0.015 (P = 0.031 when patients were considered as individual samples) ( and ).
Table 6 Chi-Square Test for Bleeding on Probing Means of the Two Groups A and B (Each Sample Represents an Implant)
Table 7 Chi-Square Test for Bleeding on Probing Means of the Two Groups A and B (Each Sample Represents a Patient)
Discussion
Both groups A and B had a mean of PI more than 1 ( and ). Group A (systemically healthy patients) showed worse scores with regard to PPD and BOP when compared to group B (patients with systemic diseases) as seen in ( and ). While a difference between both groups’ IMI scores could not be proven, when the implant was taken as an individual sample and when each separate patient was taken as an individual sample ( and ).
Most, if not all, studies agree on the fact that systemic diseases, especially diabetes mellitus and dental plaque, are causative and risk factors for the development of peri-implant diseases.Citation11,Citation27
Results of this study partially agree with Schimmel et al who found no statistical effect of medical conditions on peri-implant tissue condition, instead he found that psychological factors played a more important role especially in geriatric patients.Citation39 In contrast, Singh et al found that 20.3% of implants in hypertensive patients failed (among 832 implants).Citation26
That can be answered by Seki et al who found a negative effect of anti-hypertensive drugs on dental implants and hypothesized that bone metabolism is affected and thus deeper pockets were observed and more bone resorption.Citation40 That contradicted other authors who believed that drugs used for systemic diseases namely the anti-coagulants (that could be taken by some hypertensive patients) might have some protective role against peri-implant diseases, due to their secondary anti-inflammatory effects, this is what Romandini et al believed,Citation41 Nemati et al found out an anti-inflammatory effect for the anti-hypertensive drugs could also enhance the function of PMN’s immune cells,Citation42 this could contribute to a better understanding of the better scores of the patients with systemic diseases when compared to systemically healthy patients in this article.
The tendency of hypertensive patients to develop severe forms of gingivitis and even attachment loss was linked to the augmented immune response, this augmentation can be traced back to the release of inflammatory mediators like interleukins, c-reactive proteins, tumor necrosis factor-α and metalloprotease in patients with hypertension and coronary heart disease,Citation31,Citation43 Isola et al observed an increase in the serum and salivary levels of Galectin-3 (which is an inflammatory mediator and a member of the beta-glycoside binding proteins expressed in fibroblasts) in patients with both periodontitis and coronary heart disease.Citation43
Uncontrolled diabetic patients, since the National Institutes of Health (NIH) conference in 1988, are prohibited from dental implantology, because of diabetes mellitus related microvasculature problems, as well as the delayed wound healing and liability to infection, Heber Arbildo et al found that well controlled diabetic patients have the same implant survival rate as non-diabetic patients.Citation24 This harmonizes with our findings that diabetic patients of poor oral hygiene did not show a worse IMI score. Even more; in terms of statistical significance, their PPD and BOP were less than systemically healthy patients as seen in and .
This research agrees with Jepsen et al in 2015 who stated that plaque accumulation led to the development of mucositis, and even more if left as it is without maintenance or re-enforcement on plaque control, 43.9% of cases were presented with peri-implantitis after five years.Citation44
One of the explanations of plaque-induced periodontal diseases is related to the isolation of several biomarkers from peri-implant mucositis sulcus; bio-markers such as: AdpB: which is a distinctive surface protein with broad-spectrum extracellular matrix binding abilities associated with Prevotella species and FadA: which is an adhesin involved in tissue invasion associated with Fusobacterium species.
Other Noxious/Proinflammatory Cytokines
Interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), macrophage inflammatory protein (MIP-1α), Interleukin-8 (IL-8), Interleukin-6 (IL-6), type I collagen degradation product (ICTP), matrix metalloproteinase (MMP-1) and cathepsins.
Proteins Associated with Connective Tissue Destruction
Keratins, titin, actin- and microtubule-associated proteins, l-plastin, histone (H4, H1.2) apolipoprotein and others.Citation44,Citation45
These pro-inflammatory markers promote the inflammatory process in gingiva around the implant and peri-implant mucositis is initiated.
If plaque control is not followed properly, Pokrowiecki et al suggested more serious markers to be released, such as receptor activator of nuclear factor (NF)-kappa B ligand (RANKL) which is also known as the osteoclast differentiation factor that promotes bone resorption and the conversion from peri-implant mucositis to peri-implantitis,Citation45 the expression of RANKL in periodontal diseases was studied by Matarese et al who found out that RANKL and Osteoprotegerin (OPG) are linked to Transglutaminase-2 (TG2), an enzyme that is produced by periodontal cells in periodontal disease progression, OPG is produced by periodontal ligament fibroblasts to hinder/prevent bone resorption by inactivating pre-osteoclast differentiation, this enzyme (TG2) disturbs the RANKL/OPG ratio, favoring the RANKL expression, leading to serious tissue changes such as alveolar bone resorption.Citation46
Limitations
The use of the CPITN probe, a better result and also safer for the implants and implant/tissue interface is to use plastic and/or carbon probes designed for this purpose.
Cross-sectional design.
Limited external validity.
Conclusion
In absence of proper plaque control, systemic diseases showed no impact on the initiation and severity of peri-implant mucositis when compared to patients without systemic diseases, with regard to probing pocket depth and bleeding on probing. Thus, prioritizing plaque control and supportive periodontal/peri-implant therapy is recommended.
Disclosure
The authors declare that they have no conflict of interests related to this work.
References
- Alam MK, Rahaman SA, Basri R, Sing Yi TT, Si-Jie JW, Saha S. Dental implants – perceiving patients’ satisfaction in relation to Clinical and Electromyography Study on implant patients. PLoS One. 2015;10(10):e0140438. doi:10.1371/journal.pone.0140438
- Takemae R, Uemura T, Okamoto H, et al. Changes in mental health and quality of life with dental implants as evaluated by General Health Questionnaire (GHQ) and Health Utilities Index (HUI). Environ Health Prev Med. 2012;17(6):463–473. doi:10.1007/s12199-012-0275-9
- Kern J-S, Kern T, Wolfart S, Heussen N. A systematic review and meta-analysis of removable and fixed implant-supported prostheses in edentulous jaws: post-loading implant loss. Clin Oral Implant Res. 2016;27:174–195. doi:10.1111/clr.12531
- Pan YH, Lin HK, Jerry C-Y, et al. Evaluation of the peri-implant bone level around platform-switched dental implants: a retrospective 3-year Radiographic Study. Int J Environ Res Public Health. 2019;16(14):2570. doi:10.3390/ijerph16142570
- Jandta KD, Wattsd DC. Nanotechnology in dentistry: present and future perspectives on dental nanomaterials. Dent Mater. 2020;36(11):1365–1378. doi:10.1016/j.dental.2020.08.006
- Cervino G, Fiorillo L, Arzukanyan AV, Spagnuolo G, Cicciù M. Dental restorative digital workflow: digital smile design from aesthetic to function. Dent J. 2019;7(2):30. doi:10.3390/dj7020030
- Smeets R, Stadlinger B, Schwarz F, et al. Impact of dental implant surface modifications on osseointegration. Biomed Res Int. 2016;2016:6285620. doi:10.1155/2016/6285620
- Geraets W, Zhang L, Liu Y, Wismeijer D. Annual bone loss and success rates of dental implants based on radiographic measurements. Dentomaxillofac Radiol. 2014;43(7):20140007. doi:10.1259/dmfr.20140007
- Lehmijoki M, Holming H, Thorén H, Stoor P. Rehabilitation of the severely atrophied dentoalveolar ridge in the aesthetic region with corticocancellous grafts from the iliac crest and dental implants. Med Oral Patol Oral Cir Bucal. 2016;21(5):e614–20. doi:10.4317/medoral.21146
- Balasubramaniam AS, Raja SV, Thomas LJ. Peri-implant esthetics assessment and management. Dent Res J. 2013;10(1):7–14.
- Rokaya D, Srimaneepong V, Wisitrasameewon W, Humagain M, Thunyakitpisal P. Peri-implantitis update: risk indicators, diagnosis, and treatment. Eur J Dent. 2020;14(4):672–682. doi:10.1055/s-0040-1715779
- Costa FO, Takenaka‐Martinez S, Cota LO, Ferreira SD, Silva GL, Costa JE. Peri-implant disease in subjects with and without preventive maintenance: a 5-year follow-up. J Clin Periodontol. 2012;39(2):173–181. doi:10.1111/j.1600-051X.2011.01819.x
- Ferreira SD 1, Silva GLM, Cortelli JR, Costa JE, Costa FO. Prevalence and risk variables for peri-implant disease in Brazilian subjects. J Clin Periodontol. 2006;33(12):929–935. doi:10.1111/j.1600-051X.2006.01001.x
- Berglundh T, Armitage G, Araujo MG, et al. Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Periodontol. 2018;89(Suppl. 1):S313–S318. doi:10.1002/JPER.17-0739
- Kormas I, Pedercini C, Pedercini A, Raptopoulos M, Alassy H, Wolff LF. Peri-implant diseases: diagnosis, clinical, histological, microbiological characteristics and treatment strategies. A narrative review. Antibiotics. 2020;9(11):835. doi:10.3390/antibiotics9110835
- Derks J, Schaller D, Håkansson J, Wennström JL, Tomasi C, Berglundh T. Effectiveness of implant therapy analyzed in a swedish population: prevalence of peri-implantitis. J Dent Res. 2016;95(1):43–49. doi:10.1177/0022034515608832
- Ghensi P, Manghi P, Zolfo M, et al. Strong oral plaque microbiome signatures for dental implant diseases identified by strain-resolution metagenomics. NPJ Biofilms Microbiomes. 2020;6:47. doi:10.1038/s41522-020-00155-7
- AbdulAzeez AR, Mahmood MS, Ali WM. Phototoxic effect of visible blue light on aggregatibacter actinomycetemcomitans and porphyromonas gingivalis in patients with chronic periodontitis: an in - Vitro Study. J Bagh Coll Dent. 2015;27(1):144–150. doi:10.12816/0015279
- Wada M, Mameno T, Onodera Y, Matsuda H, Daimon K, Ikebe K. Prevalence of peri-implant disease and risk indicators in a Japanese population with at least 3 years in function-a multicentre retrospective study. Clin Oral Implants Res. 2019;30(2):111–120. doi:10.1111/clr.13397
- Caton J, Armitage G, Berglundh T, et al. A new classification scheme for periodontal and peri‐implant diseases and conditions–introduction and key changes from the 1999 classification. J Clin Periodontol. 2018;45(Suppl20):S1–S8. doi:10.1111/jcpe.12935
- Genco RJ, Sanz M. Clinical and public health implications of periodontal and systemic diseases: an overview. Periodontol. 2020;83(1):7–13. doi:10.1111/prd.12344
- Leon BM, Maddox TM. Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes. 2015;6(13):1246–1258. doi:10.4239/wjd.v6.i13.1246
- Dubey RK, Gupta DK, Singh AK. Dental implant survival in diabetic patients; review and recommendations. Natl J Maxillofac Surg. 2013;4:142–150. doi:10.4103/0975-5950.127642
- Arbildo H, Lamas C, Camara D, Vásquez H. Dental implant survival rate in well-controlled diabetic patients. A systematic review. J Oral Res. 2015;4(6):404–410. doi:10.17126/joralres.2015.077
- Whelton PK, Carey RM, Aronow WS, et al. High blood pressure clinical practice guideline. J Am Coll Cardiol. 2018;71(19):e127–e248.
- Singh R, Parihar AS, Vaibhav V, et al. A 10 years retrospective study of assessment of prevalence and risk factors of dental implants failures. J Family Med Prim Care. 2020;9(3):1617–1619. doi:10.4103/jfmpc.jfmpc_1171_19
- Fernandes CP, Oliveira FA, De barros silva PG, et al. Molecular analysis of oral bacteria in dental biofilm and atherosclerotic plaques of patients with vascular disease. Int J Cardiol. 2014;174(3):710–712. doi:10.1016/j.ijcard.2014.04.201
- Gunpinar S, Meraci B, Karas M. Analysis of risk indicators for prevalence of peri-implant diseases in Turkish population. Int J Implant Dent. 2020;6:19. doi:10.1186/s40729-020-00215-9
- Mahmood AA, AbdulAzeez AR, Hussein HM. The effect of smoking habit on apical status of adequate endodontically treated teeth with andwithout periodontal involvement. Clin Cosmet Investig Dent. 2019;11:419–428. doi:10.2147/CCIDE.S236747
- Salvi GE, Aglietta M, Eick S, Sculean A, Lang NP, Ramseier CA. Reversibility of experimental peri‐implant mucositis compared with experimental gingivitis in humans. Clin Oral Implant Res. 2012;23(2):182–190. doi:10.1111/j.1600-0501.2011.02220.x
- Khocht A, Rogers T, Janal MN, Brown M. Gingival fluid inflammatory biomarkers and hypertension in African Americans. JDR Clin Trans Res. 2017;2(3):269–277. doi:10.1177/2380084417694335
- Romandini M, Lima C, Pedrinaci I, Araoz A, Soldini MC, Sanz M. Clinical signs, symptoms, perceptions, and impact on quality of life in patients suffering from peri-implant diseases: a university-representative cross-sectional study. Clin Oral Implants Res. 2021;32(1):100–111. doi:10.1111/clr.13683
- Matarese G, Ramaglia L, Fiorillo L, Cervino G, Lauritano F, Isola G. Implantology and periodontal disease: the panacea to problem solving? Open Dent J. 2017;11:460–465. doi:10.2174/1874210601711010460
- Chen J, Cai M, Yang J, Aldhohrah T, Wang Y. Immediate versus early or conventional loading dental implants with fixed prostheses: a systematic review and meta-analysis of randomized controlled clinical trials. J Prosthet Dent. 2019;122:516–536. doi:10.1016/j.prosdent.2019.05.013
- Löe H. The gingival index, the plaque index and the retention index system. J Periodontol. 1967;38(6):610–616. doi:10.1902/jop.1967.38.6_part2.610
- French D, Grandin HM, Ofec R. Retrospective cohort study of 4,591 dental implants: analysis of risk indicators for bone loss and prevalence of peri-implant mucositis and peri-implantitis. J Periodontol. 2019;90:691–700. doi:10.1002/JPER.18-0236
- Doornewaard R, Jacquet W, Cosyn J, De Bruyn H. How do peri‐implant biologic parameters correspond with implant survival and peri‐implantitis? A critical review. Clin Oral Implant Res. 2018;29(Suppl. 18):100–123. doi:10.1111/clr.13264
- Lopes GD, Feitosa AC, Suaid FF, et al. Evaluation of peri‑implant condition in periodontally compromised patients. J Indian Prosthodont Soc. 2019;19:283–289. doi:10.4103/jips.jips_197_19
- Schimmel M, Srinivasan M, McKenna G, Müller F. Effect of advanced age and/or systemic medical conditions on dental implant survival: a systematic review and meta‐analysis. Clin Oral Implant Res. 2018;29(Suppl. 16):311–330. doi:10.1111/clr.13288
- Seki K, Hasuike A, Iwano Y, Hagiwara Y. Influence of antihypertensive medications on the clinical parameters of anodized dental implants: a retrospective cohort study. Int J Implant Dent. 2020;6(1):32. doi:10.1186/s40729-020-00231-9
- Romandini M, Lima C, Pedrinaci I, Araoz A, Soldini MC, Sanz M. Prevalence and risk/protective indicators of peri-implant diseases: a university-representative cross-sectional study. Clin Oral Implants Res. 2021;32(1):112–122. doi:10.1111/clr.13684
- Nemati F, Rahbar-Roshandel N, Hosseini F, Mahmoudian M, Shafiei M. Anti-inflammatory effects of anti-hypertensive agents: influence on interleukin-1β secretion by peripheral blood polymorphonuclear leukocytes from patients with essential hypertension. Clin Exp Hypertens. 2011;33(2):66–76. doi:10.3109/10641963.2010.496521
- Isola G, Polizzi A, Alibrandi A, Williams RC, Lo Giudice A. Analysis of galectin-3 levels as a source of coronary heart disease risk during periodontitis. J Periodont Res. 2021;56:597–605. doi:10.1111/jre.12860
- Jepsen S, Berglundh T, Genco R, et al. Primary prevention of peri-implantitis: managing peri-implant mucositis. J Clin Periodontol. 2015;42(Suppl.16):S152–S157. doi:10.1111/jcpe.12369
- Pokrowiecki R, Mielczarek A, Zaręba T, Tyski S. Oral microbiome and peri-implant diseases: where are we now? Ther Clin Risk Manag. 2017;13:1529–1542. doi:10.2147/TCRM.S139795
- Matarese G, Currò M, Isola G, et al. Transglutaminase 2 up‑regulation is associated with RANKL/ OPG pathway in cultured HPDL cells and THP‑1‑differentiated macrophages. Amino Acids. 2015;47(11):2447–2455. doi:10.1007/s00726-015-2039-5