Abstract
Background
Periodontal disease is characterized by the inflammation of all tissues that surround and support the teeth, and it is the most common public health problem worldwide. It has many different contributing factors, such as poor oral hygiene, smoking, anemia, bacterial plaque, poor economic status, and nutritional deficiency. It also causes different feto-maternal complications like preeclampsia, gestational diabetes, preterm labor, low birth weight, and early abortion. Thus, this study aimed to assess the prevalence and associated factors of periodontal disease among pregnant women.
Methods
A cross-sectional study was conducted among 618 women at public hospitals in the Southwest Ethiopia. Data were collected using a systematic random sampling method and a structured interviewer administered questionnaire. The data were entered into Epi-Data Manager 4.2 and then transferred to a statistical package for social science (SPSS) version 21 for analysis. The logistic regression analysis was done to see the significant association between each predictor with periodontal disease by considering a p-value of <0.05 and a CI of 95%.
Results
A total of 618 women attending ANC with a 100% response rate were enrolled in this study. The mean and standard deviation of the age of the study participants was 31.07 ± 7.8 years. The periodontal disease was observed among 240 (38.8%) of the total participants with 95% C.I of (35%, 43%). Predictors like rural residency, women who could not read and write, women with poor wealth index, nutritional status of underweight, had no information about periodontal disease, depression, history of diabetes mellitus, self-perceived halitosis, no history of ANC, and lower gestational age were found to be significantly associated with periodontal disease.
Conclusion
In this study, the prevalence of periodontal disease was found to be significantly higher. Therefore, in order to minimize the burden of periodontal disease among pregnant women, oral, medical, and mental health education should be promoted.
Background
Periodontal disease (PD) is the most common public health problem worldwide that can affect up to 90%Citation1,Citation2 of populations, and it is characterized by an inflammation of all the tissues that surround the teeth including the gingiva (gum tissue), cementum (outer layer of the roots of teeth), alveolar bone (sockets into which the teeth are anchored), and periodontal ligaments (connective tissue fibers between the cementum and the alveolar bone).Citation3
It is a common oral infection that comprises gingivitis and periodontitis.Citation4 Gingivitis (gum inflammation) is a mild, early form of periodontitis (gum disease) that occurs when bacteria and plaque build-up in the mouth and lead to infection. It is also characterized by bleeding, swollen gums, and pain, whereas if these conditions are left untreated and progress to loss of periodontal attachment and supporting bone, it is said to be periodontitis.Citation5
Oral diseases affect nearly 3.5 billion people worldwide.Citation6 Of these, untreated dental caries (tooth decay) in permanent teeth is the most common health condition. If the dental caries are left untreated early, it will proceed to severe periodontal (gum) disease. Severe periodontal disease was the 11th most prevalent condition in the world,Citation7 in which its prevalence was ranged from 20% to 50% around the world.Citation8 In most high-income countries, dental treatment averages 5% of total health expenditure.Citation9
The prevalence of the periodontal disease is varied across different settings and populations, for instance, the reported prevalence of PD in the United States (US),Citation10 Latin America,Citation11 and IndiaCitation12 was 50%, 40–80%, and 96.3%, respectively. The prevalence of periodontitis among pregnant women is also varied across different parts of the worldCitation13–Citation15 such as 11% in Brazil,Citation10 60.5% in Rwanda,Citation16 24.0% in Sudan,Citation17 and 42.4% in Northwest Ethiopia.Citation18
Periodontal diseases are highly common during pregnancy and lactation,Citation19 due to hormonal factors (high concentration of estrogen and progesterone) and the subsequent impact on the gingival arteries compared with non-pregnant and non-lactating women.Citation20 Periodontal disease is more prevalent at eight months of pregnancy and its rate begins to return to normal during two months postpartum.Citation21,Citation22 Pregnant women with periodontal disease are more susceptible to poor maternal and perinatal outcomes, such as preeclampsia,Citation23 gestational diabetes,Citation24 vulvovaginitis, preterm labor (gestational age of <37 weeks), fetal growth restriction,Citation25 low birth weight (<2500 g),Citation26 perinatal mortality,Citation27 early abortion, and a higher risk of early neonatal infection.Citation28 Therefore, different studies suggested that scaling and root planning during the second trimester of pregnancy reduce the risk of periodontal disease complications.Citation29–Citation31
There are different contributing factors, such as age, parity, lower level of education, and anemia were identified as risk factors for periodontal disease.Citation32 Moreover, poor oral hygiene,Citation33 smoking,Citation33–Citation35 ethnicities,Citation1,Citation3 body mass index, bacterial plaque,Citation10 lower gestational age,Citation17 residency, systemic disease,Citation18 psychosocial factors (depression, anxiety, and stress),Citation36,Citation37 and poor economic statusCitation1,Citation3,Citation38–Citation40 have been reported as risk factors for periodontal disease. Due to different factors, PD is complicated with tooth loss which can compromise intake of food, aesthetics, self-confidence, and quality of life.Citation41 This condition also negatively affects the nutritional status and the general health of the women.Citation41,Citation42 Conversely, nutritional deficiencies, specifically the shortage of vitamin D groups: vitamins D2 (cholecalciferol) and D3 (ergocalciferol), were found to be a cause of periodontal inflammation and a delay in post-surgical periodontal healing.Citation43,Citation44 In addition to nutritional deficiency, obesity can also be associated with periodontitis through the decrement of antioxidant substances in the periodontal tissues and excessive production of reactive oxygen species (ROS), which in turn causes chronic activation of inflammation and tissue destruction.Citation45 This condition further contributed to the development of Insulin Resistance (IR), which is known to cause periodontitis.Citation46
Periodontal disease (PD) is a cause for the release of metalloproteinase and prostaglandins (PgE2),Citation47 which stimulates the production of cytokines and pharmacologically active mediators, such as interleukin 1-beta, interleukin-6, alpha tumor necrosis factor.Citation48 The spread of these infectious agents from dental plaque and inflammatory mediators from periodontal tissues to other organs of the body across the junctional epithelium is believed to cause different systemic diseases like; Alzheimer’s disease,Citation49 diabetes, cardiovascular disease, feto-maternal complications, rheumatoid arthritis, and chronic obstructive pulmonary disease.Citation50–Citation56
Despite the World Health Organization (WHO) recommending the inclusion of oral health in a political declaration on universal health coverage in 2019, the periodontal disease remained disproportionately affecting poor and socially disadvantaged group of society.Citation57 Due to the nature of the disease also, the early stages of periodontitis usually remain asymptomatic,Citation58 and so that most women are complicated by feto-maternal complications associated with severe periodontitis. Therefore, studies related to periodontal disease will contribute for the maintenance of periodontal health through an early diagnosis and treatment. Moreover, there is a paucity of data regarding periodontal disease and there are high rates of maternal and perinatal adverse outcomes in Ethiopia, particularly in Southwest Ethiopia.Citation59 Hence, determining the prevalence of the periodontal disease and identifying the factors associated with periodontal disease is useful for policy development and the allocation of financial and human resources for preventive measures and the provision of treatment. Therefore, this study aimed to assess the prevalence of periodontal disease and its associated factors among pregnant women attending antenatal care (ANC) in public hospitals, Southwest Ethiopia.
Methods and Materials
Study Design and Period
A cross-sectional study was conducted among women attending ANC clinics at public hospitals in Southwest Region, Ethiopia, from December 1st, 2021 to February 30th, 2022.
Study Setting
This study was carried out in three public hospitals of Southwest Ethiopia: Mizan-Tepi University Teaching Hospital (MTUTH), Tepi General Hospital (TGH), and Gebretsadik Shawo General Hospital (GSGH). These hospitals are located in Benchi-Sheko Zone, Sheka Zone, and Kefa Zone, respectively.
Mizan Tepi University Teaching Hospital was established in 1986 GC. Previously during the Derg regime, this hospital was named “Mizan-Teferi General Hospital”. Then, until 2016 GC, it was named “Mizan Aman General Hospital”. Then, during the 2016 GC, this hospital was incorporated into Mizan-Tepi University & named “Mizan Tepi University Teaching Hospital”. This hospital was located in a town called Mizan Teferi (recently named Mizan-Aman Town) which is the capital town of the Bench Sheko Zone (previously the capital of the Bench Maji Zone). It is located 565 km away from Addis Ababa, the Capital City of Ethiopia. Currently, Mizan-Tepi University Teaching Hospital is expected to provide services to more than one million people.
Tepi General Hospital is also one of the hospitals in the southwest region, which is about 615 km away from Addis Ababa, the Capital City of Ethiopia. This hospital serves more than 500,000 people. While Gebretsadik Shawo General Hospital was first established in the 1990 EC and named “Gebretsadik Shawo Primary Hospital”. Then, in 2005 EC, this hospital was named “Gebretsadik Shawo General Hospital”. Currently, the Gebretsadik Shawo General Hospital is expected to provide care for more than 500,000 people.Citation60 In the last one year, about 2640, 1344, and 1824 women attended ANC clinic in Mizan-Tepi University Teaching Hospital, Tepi General Hospital, and Gebretsadik Shawo General Hospital, respectively (Unpublished reports).
Population
The source population was all pregnant women attending ANC at the public hospitals, and all selected pregnant women attending ANC at public hospitals during the study period were the study populations.
Inclusion and Exclusion Criteria
All pregnant women with a history of at least one child pregnancy were included in the study. Those who were seriously ill (unable to respond to an interview), on orthodontic treatment, on periodontal treatment, and had incomplete ANC follow-up charts (anthropometric measurement at early pregnancy) were excluded from the study.
Sample Size Determination
The sample size was estimated using Epi Info™ 7 software and considering assumptions of double population proportion formulas such as ratio = 1:1, confidence interval (CI = 95%), power (P = 80%), and using the proportions of outcome in unexposed group and odds ratio of different risk factors of previous researchCitation18 such as income (p = 29.3% and OR = 3.12), tooth brushing (p = 69.2% and OR = 4.85), carbohydrate intake (p = 56.36% and OR = 2.16), self-perceived halitosis (p = 36.44% and OR = 1.64), and systemic disease (p = 40.78% and OR = 1.96), the final sample size with 10% non-response rate would be 130, 125, 288, 618, and 334, respectively. Therefore, in order to increase the generalizability, its precession, and minimize the error, a larger sample size of 618 was taken.
Sampling Procedure
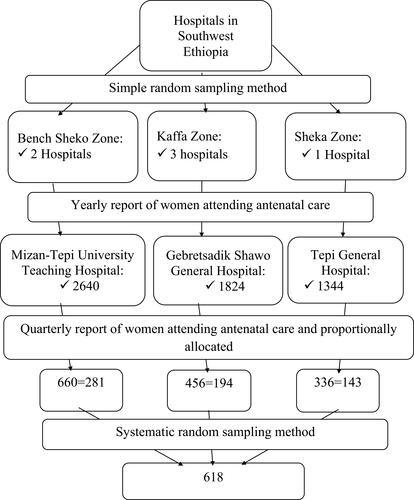
A systematic random sampling method was applied to select study participants. First, the average three-month report was taken from a yearly report of women attending ANC in each hospital (660 from MTUTH, 336 from TGH, and 456 from GSGH). Then, the sample size was proportionally allocated to each hospital (46 from MTUTH, 23 from TGH, and 31 from GSGH). The sampling interval (kth) was calculated as the total number of women attending ANC in the three hospitals divided by the sample size (1452/618=2). Then, every 2nd participant was selected after the first eligible participant was selected by the lottery method [].
Data Collection Tool, Procedures, and Quality Control
The data were collected using a structured interviewer-administered questionnaire adopted from the World Health Organization (WHO) tool,Citation61,Citation62 and other socioeconomic, behavioral, obstetrical, and nutritional variables are adapted from different reviewed literatures.Citation14,Citation17,Citation18,Citation35
WHO proposed the use of community periodontal index (CPI).Citation61,Citation62 CPI has also been provided by the American Academy of Periodontology (the “Periodontal Screening Record”, PSR), and the British Society of Periodontology (the “Basic Periodontal Examination”, BPE).Citation63–Citation65
The household wealth index was assessed by a measurement tool for wealth index adopted from Ethiopian DHS-2016.Citation66
Data on household food insecurity was measured using the Household Food Insecurity Access Scale (HFIAS), a structured, standardized, and validated tool developed by Food and Nutrition Technical Assistance (FANTA) which has nine questions with nine frequency of occurrence questions.Citation67 If a participant responds no to all question items 1–9 or responds yes to question item 1, and rarely experience in the past four weeks, it indicates for food secured household, whereas if household heads respond at least yes to question item 1 and sometimes experience in the past four weeks, it indicates for food insecured household.Citation68
Substance use was assessed using alcohol, smoking, and substance involvement screening tool (ASSIST), and if an individual used at least one of a specific substance (Alcohol, Khat, Cigarette and Others) for nonmedical purposes within the last 3 months, it is said to be current substance use, whereas if an individual used at least one of any specific substance (Alcohol, Khat, Cigarette and Others) for the nonmedical purpose at least once in a lifetime according to ASSIST, it is said to be lifetime substance use.Citation69
Depression, anxiety, and stress were assessed using the DAS scale and a score of 10 and above in DASS-D indicates the presence of depression, a score of 8 and above in DASS-A indicates the presence of anxiety, and a score of 15 and above in DASS-S indicates the presence of stress.Citation70
Body mass index (BMI) was computed using height, and weight measurement is taken at early pregnancy or first trimester. Accordingly, underweight, normal weight, overweight and obesity were defined as a body mass index (BMI) of <18.5 kg/m2, 18.5–24.9 kg/m2, 25–29.9 kg/m2 and ≥30 kg/m2, respectively.Citation71
Intra-Oral Examination
First, gingival tissues were assessed for the presence or absence of gingival inflammation. Then, the probing depth and clinical attachment loss were assessed using the World Health Organization (WHO) Community Periodontal Index (CPI) probe. The WHO-CPI probe is a 0.5 mm ball tip (help to detect the calculus and reduce soft tissue injury), a black band between 3.5 and 5.5 mm, and rings at 8.5 and 11.5 mm.Citation72
For study subjects whose age were 20 years and above, the ten permanent index teeth (17, 16, 11, 26, 27, 37, 36, 31, 46, and 47) were evaluated, whereas, for study subjects whose ages were adolescent (16, 11, 26, 36, 31, and 46) were evaluated.Citation73 Then, depending on the most affected teeth, the highest scores were assigned to each sextant (mesio-buccal/facial, mid-buccal/facial, disto-buccal/facial, mesio-lingual/palatinal, mid-lingual/palatinal, disto-lingual/palatinal) excluding the third molar. Following probing, the gingival bleeding index (GBI) was recorded as present or absent at each examination by observing bleeding on probing (BOP).Citation74
The clinical attachment loss is determined using the distance from the cementoenamel junction (CEJ) to the base of sulcus/pocket (junctional epithelium), or this can also be determined by adding the probing depth measurements with recession measurements.Citation75 Probing depth (PD) was also determined using the distance from the gingival margin to the base of the sulcus or pocket.Citation76
Accordingly, periodontal disease is determined. Then, the severity of periodontal disease was determined using the CPI score. CPI score ranges from 0 to 4 and a score 0; healthy periodontal conditions or no periodontal disease (black band completely visible or probing depth <3.5 mm); score 1; gingival bleeding (black band completely visible or probing depth <3.5 mm); score 2; calculus and bleeding (black band completely visible or probing depth <3.5 mm); score 3; probing depth of 3.5–5.5 mm (black band partially visible, indicating pocket of 4–5 mm); and a score 4; Probing depth >5.5 mm (black band entirely within the pocket, indicating pocket of 6 mm or more).Citation77 Then, study subjects with CPI 1 and 2 scores were identified as having “gingivitis”, and study subjects with CPI 3 and 4 scores were considered as having “periodontitis”.Citation78
Furthermore, periodontitis can be classified into; mild periodontitis: when there are two or more interproximal sites with attachment loss of ≥3 mm and probing depths of ≥4 mm, not on the same tooth, moderate periodontitis: when there are two or more interproximal sites with attachment loss of ≥4 mm, and probing depths of ≥5 mm, not on the same tooth, and severe periodontitis: when there are two or more interproximal sites with attachment loss of ≥6 mm and probing depth of ≥5 mm.Citation79,Citation80
Generally, to maintain the quality of the result, three days of training was given for data collectors and supervisors regarding study objectives, content, context and filling of questionnaires, interviewing technique, confidentiality issues, study participants’ rights, consenting, respondents approaching technique, and anthropometric measurement and reading. Moreover, pretest, close supervision of data collection, and double-entry of data were done.
Data Processing and Analysis
The data were checked for their completeness, and then the data were entered into Epi-Data Manager 4.2 and then transferred to a statistical package for social science (SPSS) version 21 for analysis. Descriptive statistics (frequency, percentage, mean and standard deviation) were used to describe the socio-demographic variables, behavioral variables, obstetrical variables, nutritional variables, and clinical and anthropometric measurements.
Multicollinearity among independent predictors was checked; a predictor with a variance inflation factor (VIF) of >2 was dropped from the analysis.
Binary logistic regression analysis was done to see the significant association between each predictor and periodontal disease. Then, predictors with a p-value of <0.05 were included in a multivariable logistic regression model to control all possible confounders and to identify the strong predictors significantly associated with periodontal disease. The model fitness was checked using the Hosmer–Lemeshow goodness-of-fit test and a p-value of >0.05 was considered as a good fit. Then, after computing for the measure of association both before and after adjusting for potential confounding factors and if there is a 10% or more difference between the two measures of association, the predictor is considered as a confounder. Finally, significant predictors associated with periodontal disease were determined at a p-value of <0.05 and a CI of 95%.
Results
Socio-Demographic Characteristics of the Respondents
A total of 618 women attending ANC with a 100% response rate were enrolled in this study. The mean (±SD) age of the study participants was 31.07 ± 7.8 years with most 160 (25.9%) of participants were in the age range of 18–24 years. More than half 336 (54.4%) and 338 (54.7) of the total participants were urban residents and married, respectively. Regarding their ethnicity, 158 (25.6%), 146 (23.6%), and 126 (20.4%) of the total participants were Bench, Kaffa, and Amhara, respectively. Nearly half of the participants, 279 (45.1%) could not read and write. Regarding participants’ economic status, a poor wealth index was observed among 230 (37.2%) of the total participants [].
Table 1 Socio-Demographic Characteristics of Women Attending ANC (n = 618) in Public Hospitals, South West Ethiopia, 2022
Oral Hygiene and Nutritional Status Related Characteristics
Of the total participants, about 237 (38.3%) were received health education regarding oral hygiene, and most of them, 104 (16.8%) were received health education from nurses. About 233 (37.7%) of the participants ever visited a dental clinic or dentist, of which 84 (13.6%), and 65 (10.5%) of participants have visited a dentist due to toothache and decayed teeth, respectively. Concerning tooth care, about 415 (67.2%) of the participants have been practicing brushing of teeth. Of those who practiced tooth brushing, 248 (40.1%) participants were brushed their teeth two or fewer times per day. Regarding their nutritional status, most of the participants 456 (73.8%) had normal weight, whereas 85 (13.8%) and 59 (9.5%) had overweight and underweight, respectively [].
Table 2 Oral Hygiene and Nutritional Status-Related Characteristics of Women Attending ANC in Public Hospitals, South West Ethiopia, 2022
Substance, Obstetrical, and Psychosocial Related Characteristics
Of the total participants, half of the participants 314 (50.8%) were ever used substances. Regarding their obstetrical characteristics, about 347 (56.1%) of participants have a history of gravida one, whereas 354 (57.3%) of participants have a history of para one and less. Concerning their psychosocial related characteristics, about 138 (22.3%), 158 (25.6%), and 174 (28.2%) of participants had depression, anxiety, and stress, respectively [].
Table 3 Substance, Obstetrical, and Psychosocial-Related Characteristics of Women Attending ANC in Public Hospitals, South West Ethiopia, 2022
Prevalence of Periodontal Disease
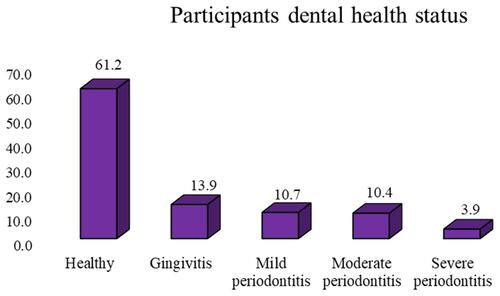
Periodontal disease was observed among 240 (38.8%) of the total participants with 95% C.I of (35%, 43%), and among these, gingivitis, mild periodontitis, moderate periodontitis, and severe periodontitis were observed among 86 (35.8%), 66 (27.5%), 64 (26.7%), and 24 (10%) of participants, respectively. Of the total study participants, healthy participants, participants with gingivitis, mild periodontitis, moderate periodontitis, and severe periodontitis were observed among 378 (61.2%), 86 (13.9%), 66 (10.7%), 64 (10.4%), and 24 (3.9%) of participants [].
Predictors Associated with Periodontal Disease
In bivariate logistic regression analysis, the association of different predictors related to socio-demographic characteristics, chronic medical illnesses, anthropometric measurements, and obstetrical characteristics with periodontal disease among pregnant women were checked. Then, predictors, such as the age of participant, residence, educational status, wealth index, nutritional status, heard about periodontal disease, frequency of toothbrush, lifetime use of chat, depression, history of diabetes mellitus, self-perceived halitosis, history of ANC, gestational age, and gravidity, was found to be significantly associated with the periodontal disease among pregnant women.
These eligible predictors were adjusted for possible confounders using multivariate logistic regression analysis. Accordingly, women who were rural residents were almost three times more likely to develop PD compared to those who lived in urban (AOR = 3.01, 95% CI = [1.79–5.08]). Similarly, those women who could not read and write (AOR = 5.05, 95% CI = [1.67–15.31]) and those women with poor wealth index (AOR = 3.12, 95% CI = [1.66–5.87]) were 5.05 and 3.12 times more likely to develop the periodontal disease compared to women with educational status of the certificate and above, and rich wealth index, respectively. Those women with nutritional status of underweight (AOR = 4.67, 95% CI = [2.12–30.59]), and those who had no information about periodontal disease (AOR = 2.79, 95% CI = [1.39–5.59]) were also 4.67, and 2.79 times more likely to develop the periodontal disease compared to women with nutritional status of obesity and women who had information about PD, respectively.
Moreover, women who had depression (AOR = 2.37, 95% CI = [1.33–4.22]), had history of diabetes mellitus (AOR = 3.99, 95% CI = [1.08–14.81]), had self-perceived halitosis (AOR = 2.28, 95% CI = [1.15–4.52]), were 2.37, 3.99, and 2.28 times more likely to develop periodontal disease compared to their counterparts, respectively. Obstetrical variables like; women who had no history of ANC (AOR = 2.25, 95% CI = [1.34–3.77]), and women had gestational age at second trimester (AOR = 1.71, 95% CI = [1.00–2.92]) were also 2.25, and 1.71 times more likely to have periodontal disease among pregnant women compared with their counterparts, respectively [].
Table 4 Independent Factors Associated with Periodontal Disease Among Women Attending ANC in Public Hospitals, South-West Ethiopia, 2022
Discussion
Periodontal disease is a public health problem that causes morbidity and mortality of all age groups.Citation81,Citation82 In the current study, we have assessed the prevalence of periodontal disease and its contributing factors. The findings could be beneficial for policymakers and programmers to minimize the burden of periodontal disease. Therefore, the aim of this study was to determine the prevalence of periodontal disease and its predictors among women attending ANC in public hospitals, southwest Ethiopia.
According to this study, the overall prevalence of periodontal disease was 240 (38.8%) with 95% C.I of (35%, 43%). This finding was found to be higher than the studies conducted in Sudan, 24.0%,Citation17 in Tanzania, 14.2%,Citation14 in Brazil, 17.24%,Citation83 and in Jordan, 31%.Citation15 In contrast to this, this study was found to be lower as compared with the studies conducted in Amsterdam, 67%,Citation84 in India, 54.8%,Citation13 and in China, 84.7%.Citation85 This discrepancy might be due to different populations, study designs, variation in sample size, and the use of different tools that may contribute to the differences observed. This study was also found to be consistent with the studies conducted in Northwest Ethiopia, 42.4%.Citation18 This similarity might be due to the similar oral health care practice and health care system availability in Ethiopia.
Accordingly, in this study, women who were rural residents were almost three times more likely to develop PD compared to those who lived in urban areas. This study is consistent with the studies conducted in India,Citation86 and in Northwest Ethiopia.Citation18 This is due to the fact that individuals from rural areas do not pay attention to oral health due to lack of awareness, inadequate availability of dental health services, and lack of an organized healthcare system.Citation87
Similarly, this study also found that predictors such as low educational status, poor wealth index, and no information about periodontal disease were more likely to develop periodontal disease. The study's findings are in line with the studies conducted in the United States,Citation88 and Northwest Ethiopia.Citation18 These disparities in socio-economic status indicators (ie, education and income) are known to affect periodontal health in which individuals’ educational status could determine their employment status, job position, and earned income and even affect their awareness and behaviors towards periodontal disease and periodontal health service utilization.Citation89
In this study, those women with poor nutritional status were more likely to develop periodontal disease. This study is corroborated with the studies conducted in Southeast Queensland, Australia,Citation90 and in Germany.Citation91 This situation is justified by the reason that being malnourished, or having a lack of proper nutrients like vitamin D, can negatively affect oral health (eg, teeth, gums, and bone that support the tooth) leading to increased risk of periodontal disease and other oral health-related problems.Citation92
Moreover, psycho-social factors like women with self-reported scoring of depression are associated with periodontal disease. This study is supported by the studies conducted in the Finger Lakes region, New York State,Citation93 and in India.Citation94 This biological plausibility for an association between depression and periodontal disease is due to the fact that depression affects the immune system due to the disturbance in the hypothalamic-pituitary-thyroid system.Citation95 Similarly, factors related to medical illness like a history of diabetes mellitus are associated with periodontal disease. This finding is consistent with the studies conducted in Sudan,Citation96 and in Indonesia.Citation97 This could be explained by the fact that diabetes contributes to the development of periodontal disease, due to an increase in the levels of inflammatory mediators, such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α).Citation98
In this study, self-perceived halitosis is also associated with periodontal disease. This finding is supported by the study conducted in Ilala and Temeke municipals, Dar-es-Salaam, Tanzania,Citation99 and in Spain.Citation100 This is due to the reason that halitosis arising from the oral cavity might be due to causative organisms residing deep in periodontal pockets and on the dorsal part of the tongue. Therefore, this film of bacteria called plaque can cause halitosis, irritate the gums, and cause inflammation to the periodontium, which finally causes periodontal disease.Citation101
In this study, obstetrical variables like women who had no history of ANC and women with lower gestational age were more likely to have periodontal disease. This finding is consistent with the studies conducted in Nigeria,Citation102 and in Khartoum, Sudan.Citation17 This is explained by the reason that the regular ANC visit provides an opportunity for women to be screened for different medical problems associated with oral health problems, especially during an early stage of pregnancy. Therefore, early, regular, and repeated visits of ANC could minimize the risk of periodontal disease associated with hormonal change during lower gestational age.Citation103
This study has its own strengths. First, since this study was conducted in the three public hospitals, it increases the probability of generalizability of periodontal disease among pregnant women. Second, this study was done using the modified version of the Community Periodontal Index of Treatment Need (CPITN) which is the Community Periodontal Index (CPI) tool by the inclusion of measurement of “Loss of attachment”. Third, this study also identified factors related to substance, obstetrical, and psychosocial characteristics of the respondents associated with the periodontal disease, which were not included in the previous studies conducted in Ethiopia. However, this study has its own limitations. First, this study did not examine the pattern and extent of alveolar bone loss using radiographic assessment but simply relied on detection of the signs of periodontal disease using periodontal probes. Second, since this study was a cross-sectional study, it did not show the cause and effect relationship of variables, and therefore, this study’s results should be interpreted with caution. Furthermore, since this study was conducted for the epidemiological purpose, it only examines the index teeth (do not examine all teeth just like for clinical purposes) and records the highest score in each sextant. Therefore, it could probably result in an underestimation of the prevalence of the disease.
Conclusion
In this study, the prevalence of periodontal disease was found to be significantly higher. Different predictors such as residence, educational status, wealth index, nutritional status, information about periodontal disease, depression, history of diabetes mellitus, self-perceived halitosis, history of ANC, and gestational age were also found to be significantly associated with the periodontal disease among pregnant women. Therefore, in order to minimize the burden of periodontal disease among pregnant women, oral, medical, and mental health education and dental examination should be included as an integral part of preconception care and antenatal care. The clinical features and feto-maternal complications associated with periodontal disease and its risks associated with medical and mental illness have to be investigated to provide appropriate management to the women attending ANC and the community at large. Moreover, comprehensive oral health cares such as promoting healthy eating habits and tooth-brushing activities, preventing tobacco/alcohol abuse in pregnant women, and promoting oral health screening programs in schools, market places and other health care institutions should be practiced among pregnant women and general population.
Abbreviations
ANC, Antenatal Care; AOR, Adjusted Odd Ratio; ASSIST, Alcohol, Smoking and Substance Involvement Screening Tool; BMI, Body mass index; BOP, Bleeding on Probing; BPE, Basic Periodontal Examination; CEJ, Cemento-Enamel Junction; COR, Crude Odd Ratio; CPI, Community Periodontal Index; CPITN, Community Periodontal Index of Treatment Need; DAS-S, Depression, Anxiety and Stress Scale; FANTA, Food and Nutrition Technical Assistance; GBI, Gingival Bleeding Index; GSGH, Gebretsadik Shawo General Hospital; HEWs, Health extension Workers; HFIAS, Household Food Insecurity Access Scale; HPs, Health professionals; IR, Insulin Resistance; MTUTH, Mizan-Tepi University Teaching Hospital; NGO, Non-governmental Organization; PD, Periodontal Disease; PSR, Periodontal Screening Record; ROS, Reactive Oxygen Species; SD, Standard Deviation; SPSS, Statistical Package for Social Science; TGH, Tepi General Hospital; US, United States; VIF, Variance Inflation Factor; WHO, World Health Organization.
Data Sharing Statement
All relevant data are included within the manuscript, but any additional data required are available from the corresponding author upon reasonable request; Email: [email protected].
Ethical Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki, and after ethical clearance was obtained from the research directorate office of the Mizan Tepi University (Ref No: MTU/CHS/12/1619/21/14). Written consent was obtained from each study participant after explaining the purpose and objectives of the study. For those participants who were illiterate, a fingerprint was used as a signature after trained interviewers had carefully explained the purpose, benefits, and potential risks before consent was obtained. The interview with study participants was conducted with strict privacy and confidentiality. The intra-oral examination using the periodontal probe and anthropometric measurements was also performed following the manufacturer’s instructions and interpreted accordingly. Then, all necessary information and the results of each study participant were communicated with their physicians for further investigations and management.
Author Contributions
Both authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Acknowledgments
We would like to thank Mizan-Tepi University for giving us this opportunity. Our appreciation also goes to Mizan Tepi University Teaching Hospital, Bonga G/tsaddik Shawo General Hospital and Tepi General Hospital administrators for giving us permission and providing us with the necessary information.
Disclosure
The authors report no conflicts of interest in relation to this work.
Additional information
Funding
References
- Helmi MF, Huang H, Goodson JM, Hasturk H, Tavares M, Natto ZS. Prevalence of periodontitis and alveolar bone loss in a patient population at Harvard School of Dental Medicine. BMC Oral Health. 2019;19(1):1–11. doi:10.1186/s12903-019-0925-z
- Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366(9499):1809–1820. doi:10.1016/S0140-6736(05)67728-8
- Periodontology AAo. Periodontal disease fact sheet; 2017. Available from: https://portal.ct.gov/-/media/Departments-and-Agencies/DPH/dph/oral_health/FactSheets/Consumer/DPH-Fact-Sheet-Consumer_Periodontal.pdf. Accessed February 18, 2022.
- Newman M, Klokkevold P, Carranza F. Chronic periodontitis. Carranzas Clin Periodontol. 2011;2011:160–212.
- World Health Organization. Oral Health Surveys: Basic Methods. World Health Organization; 2013.
- James SL, Abate D, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858.
- Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–1259.
- Sanz M, D’Aiuto F, Deanfield J, Fernandez-Avilés F. European workshop in periodontal health and cardiovascular disease—scientific evidence on the association between periodontal and cardiovascular diseases: a review of the literature. Eur Heart J Supplements. 2010;12(suppl_B):B3–B12. doi:10.1093/eurheartj/suq003
- United Nations. Political declaration of the high‐level meeting of the general assembly on the prevention and control of non‐communicable diseases. Resolution A/66/L1. 2011.
- Piscoya MD, Ximenes RA, Silva GM, Jamelli SR, Coutinho SB. Periodontitis-associated risk factors in pregnant women. Clinics. 2012;67(1):27–33. doi:10.6061/clinics/2012(01)05
- Shewale AH, Gattani DR, Bhatia N, Mahajan R, Saravanan S. Prevalence of periodontal disease in the general population of India-a systematic review. JCDR. 2016;10(6):ZE04. doi:10.7860/JCDR/2016/17958.7962
- Houshmand M, Holtfreter B, Berg MH, et al. Refining definitions of periodontal disease and caries for prediction models of incident tooth loss. J Clin Periodontol. 2012;39(7):635–644. doi:10.1111/j.1600-051X.2012.01892.x
- Govindasamy R, Dhanasekaran M, Varghese SS, Balaji V, Karthikeyan B, Christopher A. Maternal risk factors and periodontal disease: a cross-sectional study among postpartum mothers in Tamil Nadu. J Pharm Bioallied Sci. 2017;9(Suppl 1):S50. doi:10.4103/jpbs.JPBS_88_17
- Gesase N, Miranda-Rius J, Brunet-Llobet L, Lahor-Soler E, Mahande MJ, Masenga G. The association between periodontal disease and adverse pregnancy outcomes in Northern Tanzania: a cross-sectional study. Afr Health Sci. 2018;18(3):601–611. doi:10.4314/ahs.v18i3.18
- Alchalabi HA, Al Habashneh R, Jabali O, Khader Y. Association between periodontal disease and adverse pregnancy outcomes in a cohort of pregnant women in Jordan. Clin Exp Obstet Gynecol. 2013;40(3):399–402.
- Uwambaye P, Kerr M, Rulisa S, Harlan S, Munyanshongore C. Prevalence of periodontitis and associated factors among pregnant women: a cross sectional survey in Southern Province, Rwanda. Rwanda J Med Health Sci. 2021;4(1):131–150. doi:10.4314/rjmhs.v4i1.10
- Salih Y, Nasr AM, Ahmed A, Sharif ME, Adam I. Prevalence of and risk factors for periodontal disease among pregnant women in an antenatal care clinic in Khartoum, Sudan. BMC Res Notes. 2020;13(1):1–5. doi:10.1186/s13104-020-04998-3
- Tefera A, Bekele B. Periodontal disease status and associated risk factors in patients attending a tertiary hospital in northwest Ethiopia. Clin Cosmet Investig Dent. 2020;12:485. doi:10.2147/CCIDE.S282727
- Desai K, Desai P, Duseja S, Kumar S, Mahendra J, Duseja S. Significance of maternal periodontal health in preeclampsia. J Int Soc Prev Commun Dent. 2015;5(2):103. doi:10.4103/2231-0762.155734
- Tettamanti L, Lauritano D, Nardone M, et al. Pregnancy and periodontal disease: does exist a two-way relationship? Oral Implantol. 2017;10(2):112. doi:10.11138/orl/2017.10.2.112
- Amar S, Chung KM. Influence of hormonal variation on the periodontium in women. Periodontol 2000. 1994;6(1):79–87. doi:10.1111/j.1600-0757.1994.tb00028.x
- Kim EG, Park SK, Nho J-H. Factors related to maternal oral health status: focus on pregnant and breastfeeding women. Paper presented at: Healthcare; 2021.
- Khalighinejad N, Aminoshariae A, Kulild JC, Mickel A. Apical periodontitis, a predictor variable for preeclampsia: a case-control study. J Endod. 2017;43(10):1611–1614. doi:10.1016/j.joen.2017.05.021
- Kumar A, Sharma DS, Verma M, et al. Association between periodontal disease and gestational diabetes mellitus—A prospective cohort study. J Clin Periodontol. 2018;45(8):920–931. doi:10.1111/jcpe.12902
- Figueiredo MG, Takita SY, Dourado BMR, et al. Periodontal disease: repercussions in pregnant woman and newborn health—a cohort study. PLoS One. 2019;14(11):e0225036. doi:10.1371/journal.pone.0225036
- Mathew RJ, Bose A, Prasad J, Muliyil J, Singh D. Maternal periodontal disease as a significant risk factor for low birth weight in pregnant women attending a secondary care hospital in South India: a case-control study. Indian J Dent Res. 2014;25(6):742. doi:10.4103/0970-9290.152184
- Bi WG, Emami E, Luo Z-C, Santamaria C, Wei SQ. Effect of periodontal treatment in pregnancy on perinatal outcomes: a systematic review and meta-analysis. J Maternal Fetal Neonat Med. 2021;34(19):3259–3268. doi:10.1080/14767058.2019.1678142
- Offenbacher S, Jared H, O’reilly P, et al. Potential pathogenic mechanisms of periodontitis‐associated pregnancy complications. Ann Periodontol. 1998;3(1):233–250. doi:10.1902/annals.1998.3.1.233
- Ren H, Du M. Role of maternal periodontitis in preterm birth. Front Immunol. 2017;8:139. doi:10.3389/fimmu.2017.00139
- Choi SE, Choudhary A, Ahern JM, Palmer N, Barrow JR. Association between maternal periodontal disease and adverse pregnancy outcomes: an analysis of claims data. Fam Pract. 2021;38(6):718–723. doi:10.1093/fampra/cmab037
- Pockpa ZAD, Soueidan A, Koffi-Coulibaly NT, Limam A, Badran Z, Struillou X. Periodontal diseases and adverse pregnancy outcomes: review of two decades of clinical research. Oral Health Prev Dent. 2021;19(1):77–83. doi:10.3290/j.ohpd.b898969
- Grinin V, Erkanyan I, Ivanov SY. Incidence and risk factors of oral diseases in pregnant women. Stomatologiia. 2018;97(4):19–22. doi:10.17116/stomat20189704119
- Bertoldi C, Lalla M, Pradelli JM, Cortellini P, Lucchi A, Zaffe D. Risk factors and socioeconomic condition effects on periodontal and dental health: a pilot study among adults over fifty years of age. Eur J Dent. 2013;7(03):336–346. doi:10.4103/1305-7456.115418
- Harjunmaa U, Järnstedt J, Alho L, et al. Association between maternal dental periapical infections and pregnancy outcomes: results from a cross‐sectional study in Malawi. Trop Med Int Health. 2015;20(11):1549–1558. doi:10.1111/tmi.12579
- Muwazi L, Rwenyonyi CM, Nkamba M, et al. Periodontal conditions, low birth weight and preterm birth among postpartum mothers in two tertiary health facilities in Uganda. BMC Oral Health. 2014;14(1):1–8. doi:10.1186/1472-6831-14-42
- Sundararajan S, Muthukumar S, Rao SR. Relationship between depression and chronic periodontitis. J Indian Soc Periodontol. 2015;19(3):294. doi:10.4103/0972-124X.153479
- Kurer J, Watts T, Weinman J, Gower D. Psychological mood of regular dental attenders in relation to oral hygiene behaviour and gingival health. J Clin Periodontol. 1995;22(1):52–55. doi:10.1111/j.1600-051X.1995.tb01770.x
- Borrell LN, Beck JD, Heiss G. Socioeconomic disadvantage and periodontal disease: the Dental Atherosclerosis Risk in Communities study. Am J Public Health. 2006;96(2):332–339. doi:10.2105/AJPH.2004.055277
- Borrell LN, Crawford ND. Socioeconomic position indicators and periodontitis: examining the evidence. Periodontol 2000. 2012;58(1):69–83. doi:10.1111/j.1600-0757.2011.00416.x
- Tadjoedin FM, Fitri AH, Kuswandani SO, Sulijaya B, Soeroso Y. The correlation between age and periodontal diseases. J Int Dent Med Res. 2017;10(2):327.
- Tonetti MS, Jepsen S, Jin L, Otomo‐Corgel J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: a call for global action. J Clin Periodontol. 2017;44(5):456–462. doi:10.1111/jcpe.12732
- Reynolds I, Duane B. Periodontal disease has an impact on patients’ quality of life. Evid Based Dent. 2018;19(1):14–15. doi:10.1038/sj.ebd.6401287
- Antonoglou G, Knuuttila M, Niemelä O, et al. Low serum level of 1, 25 (OH) 2D is associated with chronic periodontitis. J Periodontal Res. 2015;50(2):274–280. doi:10.1111/jre.12207
- Bashutski J, Eber R, Kinney J, et al. The impact of vitamin D status on periodontal surgery outcomes. J Dent Res. 2011;90(8):1007–1012. doi:10.1177/0022034511407771
- Khosravi R, Ka K, Huang T, et al. Tumor necrosis factor-α and interleukin-6: potential interorgan inflammatory mediators contributing to destructive periodontal disease in obesity or metabolic syndrome. Mediators Inflamm. 2013;2013. doi:10.1155/2013/728987
- Genco RJ, Grossi SG, Ho A, Nishimura F, Murayama Y. A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J Periodontol. 2005;76:2075–2084. doi:10.1902/jop.2005.76.11-S.2075
- George A, Johnson M, Blinkhorn A, Ajwani S, Ellis S, Bhole S. Views of pregnant women in South Western Sydney towards dental care and an oral-health program initiated by midwives. Health Promo J Austral. 2013;24(3):178–184. doi:10.1071/HE13040
- Chapple IL, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000. 2007;43(1):160–232. doi:10.1111/j.1600-0757.2006.00178.x
- Kamer AR, Craig RG, Dasanayake AP, Brys M, Glodzik-Sobanska L, de Leon MJ. Inflammation and Alzheimer’s disease: possible role of periodontal diseases. Alzheimers Dement. 2008;4(4):242–250. doi:10.1016/j.jalz.2007.08.004
- Nazir MA. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci. 2017;11(2):72.
- Cronin A. Periodontal disease is a risk marker for coronary heart disease? Evid Based Dent. 2009;10(1):22. doi:10.1038/sj.ebd.6400634
- Graziani F, Gennai S, Solini A, Petrini M. A systematic review and meta‐analysis of epidemiologic observational evidence on the effect of periodontitis on diabetes an update of the EFP‐AAP review. J Clin Periodontol. 2018;45(2):167–187. doi:10.1111/jcpe.12837
- Ide M, Papapanou PN. Epidemiology of association between maternal periodontal disease and adverse pregnancy outcomes–systematic review. J Clin Periodontol. 2013;40:S181–S194. doi:10.1111/jcpe.12063
- Lockhart PB, Bolger AF, Papapanou PN, et al. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association? A scientific statement from the American Heart Association. Circulation. 2012;125(20):2520–2544. doi:10.1161/CIR.0b013e31825719f3
- Loos BG. Systemic effects of periodontitis. Ann R Australas Coll Dent Surg. 2006;18:27–29.
- Nagpal R, Yamashiro Y, Izumi Y. The two-way association of periodontal infection with systemic disorders: an overview. Mediators Inflamm. 2015;2015:1–9. doi:10.1155/2015/793898
- Peres M, Macpherson L, Weyant R, et al. Oral diseases: a global public health challenge. Lancet. 2019;394:249–260. doi:10.1016/S0140-6736(19)31146-8
- Jin L, Group E. Initiator paper. Interprofessional education and multidisciplinary teamwork for prevention and effective management of periodontal disease. J Int Acad Periodontol. 2015;17(1 Suppl):74–79.
- Yaya Y, Eide KT, Norheim OF, Lindtjørn B. Maternal and neonatal mortality in south-west Ethiopia: estimates and socio-economic inequality. PLoS One. 2014;9(4):e96294. doi:10.1371/journal.pone.0096294
- CSA. Ethiopia demographic and health survey 2016; 2016. Available from: https://www.healthynewbornnetwork.org/hnn-content/uploads/FR328.pdf. Accessed October 30, 2021.
- Beltrán‐Aguilar ED, Eke PI, Thornton‐Evans G, Petersen PE. Recording and surveillance systems for periodontal diseases. Periodontol 2000. 2012;60(1):40–53. doi:10.1111/j.1600-0757.2012.00446.x
- Petersen PE, Bourgeois D, Ogawa H, Estupinan-Day S, Ndiaye C. The global burden of oral diseases and risks to oral health. Bull World Health Organ. 2005;83:661–669.
- Kingdom TBSoPiasopitU. Basic Periodontal Examination (BPE) 1949. Available from: https://www.bsperio.org.uk/assets/downloads/BPE_Guidelines_2011.pdf. Accessed February 28, 2022.
- Khocht A, Zohn H, Deasy M, Chang K-M. Assessment of periodontal status with PSR and traditional clinical periodontal examination. J Am Dent Assoc. 1995;126(12):1658–1665. doi:10.14219/jada.archive.1995.0115
- Smales F, Mosedale R, Floyd P. Policy for periodontal care. Br Dent J. 1987;163(5):167–169. doi:10.1038/sj.bdj.4806229
- CSA IJAA. Ethiopia Demographic and Health Survey 2011. Ethiopia, Calverton M, USA: Central Statistical Agency; 2012:430.
- Coates J, Swindale A, Bilinsky P. Household Food Insecurity Access Scale (HFIAS) for measurement of food access: indicator guide VERSION 3; 2007. Available from: http://www.fao.org/fileadmin/user_upload/eufao-fsi4dm/doc-training/hfias.pdf. Accessed May 30, 2022.
- Coates J, Swindale A, Bilinsky P. Household Food Insecurity Access Scale (HFIAS) for measurement of food access: indicator guide: version 3. 2007.
- Saitz R. Screening and brief intervention for unhealthy drug use: little or no efficacy. Front Psychiatry. 2014;5:121. doi:10.3389/fpsyt.2014.00121
- Cheung T, Yip P. Depression, anxiety and symptoms of stress among Hong Kong nurses: a cross-sectional study. Int J Environ Res Public Health. 2015;12(9):11072–11100. doi:10.3390/ijerph120911072
- Seidell JC, Flegal KM. Assessing obesity: classification and epidemiology. Br Med Bull. 1997;53(2):238–252. doi:10.1093/oxfordjournals.bmb.a011611
- Kirch W. CPI (Community Periodontal Index). Encyclopedia of Public Health. Dordrecht: Springer; 2008. doi:10.1007/978-1-4020-5614-7_604
- Louie R, Brunelle JA, Magglore ED, Beck RW. Caries prevalence in Head Start children, 1986–87. J Public Health Dent. 1990;50(5):299–305. doi:10.1111/j.1752-7325.1990.tb02139.x
- Joss A, Adler R, Lang NP. Bleeding on probing. A parameter for monitoring periodontal conditions in clinical practice. J Clin Periodontol. 1994;21(6):402–408. doi:10.1111/j.1600-051X.1994.tb00737.x
- Lindhe J, Nyman S, Karring T. Scaling and root planing in shallow pockets. J Clin Periodontol. 1982;9(5):415–418. doi:10.1111/j.1600-051X.1982.tb02054.x
- Listgarten M. Periodontal probing: what does it mean? J Clin Periodontol. 1980;7(3):165–176. doi:10.1111/j.1600-051X.1980.tb01960.x
- World Health Organization. Oral Health Periodontal Country Profiles. Geneva, Switzerland: World Health Organization; 2005.
- Kesim S, Unalan D, Esen C, Ozturk A. The relationship between periodontal disease severity and state-trait anxiety level. J Pak Med Assoc. 2012;62(12):1304–1308.
- Page RC, Eke PI. Case definitions for use in population‐based surveillance of periodontitis. J Periodontol. 2007;78:1387–1399. doi:10.1902/jop.2007.060264
- Eke PI, Page RC, Wei L, Thornton‐Evans G, Genco RJ. Update of the case definitions for population‐based surveillance of periodontitis. J Periodontol. 2012;83(12):1449–1454. doi:10.1902/jop.2012.110664
- Zhang J, Jiang H, Sun M, Chen J. Association between periodontal disease and mortality in people with CKD: a meta-analysis of cohort studies. BMC Nephrol. 2017;18(1):1–11. doi:10.1186/s12882-017-0680-9
- Marcenes W, Kassebaum NJ, Bernabé E, et al. Global burden of oral conditions in 1990–2010: a systematic analysis. J Dent Res. 2013;92(7):592–597. doi:10.1177/0022034513490168
- Gomes‐Filho IS, Batista JET, Trindade SC, et al. Obesity and periodontitis are not associated in pregnant women. J Periodontal Res. 2020;55(1):77–84. doi:10.1111/jre.12690
- Verhulst MJ, Teeuw WJ, Bizzarro S, et al. A rapid, non-invasive tool for periodontitis screening in a medical care setting. BMC Oral Health. 2019;19(1):1–14. doi:10.1186/s12903-019-0784-7
- Wu Y-M, Liu J, Sun W-L, et al. Periodontal status and associated risk factors among childbearing age women in Cixi City of China. J Zhejiang Univ Sci B. 2013;14(3):231–239. doi:10.1631/jzus.B1200034
- Rao MVR, Katari PK, Vegi L, Bypureddy TT, Rao KSP, Tejaswi KS. Prevalence of periodontal diseases among rural population of Mustabad, Krishna District. J Int Soc Prev Commun Dent. 2016;6(Suppl 1):S59. doi:10.4103/2231-0762.181169
- Bhat M. Oral health status and treatment needs of a rural Indian fishing community. West Indian Med J. 2008;57(4):414–417.
- Dye BA, Tan S, Smith V, et al. Trends in oral health status; United States, 1988–1994 and 1999–2004. 2007.
- Elo IT, Preston SH. Educational differentials in mortality: United States, 1979–1985. Social Sci Med. 1996;42(1):47–57. doi:10.1016/0277-9536(95)00062-3
- Hugo C, Cockburn N, Ford P, March S, Isenring E. Poor nutritional status is associated with worse oral health and poorer quality of life in aged care residents. J Nurs Home Res. 2016;2:118–122.
- Woelber JP, Bremer K, Vach K, et al. An oral health optimized diet can reduce gingival and periodontal inflammation in humans-a randomized controlled pilot study. BMC Oral Health. 2017;17(1):1–8. doi:10.1186/s12903-016-0257-1
- Sheetal A, Hiremath VK, Patil AG, Sajjansetty S, Kumar SR. Malnutrition and its oral outcome–a review. JCDR. 2013;7(1):178. doi:10.7860/JCDR/2012/5104.2702
- Kopycka-Kedzierawski DT, Li D, Xiao J, Billings RJ, Dye TD. Association of periodontal disease with depression and adverse birth outcomes: results from the Perinatal database; Finger Lakes region, New York State. PLoS One. 2019;14(4):e0215440. doi:10.1371/journal.pone.0215440
- Fatima Z, Bey A, Azmi S, Gupta N, Khan A. Mental depression as a risk factor for periodontal disease: a case–control study. Eur J Gen Dent. 2016;5(02):86–89. doi:10.4103/2278-9626.179557
- Biondi M, Zannino L-G. Psychological stress, neuroimmunomodulation, and susceptibility to infectious diseases in animals and man: a review. Psychother Psychosom. 1997;66(1):3–26. doi:10.1159/000289101
- Mohamed HG, Idris SB, Ahmed MF, et al. Association between oral health status and type 2 diabetes mellitus among Sudanese adults: a matched case-control study. PLoS One. 2013;8(12):e82158. doi:10.1371/journal.pone.0082158
- Susanto H, Nesse W, Dijkstra PU, Agustina D, Vissink A, Abbas F. Periodontitis prevalence and severity in Indonesians with type 2 diabetes. J Periodontol. 2011;82(4):550–557. doi:10.1902/jop.2010.100285
- Engebretson SP, Hey‐Hadavi J, Ehrhardt FJ, et al. Gingival crevicular fluid levels of interleukin‐1β and glycemic control in patients with chronic periodontitis and type 2 diabetes. J Periodontol. 2004;75(9):1203–1208. doi:10.1902/jop.2004.75.9.1203
- Kayombo C, Mumghamba E. Self-reported halitosis in relation to oral hygiene practices, oral health status, general health problems, and multifactorial characteristics among workers in Ilala and Temeke municipals, Tanzania. Int J Dent. 2017;2017:1–10. doi:10.1155/2017/8682010
- Silveira MF, Freire BM, Martins AM, Marcopito LF. Periodontal condition of adolescents and associated factors. Revista Gaúcha de Odontologia. 2019;67. doi:10.1590/1981-86372019000043489
- Quirynen M, Dadamio J, Van den Velde S, et al. Characteristics of 2000 patients who visited a halitosis clinic. J Clin Periodontol. 2009;36(11):970–975. doi:10.1111/j.1600-051X.2009.01478.x
- Onigbinde O, Sorunke M, Braimoh M, Adeniyi A. Periodontal status and some variables among pregnant women in a Nigeria tertiary institution. Ann Med Health Sci Res. 2014;4(6):852–857. doi:10.4103/2141-9248.144876
- Wu M, Chen S-W, Jiang S-Y. Relationship between gingival inflammation and pregnancy. Mediators Inflamm. 2015;2015:1–11. doi:10.1155/2015/623427