Abstract
Endoscopic submucosal dissection (ESD) enables en bloc resection of large complex colorectal superficial neoplastic lesions, resulting in very low rates of local recurrence, high-quality pathologic specimens for accurate histopathologic diagnosis and potentially curative treatment of early adenocarcinoma without resorting to major surgical resection. The safety and efficacy of the technique, which was pioneered in the upper gastrointestinal tract, has been established by the consistently impressive outcomes from expert centers in Japan and some other eastern countries. However, ESD is challenging to perform in the colorectum and there is a significant risk of complications, particularly in the early stages of the learning curve. Early studies from western centers raised concerns about the high complication rates, and the impressive results from Japanese centers were not replicated. As a result, many western endoscopists are skeptical about the role of ESD and few centers have incorporated the technique into their practice. Nevertheless, although the distribution of expertise, referral centers and modes of practice may differ in Japan and western countries, ESD has an important role and can be safely and effectively incorporated into western practice. Key to achieving this is meticulous lesion assessment and selection, appropriate referral to centers with the necessary expertise and experience and application of the appropriate technique individualized to the patient. This review discusses the advantages, risks and benefits of ESD to treat colorectal lesions and the importance of preprocedure lesion assessment and in vivo diagnosis and outlines a pragmatic rationale for appropriate lesion selection as well as the patient, technical and institutional factors that should be considered.
Introduction
Endoscopic submucosal dissection (ESD) allows organ-preserving, en bloc, curative resection of large superficial neoplastic lesions and cancers with early submucosal invasion in the colon and rectum. ESD was first developed to treat lesions in the upper gastrointestinal tract.Citation1 However, in the colon and rectum, endoscopic mucosal resection (EMR), pioneered over 40 years ago and in established practice for several decades, was the mainstay of treatment as colorectal ESD was considered technically challenging compared to gastric or esophageal ESD because of the difficulties in scope stability and manipulation and a higher risk of perforation and fecal peritonitis due to the thin colonic wall.Citation2–Citation4 Nevertheless, following its established role in the upper gastrointestinal tract, ESD was first described in the colorectum in the late 1990s and there has since been a proliferation in its use.Citation5 In series involving several thousands of patients, it has been shown to achieve high rates of en bloc and curative resection of large lesions with low recurrence rates compared to EMR and is thought to have the added advantage of providing more accurate pathologic diagnosis and reducing the need for additional surgery.Citation6–Citation11
However, almost all of these results are obtained from large series from expert centers in Asia, primarily Japan.Citation9 The adoption of ESD in western practice has been very limited because of its technical difficulty, steep learning curve and the difference in training opportunities compared to Japan because of the relative scarcity of early gastric and esophageal cancer.Citation12–Citation14 There are concerns about the difference in outcomes and adverse events in western compared to Japanese series,Citation9,Citation15–Citation17 leading many western endoscopists to favor EMR as a safer, technically less demanding and more feasible universal strategy for tackling almost all suitable colorectal lesions.Citation12,Citation13,Citation18,Citation19
ESD, however, undoubtedly provides several advantages over EMR for appropriately selected patients, for example, where en bloc resection of lesions at high risk of containing submucosal invasion is essential.Citation20 It is also a valuable technique in resecting lesions with profound submucosal fibrosis from previous resection or heavy manipulation, which is common in western practice.Citation21 The patient population, referral patterns, case load for the relatively few experienced interventional colorectal endoscopists and lesion characteristics are likely to be substantially different from eastern practice. The key to safely and effectively incorporating ESD into western practice is the adoption of a pragmatic, lesion-specific approach using accurate preprocedure assessment and in vivo diagnosis to guide meticulous patient selection, as well as treatment by interventional endoscopists with expertise in the full range of endoscopic resection techniques.
In this review, we will consider the benefits and challenges of ESD in western practice and discuss the importance of appropriate patient selection and special patient, institutional and technical considerations when deciding the appropriateness of using the technique.
Outcomes and advantages of colorectal ESD
En bloc resection
Foremost among the suggested benefits of ESD is that it offers the possibility of en bloc resection of large lesions in the colon and rectum. Indeed, most other benefits of ESD, such as reduced recurrence, improved histopathologic diagnostic accuracy and potential curative treatment for early invasive cancers, stem from the ability to achieve en bloc resection using this technique.
There have been several systematic reviews and meta-analyses examining ESD, which have demonstrated en bloc resection rates using ESD of 88%–92%, compared to 35%–63% for EMR.Citation6–Citation9,Citation22,Citation23 Although a randomized control trial comparing ESD and EMR is in progress, there are no published randomized control trials comparing the techniques.Citation24 Nevertheless, these systematic reviews and meta-analyses include several thousands of patients and it is clear that very high rates of en bloc resection can be achieved. While en bloc resection using EMR is only consistently achieved for lesions <2 cm,Citation25 ESD can achieve en bloc resection for much larger lesions, and successful en bloc resection of massive lesions over 10 cm has been reported.Citation26,Citation27 Furthermore, hybrid techniques using ESD to perform a circumferential mucosal incision with partial submucosal dissection, followed by EMR, can often be used where complete ESD is either too technically challenging or sufficient expertise is lacking to achieve en bloc resection in a greater proportion of lesions >2 cm than would otherwise be possible with EMR alone.Citation28,Citation29
Low recurrence rates
As a result of these high rates of en bloc resection, few local recurrences are seen after ESD, despite the often large lesion size. A multicenter study found that, regardless of the technique employed, piecemeal resection was the most important risk factor for recurrence; also, a meta-analysis of risk factors for local recurrence found that piecemeal resection was the only independent risk factor for recurrence.Citation30,Citation31 Akintoye et al included several thousands of cases of ESD in a meta-analysis and found a pooled recurrence rate of only 1%.Citation9 Other meta-analyses have consistently demonstrated rates of local recurrence after ESD of 0.7%–1.2%, compared to 10.4%–12.7% after EMR.Citation6,Citation8,Citation22,Citation23
Accurate diagnosis and curative resection of early adenocarcinoma
En bloc resection to aid the accuracy of histopathologic diagnosis is of particular importance in the presence of adenocarcinoma.Citation32–Citation35 Piecemeal resection can make accurate assessment of the depth of invasion of an adenocarcinoma difficult, which could potentially lead to subsequent incorrect over- or undertreatment of a lesion. It has been suggested that inaccuracies in the assessment of depth of invasion due to piecemeal resection have led to invasive recurrences of adenocarcinoma, although there is, to date, no definitive evidence for this assertion.Citation36 Aiding the accurate histopathologic diagnosis of specimens is one of the primary reasons for recommending ESD in guidelines from both Japan and Europe, and the types of lesion where this is particularly recommended will be discussed in further detail below.Citation20,Citation35,Citation37 In addition, en bloc resection allows curative endoscopic resection of adenocarcinoma with early submucosal invasion, as lymph node metastases are rare in adenocarcinoma with only superficial submucosal invasion or invading <1000 µm from the muscularis mucosae.Citation38,Citation39 In fact, several series have demonstrated that, in the absence of high-risk histopathologic factors including lymphovascular invasion, poor differentiation or tumor budding, early colorectal cancer with only superficial submucosal invasion or invasion to a depth of <1000 µm is associated with a risk of lymph node metastases approaching 0%.Citation40–Citation46 As a result, ESD is recommended where there is a risk of only superficial submucosal invasion and curative endoscopic resection may be possible.Citation35 In the absence of any high-risk histopathologic features, ESD has been shown to provide excellent oncologic results and a potentially better safety profile compared to standard surgical segmental resection.Citation47
However, it is worth noting that EMR has also been used for many years to effectively treat early colorectal cancers without high-risk features and that, despite the sound scientific rationale behind recommending en bloc resection using ESD to aid diagnosis and curative resection, no convincing evidence of its benefit over EMR in oncologic outcomes has been published.Citation44–Citation46
Disadvantages of ESD
Colorectal ESD is a technically demanding modality for endoscopic resection, with an increased risk of adverse events as a result of difficulties achieving scope stability due to colonic looping; peristalsis, flexions and mucosal folds making manipulation of the knife challenging, the thin colonic wall allowing little margin of error during dissection and the risk of peritonitis from any perforation.Citation4,Citation9,Citation48
Complications
One of the primary concerns about ESD has been the increased risk of perforation compared to EMR. Even in the expert hands of experienced Japanese endoscopists, initial case series of ESD reported relatively high perforation rates with early experience of ~10%–12%.Citation49–Citation51
However, several points about the risk of perforation during ESD are worth noting. First, the risk appears to significantly reduce with experience, with one series demonstrating a reduction in perforations from 12% in its first 100 cases to 2% in the subsequent 100 cases.Citation51 Large series of colorectal ESD suggest that the perforation rate in the hands of experienced practitioners is now in the range of 3.1%–5.6%.Citation9–Citation11,Citation52–Citation57 Second, despite an apparently higher perforation rate, the overwhelming majority of cases are recognized immediately and successfully managed by closing the defect with endoscopic clips or with conservative management and only a small fraction require surgery.Citation10,Citation11,Citation52,Citation53 One caveat to this observation is that, although these patients avoided surgery, there is very little reporting in these studies of any subsequent outcomes such as delayed recovery, longer hospital stay, increased costs or antibiotic requirements. Third, comparisons of perforation rates between ESD and EMR are difficult due to differences in important factors such as lesion size between different cohorts.
Although meta-analyses confirm that colorectal ESD is associated with a significantly greater risk of perforation with rates ranging from 4.8% to 5.7% for ESD compared to 0.9%–1.4% for EMR, these are almost exclusively based on retrospective studies; also, lesions resected using ESD were larger than those resected using EMR and these studies do not report other details of the lesions that may have affected perforation.Citation6–Citation8,Citation22,Citation23
Significant bleeding is a relatively infrequent complication of ESD, and it appears that the risk of bleeding is similar regardless of the resection technique. Although definitions of significant bleeding vary between studies, it reportedly occurs in 0.5%–2.75% of cases.Citation9,Citation11,Citation52,Citation54,Citation56
Procedure time
ESD is a technically challenging procedure, which is reflected in the significantly longer procedure time compared to EMR.Citation6–Citation8,Citation36,Citation58 This has implications not only for institutions’ service provision, but also for patients who usually have the procedure performed under conscious sedation. Data recording patient experience of ESD compared to other procedures are lacking.
Hospital admission
In expert centers in Japan, patients undergoing ESD are usually admitted to hospital for a significant period of time.Citation59,Citation60 Even within a special clinical pathway designed to reduce hospital stay, the mean length of stay was 5 days.Citation61 By contrast, EMR is usually performed as a day case procedure.Citation62
Availability of expertise and opportunities for training
The availability of expert endoscopists with the necessary experience in ESD is of particular relevance in western practice. Related to this is the added difficulty of ensuring appropriate training opportunities in a western setting.Citation63 In Japan, well-defined training pathways have been established, which begin with technically easier and safer procedures in the stomach, progressing to the esophagus and finally to the rectum and colon.Citation64–Citation66 Suitable upper gastrointestinal lesions are far less common in the west. These issues, together with concerns about the impact on interventional endoscopy services due to the added length of procedure and risk of complications, have led to slow uptake of ESD in western practice. Reluctance to adopt ESD was compounded by some early western series on colorectal ESD reporting significant and concerning complication rates,Citation16,Citation67,Citation68 although, as noted above, there appears to be a clear learning curve with reduction of complications with increasing experience, which has also been demonstrated in a western setting.Citation15 Emerging evidence from Europe suggests comparable en bloc resection and perforation rates to eastern practice.Citation69,Citation70
Indications for ESD
Although several international authorities have published guidance on endoscopic resection in the colorectum, the Japanese Gastroenterological Society and several expert centers in Japan are the only authorities to define clear recommendations for colorectal ESD. These criteria may be applicable in Japan where the necessary skills and institutional experience exist, but their relevance in western practice is less clear. Nevertheless, they provide a useful starting point when considering appropriate patient and lesion selection for ESD. In most guidelines, ESD is recommended based on the size of the lesion (and, therefore, the risks of piecemeal resection) and the risk of adenocarcinoma.
Risk of invasive adenocarcinoma
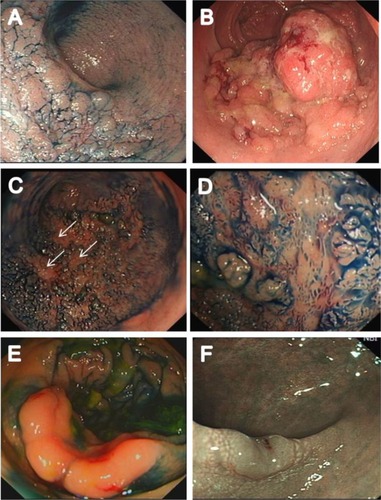
As the primary advantage of ESD is en bloc resection of lesions allowing accurate diagnosis and potentially curative resection of adenocarcinoma, one of the central tenants in selecting suitable lesions for ESD is that the risk of a large lesion containing adenocarcinoma varies according to its morphology and surface (pit) pattern. Lesions <2 cm can be resected en bloc using EMR, and so ESD is primarily of relevance for lesions >2 cm which are most commonly laterally spreading tumors (LST). shows the different morphological subtypes of LST. The incidence of adenocarcinoma in LST granular (LST G) homogenous type lesions is low, whereas it is higher in LST G mixed-nodular type lesions and, when present, tends occur under a large nodule. The incidence is highest in LST nongranular (LST NG) type lesions, particularly the pseudodepressed type, and also tends to be multifocal in these lesions.Citation33,Citation71,Citation72 In addition, the risk of submucosal invasion can be accurately predicted using magnification chromoendoscopy to determine the presence of type V pit pattern as well as other image enhancement techniques such as narrow band imaging (NBI).Citation73–Citation77
Figure 1 Different morphological subtypes of LST.
Abbreviation: LST, laterally spreading tumors.
Risk of recurrence
ESD may be selected based on lesion size. The risk of recurrence is consistently related to the size of the lesion and piecemeal resection in studies, and en bloc resection is not consistently achieved for lesions >2 cm using EMR.Citation25,Citation31
Guidelines for selecting ESD
The Japanese Gastroenterological Society and expert centers in Japan, therefore, recommend ESD for lesions where en bloc resection is necessary and would be difficult to achieve with EMR, including LST NG larger than 2 cm, LST G larger than 3 cm (especially mixed-nodular type), Vi pit pattern or suspicion of only superficial submucosal invasion, recurrent lesions and lesions with submucosal fibrosis.
Other international guidance, although adhering to similar principles, is less definitive owing largely to the paucity of expert centers and endoscopists performing ESD in western practice.Citation20,Citation37,Citation78 Guidance from societies in the UK and Europe both not only acknowledge the potential role of ESD in en bloc resection where adenocarcinoma is suspected, but also note that piecemeal EMR is safe and highly effective for the majority of large colorectal superficial neoplastic lesions.Citation20,Citation37
Controversies regarding the indications for ESD
A liberal interpretation of the indications for ESD could include any lesion with a risk of harboring adenocarcinoma or where the risk of recurrence is significant and this means that in expert centers in Japan, where the expertise exists and the case load allows, many large colorectal lesions are resected using ESD.Citation79 However, some western endoscopists question the value of ESD for the majority of colorectal superficial neoplastic lesions. Aside from the lack of opportunities for training and gaining sufficient experience of ESD already discussed, they take the view that EMR and piecemeal EMR are highly effective and safe procedures for resecting large superficial colorectal lesions in over 90% of patients. Although the recurrence rate may be higher than after en bloc resection by ESD, this is successfully treated by EMR in most cases and is a low-risk and relatively simple procedure which does not usually require hospital admission.Citation62,Citation80 In addition, EMR is widely practiced and the necessary skills and experience are easier to attain.Citation37
Some argue that the additional benefit of performing ESD is minimal for most lesions as evidenced by the fact that few lesions contain adenocarcinoma and, for those resected en bloc by ESD, approximately half require surgery as they contain high-risk features.Citation11,Citation13,Citation14,Citation19 This leaves only a fraction of patients potentially receiving curative resection by ESD, and the costs and resource burden may not justify the universal use of ESD for large lesions, especially where the benefit is small, such as lesions in the right colon when the morbidity associated with laparoscopy is minimal.Citation12,Citation19,Citation81
Some have argued that there is no evidence that fewer patients require surgery after treatment with ESD compared to EMR, despite the claim that ESD may afford curative resection to more patients and reduce recurrence.Citation82 There are several limitations in the studies comparing techniques, but in many, the requirement for surgery following ESD is either similar or more frequent compared to EMR.Citation8,Citation23 Some authors feel it is safest to generally recommend surgery when adenocarcinoma is found and, as a result, EMR is sufficient.Citation81
However, even authors who note the limited uptake of ESD in the west or question the extent to which ESD should be used generally agree that there is an important role for the technique in resecting particularly high-risk lesions en bloc or tackling difficult fibrotic lesions, and it is likely that it will be increasingly employed in western practice in the future.Citation18,Citation19,Citation79
Patient selection
Given the advantages and efficacy of both ESD and EMR, it is necessary to arrive at a pragmatic approach to patient and lesion selection which will allow the best technique to be delivered to the right patient, as well as allow the most effective use of ESD within the resources available in the majority of centers.
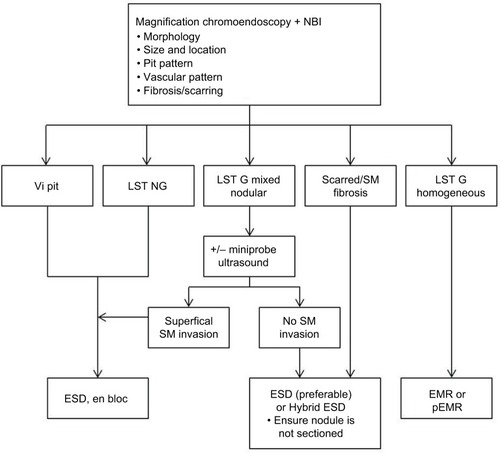
shows the approach we have adopted in selecting lesions for ESD. Meticulous assessment prior to resection in order to make an accurate endoscopic prediction of the risk of invasive adenocarcinoma is of paramount importance in selecting the most appropriate resection technique. Magnification chromoendoscopy and NBI are invaluable in this decision-making process.Citation35,Citation71,Citation75,Citation76 If the expertise is available, colonoscopic ultrasound may also be used to supplement this process in lesions where type Vi pit is discovered or where the risk of submucosal invasion is high, such as LST NG or LST G mixed-nodular lesions, in an attempt to exclude deep invasion before resection is attempted.Citation83–Citation85 Endoscopic resection should not be considered for lesions which obviously have deeply invasive adenocarcinoma, for example, lesions displaying Vn pit pattern or where colonoscopic ultrasound suggests deep invasion.
Figure 2 Process of lesion-specific selection of resection technique at King’s College Hospital.
Definitive indications for en bloc resection
En bloc resection should be performed where there is a high risk of adenocarcinoma with superficial invasion. This includes LST NG lesions, particularly pseudodepressed type, and lesions displaying Vi pit. All such lesions >2 cm should be resected using ESD, although ESD should also be used for smaller LST NG lesions where en bloc resection by EMR may be compromised by submucosal fibrosis. If ESD is not performed locally, then these patients should be referred to a center where the expertise is available.
Lesions for which ESD is preferable
Lesions with a significant risk of harboring invasive adenocarcinoma should be resected using ESD where possible. This includes LST G mixed-nodular lesions. However, en bloc resection using ESD may not be possible due to factors such as extreme lesion size, patient frailty and comorbidity precluding a lengthy procedure, deeply scarred lesions from previous attempts at resection or a lack of suitable expertise in ESD. When invasive adenocarcinoma occurs in such lesions, it is almost invariably present under the largest dominant nodule or under depressed areas ().Citation33,Citation71,Citation76 Therefore, at a minimum, the resection strategy for these lesions should be removal of these areas in one piece to avoid sectioning an area of adenocarcinoma before piecemeal resection to complete the removal of the remaining granular portion of the lesion.Citation10,Citation33,Citation35,Citation48,Citation71,Citation86,Citation87
Lesions for which ESD is not essential
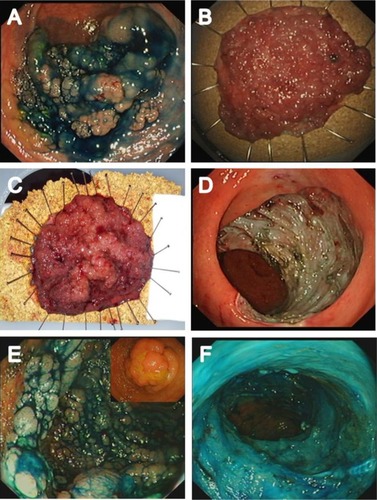
Although en bloc resection is always preferable, the practical realities of advanced interventional endoscopy, particularly outside of Japan, are that piecemeal EMR is the most viable strategy for safe and effective resection in some patients. LST G homogenous lesions have a very low incidence of unrecognized adenocarcinoma, even at a large size, and therefore can be resected using piecemeal EMR.Citation10,Citation33 Lesions of any morphology <2 cm can, in principle, be resected en bloc using EMR, although if there is any suspicion of superficial submucosal invasion, we would recommend considering ESD even for these smaller lesions because it allows consistent en bloc resection and also enables accurate submucosal dissection under vision, ensuring R0 resection and a high-quality pathologic specimen. shows examples of appropriate selection of resection technique specific to the patient and lesion characteristics.
Figure 3 Examples of appropriate selection of resection technique.
Abbreviations: ESD, endoscopic submucosal dissection; LST, laterally spreading tumors; pEMR, piecemeal endoscopic mucosal resection.
Lesions for which ESD is a valuable technique
There are situations for which ESD is a useful technique to allow effective treatment of a benign colorectal lesion, but where it is not required specifically for en bloc resection.
Lesions which are deeply scarred as a result of recurrence after previous resection present a particular challenge (). Although these can be effectively treated by EMR, especially if diminutive in size, many larger recurrent or residual lesions are resistant to submucosal lifting and snare capture.Citation62,Citation80,Citation88 ESD may be the only effective resection strategy for these, either as the sole modality or as part of a hybrid technique. Several small series have demonstrated that ESD can be used to achieve safe resection of recurrent lesions with impressive en bloc resection rates, or as part of a hybrid procedure to enable effective snare capture.Citation89–Citation93 However, significant submucosal fibrosis has been consistently identified as a risk factor for recurrence and perforation during ESD, as well as adding to the technical difficulty of the procedure, and therefore, the use of ESD for these particularly challenging lesions should be reserved for interventional endoscopists with sufficient experience.Citation94–Citation96
Lesions extending to the anorectum and dentate line are especially challenging to resect with endoscopic techniques as a result of difficult access and visualization and pain due to the somatic sensory innervation, and there may be a lower threshold for surgical management in these cases.Citation97,Citation98 ESD is a safe and highly effective technique for such lesions, even in the presence of hemorrhoids, with minimal risk of bleeding and very low risk of recurrence.Citation98–Citation100
Technical, institutional and patient considerations
Detailed discussion of the endosurgical knives and equipment available for performing ESD is beyond the scope of this review and has been detailed elsewhere; however, the development of certain techniques has greatly assisted successful ESD for large tumors.Citation32,Citation101,Citation102
Dissection techniques – pocket-creation method
The technique for colorectal ESD has evolved to one in which a partial mucosal incision is initially performed followed by variable amounts of submucosal dissection instead of an initial circumferential mucosal incision, as this maintains mucosal tension which assists insertion of the distal attachment and prevents leakage of injection fluid. This was further developed into the pocket-creation method in which, following a minimal partial mucosal incision, a large submucosal pocket is created under the lesion before completing the mucosal incision circumferentially.Citation103–Citation105 This has the advantage of maintaining a thick submucosal layer under the tumor, resulting in a high-quality pathologic specimen. Also, insertion of the distal attachment into the submucosal pocket results in the tip being spontaneously fixed and, therefore, synchronized with breathing or heart beats, thus maintaining the tip of the knife at a stable distance from the submucosa.Citation103,Citation104 A further modification of this technique for lesions complicated by submucosal fibrosis is the formation of multiple pockets which meet at the area of fibrosis, allowing accurate identification of the muscle layer and precise dissection of the muscle.Citation105
Retraction techniques
One of the difficulties with ESD is maintaining adequate traction. Patient position change to make optimal use of gravity is the most commonly employed method.Citation106,Citation107 A variety of innovations have been described to assist with traction, most involving endoscopic clips.Citation108 One of the simplest and most cost-effective is the clip with line method.Citation107,Citation109 An endoscopic hemoclip is inserted into the colonoscope, a length of dental floss or silk is tied to one arm, the clip is then withdrawn back into the endoscope which is reinserted, and the clip is deployed onto the mucosal flap where traction is required. Counter traction is then achieved by pulling the dental floss or silk gently or applying a light weight to the opposite end. Various directions of counter traction can be achieved using modifications of the technique by creating a pulley effect using a second clip to “capture” the line and then fixing this second clip to the opposite side of the lumen. The dental floss is then drawn through this second clip, altering the direction of pull on the first clip.Citation107
Another simple method is the clip-flap method in which clips are applied to the incised mucosal edge soon after mucosal incision and these act to assist gravity in lifting the mucosal edge, improving visualization of the submucosal plane to allow insertion of the distal attachment.Citation101
Several other techniques have been described using additional endoscopes, external forceps or novel devices, but have not been widely applied, especially in the colorectum.Citation110–Citation112
Institutional factors
In order to safely and effectively incorporate ESD into an advanced interventional endoscopy practice, an adequate case load is essential. It has been demonstrated that complication rates for colorectal ESD are significantly lower in institutions performing a high volume of procedures.Citation11 Clearly, it is not appropriate for every colonoscopist to attempt to perform ESD or, indeed, for every institution to offer ESD. Rather, it should be carried out by experienced practitioners in central referral hospitals to maintain the necessary experience which should translate into effective treatment.Citation113
It may be useful, particularly in western practice, to introduce a complex polyp multidisciplinary meeting to ensure the correct treatment is given to patients in environments where surgery is often still performed for benign colorectal lesions and also to assist in correct decision making with regard to the resection technique or the need for referral.Citation37,Citation114
Effective models for ESD training in the west need to be established and validated. Various training pathways have been proposed involving the use of porcine models, a period of observation of Japanese experts and progressive use of the technique beginning in the rectum and progressing into the colon, as safety and competence are proven, preferably under the supervision of an expert.Citation68,Citation115–Citation118 It is difficult to determine the exact learning curve for colorectal ESD as most studies involve endoscopists already experienced in upper gastrointestinal ESD. However, training in ESD is feasible without significant prior experience of upper gastrointestinal ESD.Citation119,Citation120
Patient considerations
It is important that the correct treatment is offered on an individual basis to patients. ESD may be indicated on the basis of specific lesion characteristics, but it may not be the most suitable treatment for the patient. Guidelines across nations are consistent in stating that the risks and benefits of a treatment should be carefully considered for each patient, especially the elderly.Citation35,Citation37 Elderly frail patients may be ill-equipped to withstand a lengthy procedure with the medications for conscious sedation which may be required for ESD.
Conclusion
A large body of evidence from Japan and other eastern expert centers, and emerging evidence from some western centers, has demonstrated that ESD is a highly effective technique to achieve en bloc resection of large colorectal superficial neoplastic lesions, allowing accurate histopathologic diagnosis and staging of adenocarcinoma and offering potentially curative organ-preserving, minimally invasive treatment for early cancer. With the appropriate expertise and experience, this can be achieved with minimal morbidity. However, accurate preprocedure assessment and in vivo diagnosis to aid appropriate lesion selection are essential to ensure the correct technique is offered to the patient, for which magnification endoscopy is an invaluable tool. There are substantial differences in training opportunities, referral patterns, knowledge among diagnostic endoscopists and modes of practice between the east and west, but ESD has been successfully introduced into some western referral centers and appropriate referral to such centers together with careful lesion and patient selection will maintain high-quality outcomes.
Disclosure
The authors report no conflicts of interest in this work.
References
- HiraoMMasudaKAsanumaTEndoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrineGastrointest Endosc19883432642693391382
- DeyhlePLargiadèrFJennySFumagalliIA method for endoscopic electroresection of sessile colonic polypsEndoscopy19735013840
- YokotaTSugiharaKYoshidaSEndoscopic mucosal resection for colorectal neoplastic lesionsDis Colon Rectum19943711110811117956578
- LaiLHChanFKEndoscopic submucosal dissection for colonic lesions: why and how should we do it?J Dig Dis201112422923321791017
- GotodaTKondoHOnoHA new endoscopic mucosal resection procedure using an insulation-tipped electrosurgical knife for rectal flat lesions: report of two casesGastrointest Endosc199950456056310502182
- WangJZhangXHGeJYangCMLiuJYZhaoSLEndoscopic submucosal dissection versus endoscopic mucosal resection for colorectal tumors: a meta-analysisWorld J Gastroenterol201420258282828725009404
- ZhangHPWuWYangSShangJLinJThe efficacy and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for colorectal tumors: a meta-analysisInt J Colorectal Dis201631379179326123699
- FujiyaMTanakaKDokoshiTEfficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissectionGastrointest Endosc201581358359525592748
- AkintoyeEKumarNAiharaHNasHThompsonCCColorectal endoscopic submucosal dissection: a systematic review and meta-analysisEndosc Int Open2016410E1030E104427747275
- SaitoYSakamotoTFukunagaSEndoscopic submucosal dissection (ESD) for colorectal tumorsDig Endosc200921Suppl 1S7S1219691740
- SaitoYUraokaTYamaguchiYA prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video)Gastrointest Endosc20107261217122521030017
- HolmesIFriedlandSEndoscopic mucosal resection versus endoscopic submucosal dissection for large polyps: a western colonoscopist’s viewClin Endosc201649545445627561263
- BourkeMCurrent status of colonic endoscopic mucosal resection in the west and the interface with endoscopic submucosal dissectionDig Endosc200921Suppl 1S22S2719691728
- Marín-GabrielJCFernández-EsparrachGDíaz-TasendeJHerreros de TejadaAColorectal endoscopic submucosal dissection from a Western perspective: Today’s promises and future challengesWorld J Gastrointest Endosc201682405526839645
- ProbstAGolgerDAnthuberMMärklBMessmannHEndoscopic submucosal dissection in large sessile lesions of the rectosigmoid: learning curve in a European centerEndoscopy201244766066722528673
- FarhatSChaussadeSPonchonTEndoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in developmentEndoscopy201143866467021623560
- FuccioLHassanCPonchonTClinical outcomes after endoscopic submucosal dissection for colorectal neoplasia: a systematic review and meta-analysisGastrointest Endosc Epub2017228
- SaundersBPTsiamoulosZPEndoscopic mucosal resection and endoscopic submucosal dissection of large colonic polypsNat Rev Gastroenterol Hepatol201613848649627353401
- BurgessNGBourkeMJEndoscopic resection of colorectal lesions: The narrowing divide between East and WestDig Endosc201628329630526212579
- Pimentel-NunesPDinis-RibeiroMPonchonTEndoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) GuidelineEndoscopy201547982985426317585
- KimHGThosaniNBanerjeeSChenAFriedlandSEffect of prior biopsy sampling, tattoo placement, and snare sampling on endoscopic resection of large nonpedunculated colorectal lesionsGastrointest Endosc201581120421325440686
- De CeglieAHassanCMangiavillanoBEndoscopic mucosal resection and endoscopic submucosal dissection for colorectal lesions: a systematic reviewCrit Rev Oncol Hematol201610413815527370173
- ArezzoAPasseraRMarcheseNGalloroGMantaRCirocchiRSystematic review and meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for colorectal lesionsUnited European Gastroenterol J2016411829
- BackesYMoonsLMGvan BergeijkJDEndoscopic mucosal resection (EMR) versus endoscopic submucosal dissection (ESD) for resection of large distal non-pedunculated colorectal adenomas (MATILDA-trial): rationale and design of a multicenter randomized clinical trialBMC Gastroenterol20161615627229709
- KimHHKimJHParkSJParkMIMoonWRisk factors for incomplete resection and complications in endoscopic mucosal resection for lateral spreading tumorsDig Endosc201224425926622725112
- JungDHYounYHKimJHParkHEndoscopic submucosal dissection for colorectal lateral spreading tumors larger than 10 cm: is it feasible?Gastrointest Endosc201581361462025440691
- AntillonMRBartalosCRMillerMLDiaz-AriasAAIbdahJAMarshallJBEn bloc endoscopic submucosal dissection of a 14-cm laterally spreading adenoma of the rectum with involvement to the anal canal: expanding the frontiers of endoscopic surgery (with video)Gastrointest Endosc200867233233718226698
- HongYMKimHWParkSBChoiCWKangDHEndoscopic mucosal resection with circumferential incision for the treatment of large sessile polyps and laterally spreading tumors of the colorectumClin Endosc2015481525825674527
- BaeJHYangDHLeeSOptimized hybrid endoscopic submucosal dissection for colorectal tumors: a randomized controlled trialGastrointest Endosc201683358459226320696
- OkaSTanakaSSaitoYLocal recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in JapanAm J Gastroenterol2015110569770725848926
- BelderbosTDLeendersMMoonsLMSiersemaPDLocal recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysisEndoscopy201446538840224671869
- BhattAAbeSKumaravelAVargoJSaitoYIndications and Techniques for Endoscopic Submucosal DissectionAm J Gastroenterol2015110678479125623656
- OkaSTanakaSKanaoHObaSChayamaKTherapeutic strategy for colorectal laterally spreading tumorDig Endosc200921Suppl 1S43S4619691733
- TakeuchiYOhtaTMatsuiFNagaiKUedoNIndication, strategy and outcomes of endoscopic submucosal dissection for colorectal neoplasmDig Endosc201224Suppl 1100104
- TanakaSKashidaHSaitoYJGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resectionDig Endosc201527441743425652022
- SaitoYFukuzawaMMatsudaTClinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resectionSurg Endosc201024234335219517168
- RutterMDChattreeABarbourJABritish Society of Gastroenterology/Association of Coloproctologists of Great Britain and Ireland guidelines for the management of large non-pedunculated colorectal polypsGut201564121847187326104751
- BeatonCTwineCPWilliamsGLRadcliffeAGSystematic review and meta-analysis of histopathological factors influencing the risk of lymph node metastasis in early colorectal cancerColorectal Dis201315778879723331927
- ChoiJYJungSAShimKNMeta-analysis of predictive clinicopathologic factors for lymph node metastasis in patients with early colorectal carcinomaJ Korean Med Sci201530439840625829807
- KitajimaKFujimoriTFujiiSCorrelations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative studyJ Gastroenterol200439653454315235870
- TanakaSHarumaKTeixeiraCREndoscopic treatment of submucosal invasive colorectal carcinoma with special reference to risk factors for lymph node metastasisJ Gastroenterol19953067107178963387
- YasudaKInomataMShiromizuAShiraishiNHigashiHKitanoSRisk factors for occult lymph node metastasis of colorectal cancer invading the submucosa and indications for endoscopic mucosal resectionDis Colon Rectum20075091370137617661146
- TominagaKNakanishiYNimuraSYoshimuraKSakaiYShimodaTPredictive histopathologic factors for lymph node metastasis in patients with nonpedunculated submucosal invasive colorectal carcinomaDis Colon Rectum20054819210015690664
- BelderbosTDGvan ErningFNde HinghIHJTvan OijenMGHLemmensVEPPSiersemaPDLong-term recurrence-free survival after standard endoscopic resection versus surgical resection of sub-mucosal invasive colorectal cancer: a population-based studyClin Gastroenterol Hepatol2017153403411.e127609703
- YoshiiSNojimaMNoshoKFactors associated with risk for colorectal cancer recurrence after endoscopic resection of T1 tumorsClin Gastroenterol Hepatol2014122292302.e323962552
- YodaYIkematsuHMatsudaTA large-scale multicenter study of long-term outcomes after endoscopic resection for submucosal invasive colorectal cancerEndoscopy201345971872423918621
- KiriyamaSSaitoYYamamotoSComparison of endoscopic submucosal dissection with laparoscopic-assisted colorectal surgery for early-stage colorectal cancer: a retrospective analysisEndoscopy201244111024103023012216
- SaitoYOtakeYSakamotoTIndications for and technical aspects of colorectal endoscopic submucosal dissectionGut Liver20137326326923710305
- NishiyamaHIsomotoHYamaguchiNEndoscopic submucosal dissection for laterally spreading tumors of the colorectum in 200 consecutive casesSurg Endosc201024112881288720419319
- YoshidaNWakabayashiNKanemasaKEndoscopic submucosal dissection for colorectal tumors: technical difficulties and rate of perforationEndoscopy200941975876119746316
- HisabeTNagahamaTHiraiFMatsuiTIwashitaAClinical outcomes of 200 colorectal endoscopic submucosal dissectionsDig Endosc201224Suppl 110510922533763
- LeeE-JLeeJBLeeSHEndoscopic submucosal dissection for colorectal tumors-−1,000 colorectal ESD cases: one specialized institute’s experiencesSurg Endosc2013271313922729707
- FujishiroMYahagiNKakushimaNOutcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms in 200 consecutive casesClin Gastroenterol Hepatol200756678683 quiz 64517466600
- TanakaSToyonagaTMoritaYFeasibility and safety of endoscopic submucosal dissection for large colorectal tumorsSurg Laparosc Endosc Percutan Tech201525322322825738701
- HayashiNTanakaSNishiyamaSPredictors of incomplete resection and perforation associated with endoscopic sub-mucosal dissection for colorectal tumorsGastrointest Endosc201479342743524210654
- PatelNPatelKAshrafianHAthanasiouTDarziATeareJColorectal endoscopic submucosal dissection: Systematic review of mid-term clinical outcomesDig Endosc201628440541626710317
- TanakaSTamegaiYTsudaSSaitoYYahagiNYamanoHOMulti-center questionnaire survey on the current situation of colorectal endoscopic submucosal dissection in JapanDig Endosc201022Suppl 1S2S820590765
- TajikaMNiwaYBhatiaVComparison of endoscopic submucosal dissection and endoscopic mucosal resection for large colorectal tumorsEur J Gastroenterol Hepatol201123111042104921869682
- WangSGaoSYangWGuoSLiYEndoscopic submucosal dissection versus local excision for early rectal cancer: a systematic review and meta-analysisTech Coloproctol20162011926519288
- NakamuraFSaitoYSakamotoTPotential perioperative advantage of colorectal endoscopic submucosal dissection versus laparoscopy-assisted colectomySurg Endosc201529359660625037724
- TomikiYKawaiMTakeharaKClinical pathway to discharge 3 days after colorectal endoscopic submucosal dissectionDig Endosc201527667968625756606
- MossAWilliamsSJHouriganLFLong-term adenoma recurrence following wide-field endoscopic mucosal resection (WF-EMR) for advanced colonic mucosal neoplasia is infrequent: results and risk factors in 1000 cases from the Australian Colonic EMR (ACE) studyGut2015641576524986245
- FerreiraJAkermanPColorectal endoscopic submucosal dissection: past, present, and factors impacting future disseminationClin Colon Rectal Surg201528314615126491406
- SakamotoTSaitoYFukunagaSNakajimaTMatsudaTLearning curve associated with colorectal endoscopic submucosal dissection for endoscopists experienced in gastric endoscopic submucosal dissectionDis Colon Rectum201154101307131221904147
- NiimiKFujishiroMGotoOKodashimaSKoikeKSafety and efficacy of colorectal endoscopic submucosal dissection by the trainee endoscopistsDig Endosc201224Suppl 1154158
- OhataKItoTChibaHTsujiYMatsuhashiNEffective training system in colorectal endoscopic submucosal dissectionDig Endosc201224Suppl 1848922533759
- RahmiGHotaytBChaussadeSEndoscopic submucosal dissection for superficial rectal tumors: prospective evaluation in FranceEndoscopy201446867067624977400
- CodaSTrentinoPAntonellisFA Western single-center experience with endoscopic submucosal dissection for early gastrointestinal cancersGastric Cancer201013425826321128062
- SauerMHildenbrandROyamaTSidoBYahagiNDumoulinFLEndoscopic submucosal dissection for flat or sessile colorectal neoplasia > 20 mm: A European single-center series of 182 casesEndosc Int Open201648E895E90027540580
- ProbstAEbigboAMärklBEndoscopic submucosal dissection for early rectal neoplasia: experience from a European centerEndoscopy201749322223227842423
- UraokaTSaitoYMatsudaTEndoscopic indications for endoscopic mucosal resection of laterally spreading tumors in the colorectumGut200655111592159716682427
- SaitoYFujiiTKondoHEndoscopic treatment for laterally spreading tumors in the colonEndoscopy200133868268611490384
- KudoSTamuraSNakajimaTYamanoHKusakaHWatanabeHDiagnosis of colorectal tumorous lesions by magnifying endoscopyGastrointest Endosc19964418148836710
- KudoSRubioCATeixeiraCRKashidaHKogureEPit pattern in colorectal neoplasia: endoscopic magnifying viewEndoscopy200133436737311315901
- KatoSFujiiTKobaIAssessment of colorectal lesions using magnifying colonoscopy and mucosal dye spraying: can significant lesions be distinguished?Endoscopy200133430631011315890
- YamadaMSaitoYSakamotoTEndoscopic predictors of deep submucosal invasion in colorectal laterally spreading tumorsEndoscopy201648545646426919264
- HayashiNTanakaSHewettDGEndoscopic prediction of deep submucosal invasive carcinoma: validation of the narrow-band imaging international colorectal endoscopic (NICE) classificationGastrointest Endosc201378462563223910062
- ASGE Technology CommitteeMapleJTAbu DayyehBKEndoscopic submucosal dissectionGastrointest Endosc20158161311132525796422
- UraokaTParra-BlancoAYahagiNColorectal endoscopic submucosal dissection: is it suitable in western countries?J Gastroenterol Hepatol201328340641423278302
- HassanCRepiciASharmaPEfficacy and safety of endoscopic resection of large colorectal polyps: a systematic review and meta-analysisGut201665580682025681402
- BourkeMJEndoscopic resection for mucosal neoplasia: pushing the boundaries, confronting the realityJ Gastroenterol Hepatol201126111582158422011293
- RolnyPThe need for surgery after endoscopic treatment of colorectal neoplasms is the most important outcome criterionEndoscopy2017491808227806405
- HajiAAdamsKBjarnasonIPapagrigoriadisSHigh-frequency mini probe ultrasound before endoscopic resection of colorectal polyps–is it useful?Dis Colon Rectum201457337838224509463
- GallTMHMarkarSRJacksonDHajiAFaizOMini-probe ultrasonography for the staging of colon cancer: a systematic review and meta-analysisColorectal Dis2014161O1O824119196
- HurlstoneDPBrownSCrossSSShorthouseAJSandersDSHigh magnification chromoscopic colonoscopy or high frequency 20 MHz mini probe endoscopic ultrasound staging for early colorectal neoplasia: a comparative prospective analysisGut200554111585158915964906
- SaitoYYamadaMSoEColorectal endoscopic submucosal dissection: Technical advantages compared to endoscopic mucosal resection and minimally invasive surgeryDig Endosc201426Suppl 15261
- WatanabeTItabashiMShimadaYJapanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancerInt J Clin Oncol201520220723925782566
- KimHGSethiSBanerjeeSFriedlandSOutcomes of endoscopic treatment of second recurrences of large nonpedunculated colorectal adenomasSurg Endosc20163062457246426423413
- ChedgyFJQBhattacharyyaRKandiahKLongcroft-WheatonGBhandariPKnife-assisted snare resection: a novel technique for resection of scarred polyps in the colonEndoscopy201648327728026820175
- ZhouPYaoLQinXXuMZhongYChenWEndoscopic submucosal dissection for locally recurrent colorectal lesions after previous endoscopic mucosal resectionDis Colon Rectum200952230531019279438
- KurokiYHoteyaSMitaniTEndoscopic submucosal dissection for residual/locally recurrent lesions after endoscopic therapy for colorectal tumorsJ Gastroenterol Hepatol201025111747175321039836
- HurlstoneDPShorthouseAJBrownSRTiffinNCrossSSSalvage endoscopic submucosal dissection for residual or local recurrent intraepithelial neoplasia in the colorectum: a prospective analysisColorectal Dis200810989189718355372
- SakamotoTSaitoYMatsudaTFukunagaSNakajimaTFujiiTTreatment strategy for recurrent or residual colorectal tumors after endoscopic resectionSurg Endosc201125125526020559661
- IsomotoHNishiyamaHYamaguchiNClinicopathological factors associated with clinical outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasmsEndoscopy200941867968319670135
- SatoKItoSKitagawaTFactors affecting the technical difficulty and clinical outcome of endoscopic submucosal dissection for colorectal tumorsSurg Endosc201428102959296524853849
- MatsumotoATanakaSObaSOutcome of endoscopic submucosal dissection for colorectal tumors accompanied by fibrosisScand J Gastroenterol201045111329133720626303
- HoltBABassanMSSextonAWilliamsSJBourkeMJAdvanced mucosal neoplasia of the anorectal junction: endoscopic resection technique and outcomes (with videos)Gastrointest Endosc201479111912623953401
- TamaruYOkaSTanakaSEndoscopic submucosal dissection for anorectal tumor with hemorrhoids close to the dentate line: a multicenter study of Hiroshima GI Endoscopy Study GroupSurg Endosc201630104425443126895899
- MatsumotoSMashimaHThe efficacy of endoscopic submucosal dissection for colorectal tumors extending to the dentate lineInt J Colorectal Dis201732683183728188417
- TanakaSToyonagaTMoritaYFeasibility and safety of endoscopic submucosal dissection for lower rectal tumors with hemorrhoidsWorld J Gastroenterol201622276268627527468216
- YamamotoKMichidaTNishidaTHayashiSNaitoMItoTColorectal endoscopic submucosal dissection: recent technical advances for safe and successful proceduresWorld J Gastrointest Endosc20157141114112826468335
- SakamotoTMoriGYamadaMEndoscopic submucosal dissection for colorectal neoplasms: a reviewWorld J Gastroenterol20142043161531615825473168
- ShinozakiSHayashiYLeforAKYamamotoHWhat is the best therapeutic strategy for colonoscopy of colorectal neoplasia? Future perspectives from the EastDig Endosc201628328929526524602
- HayashiYMiuraYYamamotoHPocket-creation method for the safe, reliable, and efficient endoscopic submucosal dissection of colorectal lateral spreading tumorsDig Endosc201527453453525708068
- HayashiYSunadaKTakahashiHPocket-creation method of endoscopic submucosal dissection to achieve en bloc resection of giant colorectal subpedunculated neoplastic lesionsEndoscopy201446Suppl 1 UCTNE421E42225314173
- MavrogenisGHochbergerJDeprezPShafazandMCoumarosDYamamotoKTechnological review on endoscopic submucosal dissection: available equipment, recent developments and emerging techniquesScand J Gastroenterol201752448649828050913
- OyamaTCounter traction makes endoscopic submucosal dissection easierClin Endosc201245437537823251884
- TsujiKYoshidaNNakanishiHTakemuraKYamadaSDoyamaHRecent traction methods for endoscopic submucosal dissectionWorld J Gastroenterol201622265917592627468186
- SuzukiSGotodaTKobayashiYUsefulness of a traction method using dental floss and a hemoclip for gastric endoscopic submucosal dissection: a propensity score matching analysis (with videos)Gastrointest Endosc201683233734626320698
- UraokaTIshikawaSKatoJAdvantages of using thin endoscope-assisted endoscopic submucosal dissection technique for large colorectal tumorsDig Endosc201022318619120642607
- AiharaHRyouMKumarNRyanMBThompsonCCA novel magnetic countertraction device for endoscopic submucosal dissection significantly reduces procedure time and minimizes technical difficultyEndoscopy201446542242524573770
- ImaedaHHosoeNIdaYNovel technique of endoscopic sub-mucosal dissection by using external forceps for early rectal cancer (with videos)Gastrointest Endosc20127561253125722624814
- TanakaSTerasakiMHayashiNOkaSChayamaKWarning for unprincipled colorectal endoscopic submucosal dissection: accurate diagnosis and reasonable treatment strategyDig Endosc201325210711623368854
- Le RoyFManfrediSHamonicSFrequency of and risk factors for the surgical resection of nonmalignant colorectal polyps: a population-based studyEndoscopy201648326327026340603
- BerrFPonchonTNeureiterDExperimental endoscopic sub-mucosal dissection training in a porcine model: learning experience of skilled Western endoscopistsDig Endosc201123428128921951087
- DraganovPVChangMComanRMWaghMSAnQGotodaTRole of observation of live cases done by Japanese experts in the acquisition of ESD skills by a western endoscopistWorld J Gastroenterol201420164675468024782619
- OyamaTYahagiNPonchonTKiesslichTBerrFHow to establish endoscopic submucosal dissection in Western countriesWorld J Gastroenterol20152140112091122026523097
- SpychalskiMDzikiASafe and efficient colorectal endoscopic sub-mucosal dissection in European settings: is successful implementation of the procedure possible?Dig Endosc201527336837325181427
- YangD-HJeongGHSongYThe feasibility of performing colorectal endoscopic submucosal dissection without previous experience in performing gastric endoscopic submucosal dissectionDig Dis Sci201560113431344126088371
- ShigaHKurohaMEndoKColorectal endoscopic submucosal dissection (ESD) performed by experienced endoscopists with limited experience in gastric ESDInt J Colorectal Dis201530121645165226243470
