Abstract
Diabetic gastroparesis (DMGP) is a condition of delayed gastric emptying after gastric outlet obstruction has been excluded. Symptoms of nausea, vomiting, early satiety, bloating, and abdominal pain are associated with DMGP. Uncontrolled symptoms can lead to overall poor quality of life and financial burdens on the healthcare system. A combination of antiemetics and prokinetics is used in symptom control; metoclopramide is the main prokinetic available for clinical use and is the only U.S. Food and Drug Administration-approved agent in the United States. However, a black box warning in 2009 reporting its association with tardive dyskinesia and recommending caution in chronically using this agent beyond 3 months has decreased its role in clinical practice. There is an unmet need for new prokinetics with good efficacy and safety profiles. Currently, there are several new drugs with different mechanisms of action in the pipeline that are under investigation and show promising preliminary results. Surgically combining gastric electrical stimulation with pyloroplasty is considered “gold” standard. Advances in therapeutic endoscopic intervention with gastric per-oral endoscopic pyloromyotomy have also been shown to improve gastric emptying and gastroparesis (GP) symptoms. In this review, we will comment on the challenges encountered when managing patients with DMGP and provide an update on advances in drug development and endoscopic and surgical interventions.
Introduction
Gastroparesis (GP) is a debilitating disease associated with poor quality of life (QoL) and financial burdens on the healthcare system.Citation1 There is a paucity of data on the prevalence of diabetic gastroparesis (DMGP). Based on an epidemiological study from Olmsted county in Minnesota, the risk of GP in patients with type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) is 5.2% and 1.0%, respectively.Citation2 Clinical symptoms of DMGP include nausea/vomiting, early satiety, bloating, and abdominal pain.Citation3,Citation4 The basis for the diagnosis of DMGP relies on the exclusion of gastric outlet obstruction and a delayed gastric emptying (GE) study with a radionuclide-labeled solid meal. GE is considered delayed with a food bolus retention of >60% at 2 hours and/or >10% at 4 hours.Citation5 Early satiety and postprandial fullness, two predominant symptoms in DMGP, have been associated with disease severity and delayed GE.Citation6 Patients with T1DM-related GP, who account for ~10% of the GP population, have comparable baseline symptoms to patients with T2DM-related GP. However, patients with T1DM-related GP have less clinical improvement with medical and/or surgical treatment and more vascular complications from diabetes.Citation7 Overall, patients with DMGP often have concomitant challenges with the management of hyperglycemia, and a vicious cycle is created, leading to worsening gastrointestinal neuropathy and clinical symptoms.Citation8,Citation9
The pathogenesis of DMGP remains elusive; however, it likely starts with impairment in the microcirculation of the gastric wall,Citation10 which is also seen in other complications of diabetes (i.e., nephropathy and retinopathy). Loss of gastric neurons containing nitric oxide (NO) synthetase may ensue in patients with DMGP, and an impaired NO pathway in the myenteric plexus may lead to an impaired gastric accommodation reflex with subsequent dyspeptic symptomsCitation11,Citation12 (). Damage to intrinsic/extrinsic neurons,Citation13 loss of interstitial cells of Cajal (ICC),Citation14 and loss of heme oxygenase 1 (HO1)Citation15 have also been implicated in DMGP. Lack of the protective enzyme heme oxygenase (HO1), which is expressed in type 2 macrophages against oxidative stress, has been associated with DMGP.Citation16 Loss of CD206-positive antiinflammatory macrophages (M2) in circular muscles in the bodyCitation17 and antrumCitation18 of the stomach has been seen in conjunction with loss of ICC in patients with DMGP. Refractory DMGP has been associated with fewer ganglion cells and more depleted ICC in the inner circular layer than in idiopathic GP.Citation19 Vagal cholinergics are also more often affected in DMGP than in idiopathic gastroparesis (IDGP).Citation20 Murine models have suggested a potential link between reduced insu lin/IGF-1 signaling and subsequent ICC depletion, smooth muscle atrophy, and reduced stem cell factor production.Citation21
Figure 1 Summary of the neural, myoelectrical, muscular, and cellular aspects of the pathophysiology of gastroparesis.
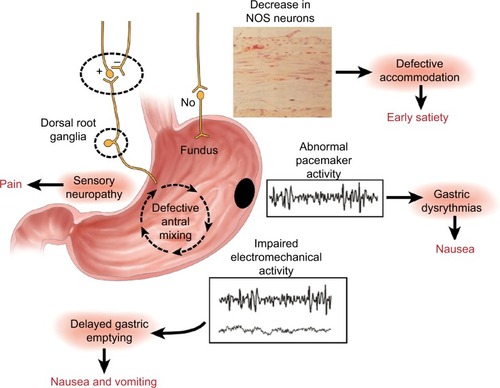
Pyloric dysfunction is an overlooked aspect in the pathogenesis of GP. There is evidence of “pylorospasm” among patients with diabetes.Citation22 Combined antral and pyloric dysfunction contribute to GP, with loss of relaxation in the pylorus and concomitant loss of antral motility. In addition, loss of ICC and smooth muscle fibrosis is more common in the pylorus than in the antrum of patients with refractory GP.Citation23 The advent of the endoscopic functional luminal imaging probe (EndoFLIP) has allowed for the evaluation of pyloric dysfunction in DMGP. Symptoms of early satiety and postprandial fullness are inversely correlated with the diameter and cross-sectional area of the pyloric sphincter.Citation24
In addition, wireless motility capsule testing has shown that there are extra-gastric delays in small bowel and colonic transit in up to 40% of patients with GP.Citation25 Symptoms related to extra-gastric delays may be suboptimally addressed when treating patients with DMGP due to their lack of awareness.
Antiemetics
A search was conducted in MEDLINE up to August 1, 2017. Mesh and non-Mesh terms were used, including “gastropare-sis,” “therapeutics,” “antiemetics,” “clonidine,” “nabinole,” “dronabinol,” “marinol,” “cannabinoids,” “ondansetron,” “granisetron,” “tropisetron,” “dolasetron,” and “aprepitant.” presents a summary of the antiemetics studied in DMGP, and shows antiemetics commonly used in DMGP but not studied in prospective or clinical trials.
Table 1 Antiemetics used in DMGP with data from prospective or controlled trials
Table 2 Antiemetics used but not studied in prospective or clinical trials
The scopolamine patch was adapted due to the inherent impediment of drug absorption during an episode of vomiting. Among 5-HT3 antagonists, granisetron is the only agent that can be administered via a transdermal patch, which is optimal during bouts of vomiting. It has been used in chemo-induced, radiation-induced, and postoperative nausea and vomiting. In a prospective study of 51 patients with GP (13 DMGP, 35 IDGP, 2 postsurgical GP, and 1 mixed connective tissue disease), 76% of patients reported clinical improvement in symptoms of nausea and vomiting (p<0.05); however, less symptom improvement was seen among those with severely delayed GE (p=0.065).Citation26 Aprepitant, a neurokinin-1 receptor antagonist, has been used for short-term treatment of chemotherapy-induced nausea, and its clinical benefit in refractory GP has been reported in a small case series.Citation27 Most recently, the APRON (aprepitant for the treatment of nausea) randomized controlled trial evaluated the efficacy of 125 mg of aprepitant daily (n=63) vs. placebo (n=63) for patients with nausea and vomiting due to suspected gastric dysfunction; 38% and 21% of the patients had diabetes in the aprepitant and placebo groups, respectively. Delayed GE was present in 46% of patients taking aprepitant and in 68% of those taking a placebo, whereas rapid GE was present in 3% of patients in each arm. Overall, the primary endpoint of reduction in nausea was not different from that of placebo (46% vs. 40%, p=0.43); however, there were improvements in overall symptom relief (p=0.001) and reductions in gastroparesis cardinal symptom index (GCSI) scores for nausea (p=0.005) and vomiting (p=0.001) in patients taking aprepitant.Citation28,Citation29 Tradipitant (VLY-686), a drug similar to aprepitant, is a neurokinin-1 receptor antagonist that works by blocking substance P; it is now undergoing clinical trial evaluation.Citation30
In a small randomized trial of patients with DMGP, oral clonidine, an alpha-2 adrenergic agonist, reduced symptoms of nausea and vomiting. Ten patients with DMGP and 10 matched controls received oral clonidine in doses ranging from 0.1 to 0.3 mg BID. GE of a solid meal was no different between the groups (p=0.62); however, symptoms of nausea and vomiting were reduced in patients with DMGP.Citation31 In a separate case series, six patients with diabetes with refractory symptoms of bloating, nausea, and vomiting received a median dose of 0.3 mg of clonidine per day and had a significant improvement in GE t1/2 of a liquid meal (p<0.025) and overall symptoms.Citation32 It is postulated that the therapeutic effect of clonidine comes from its action on the alpha-2 adrenergic receptor in the chemoreceptor trigger zone.Citation33
The impact of acupuncture on the symptoms of DMGP has been an active area of investigation. In a prospective nonrandomized, unblinded case-crossover study of eight patients with DMGP who were on domperidone (20 mg QID) for 12 weeks with a washout period of 2–3 weeks, the patients received acupuncture biweekly for 8 weeks. There was no change in GCSI scores with domperidone (p=0.77); however, there was improvement in the majority of the cardinal symptoms for GCSI (p=0.079) and QoL after acupuncture (p=0.002).Citation34 In a meta-analysis of low-quality randomized trials, acupuncture treatment had a higher response for the improvement of dyspeptic symptoms than controls (RR 1.20, CI: 1.12–1.29; p<0.00001).Citation35 These data should be regarded with caution due to the possibility of publication bias. Our research group has evaluated the effect of transcutaneous acupuncture (TEA) in the treatment of nausea in patients with GP. Using electroencephalography and electrogastrography, 11 patients with DMGP showed improvement with TEA in terms of gastric dysrhythmia and reduction of nausea (p<0.05).Citation36 To date, no clinical trials have evaluated marijuana or tetrahydrocannabinol (THC) derivatives (i.e., nabinol or dronabinol) in the treatment of GP symptoms. THC derivatives are likely to be ineffective for the long-term treatment of DMGP since they delay GECitation37 and may lead to cannabinoid hyperemesis syndrome.Citation38
Prokinetics
A search was conducted in MEDLINE up to August 1, 2017. Mesh and non-Mesh terms were used, including “gastroparesis”, “prokinetics”, “azithromycin”, “erythromycin”, “metoclopramide”, “domperidone”, and “metopimazine”. shows the prokinetics used in DMGP.
Table 3 Prokinetics in DMGP
Oral metoclopramide has been the mainstay treatment in DMGP since its approval by the U.S. Food and Drug Administration (FDA) in 1980.Citation39 Metoclopramide is a D2-dopamine receptor antagonist that increases lower esophageal sphincter (LES) pressure, GE, and transit time in the proximal small bowel. It also has an antiemetic effect via its antagonistic effects on dopamine receptors in the chemoreceptor trigger zone.Citation40 However, in 2009, a black box warning was issued on metoclopramide due to its association with tardive dyskinesia.Citation41 This black box warning led to legal implications and has decreased its use in clinical practice.Citation42
A study of genetic polymorphisms in the recipients of metoclopramide for upper gastrointestinal symptoms suggestive of GP revealed that side effects were associated with polymorphisms in CYP2D6, KCNH2, and 5-HT4 receptor HTR4 genes, and good clinical response was associated with polymorphisms in KCNH2 and ADRA1D.Citation43 In an open-label randomized trial evaluating patients with DMGP, a nasal spray containing 10 or 20 mg of metoclopramide was compared with metoclopramide 10 mg oral tablets QID×6 weeks. Total symptom scores were better with the administration of 20 mg (p=0.008) and 10 mg (p=0.03) nasal spray metoclopramide than with oral metoclopramide.Citation44 A Phase III randomized multicenter trial also evaluated nasal metoclopramide at 10 mg QID vs. placebo for 28 days. A total of 205 women with DMGP (88% T2DM) with a mean age of 52.7 years were included in the trial. The primary endpoint of reduction in baseline symptoms at week 4 was not met; however, a subgroup analysis of patients with moderate– severe symptoms at baseline showed a significant reduction of symptoms at weeks 1 and 3. There were no differences in adverse events between either arm.Citation45
Domperidone has been associated with improvement in symptoms of postprandial fullness, nausea, and vomiting.Citation46–Citation59 This dopamine 2 antagonist differs from metoclopramide in that it does neither cross the blood–brain barrier nor induces central nervous system side effects but has a similar efficacy profile to that of oral metoclopramide.Citation55 It has been utilized worldwide for over 25 years, but it is available only in the United States under an investigational new drug application through the FDA. In a prospective cohort (88 IDGP, 16 DMGP, and 9 postsurgical GP), most patients showed an improvement in GP symptoms, including nausea, vomiting, retching, early satiety, postprandial fullness, and upper abdominal pain (p<0.001).Citation56 Similarly, a recent prospective cohort of 34 patients (5 DMGP and 29 IDGP) taking domperidone at 10 mg TID for an average treatment duration of 36.9 days showed an overall GP symptom improvement (p<0.05).Citation48 In a systematic review of 28 trials (11 full articles and 17 abstracts), 64% of studies showed a symptom improvement, 60% showed an improvement in GE, and 67% of studies showed a reduction in hospital readmissions. It was concluded that there is level 3 evidence for the efficacy of domperidone in DMGP, leading to a grade C recommendation.Citation60 “New” domperidone formula tions have been developed to minimize the remote possibility of cardiotoxicity (prolonged QT interval) with the original domperidone. NG101 (metopimazine) has shown promising results in animal models and is a highly potent D2 receptor antagonist (100× more potent than metoclopramide) that does not cross the blood–brain barrier or antagonize hERG channels (cardiac channels) in vitro.Citation61 Similarly, TAK-906 is being evaluated in Phase II clinical studies to assess the safety of the drug in patients with DMGP and IDGP in a randomized double-blind trial.
Erythromycin is a macrolide antibiotic with an agonist effect on the motilin receptor,Citation62 and IV or oral erythromycin administration may improve GE by 30%–60%.Citation63 Motilin, a polypeptide hormone, increases LES pressure and initiates migrating motor complex (MMC) activity in the antrum and proximal small bowel.Citation64 Intravenous erythromycin at doses of 6 mg/kg followed by an oral erythromycin base at a dose of 500 mg TID prior to meals has been shown to improve solid meal retention at 2 hours from 85% at baseline vs. 20% following intravenous erythromycin (p<0.001) and 48% after 4 weeks of oral therapy (p<0.01).Citation65 However, a randomized trial of motilin receptor agonist (ABT-229) vs. placebo failed to show an improvement in clinical symptoms in patients with T1DM with or without delayed GE.Citation66 Furthermore, the effects of erythromycin may only be short lived due to the development of tachyphylaxis with prolonged use.Citation67 A similar macrolide, azithromycin, has also been shown to improve antral and duodenal contractions in patients with gastrointestinal dysmotility; however, unlike erythromycin, it has fewer drug–drug interactions and is not metabolized by the P450 system.Citation68,Citation69 A prospective study of 120 patients with chronic abdominal pain or suspected GP underwent solid meal GE with IV azithromycin or 250 mg of IV erythromycin given at 75–80 minutes with 15 minutes of further imaging to determine GE t1/2. GE t1/2 was comparable between azithromycin (mean GE t1/2 10.4±7.2 minutes) and erythromycin (mean GE t1/2 11.9±8.4 minutes; p=0.30). Due to the fewer drug–drug interactions, a lower incidence of QT prolongation, and a longer half-life with azithromycin, it may be a better alternative to erythromycinCitation70; however, concerns about antimicrobial stewardship may limit its long-term use.
Advances in drug development
The need for further drug development for GP continues, and several agents are poised to gain acceptance (). Traditionally, endpoints in clinical trials have focused on enhancing GE and evaluating GCSI scores. Even though prokinetics have been associated with clinical improvement and accelerated GE, establishing a correlation between symptom improvement and changes in GE has been elusive for drugs such as metoclopramide, domperidone, cisapride, erythromycin, and levosulpiride. The lack of a relationship between these two outcomes has led to questioning of the use of GE as a primary endpoint in drug development.Citation71
Table 4 Prokinetics in Phase II and III stages of development
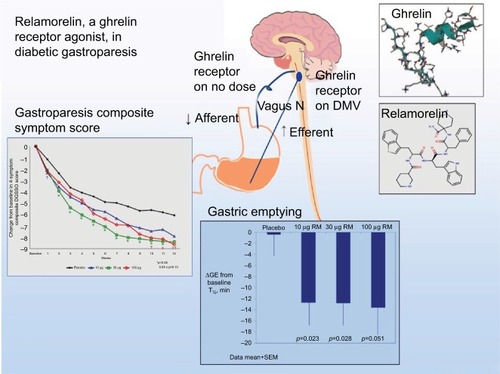
Ghrelin promotes gastric motility and has been shown to relieve GP symptoms ().Citation72,Citation73 Relamorelin (RM-131), a selective ghrelin receptor agonist, accelerated solid GE t1/2 (p=0.01) in a Phase Ib clinical trialCitation74 with a reduction in clinical symptoms (p<0.05) and improved GE in a Phase IIb randomized trial of patients with moderate–severe diabetic GP who received 10 or 30 µg of subcutaneous relamorelin BID vs. placebo (p<0.05).Citation75 Similarly, a double-blind Phase II randomized trial including 204 patients with DMGP from 27 clinical centers received 10 µg of relamorelin subcutaneously once or twice daily. Twice a day dosing accelerated GE (p<0.03) with a decrease in vomiting episodes of nearly 60% (p≤0.03).Citation76 Currently, a multicenter Phase III randomized trial has started for relamorelin at a dose of 10 µg BID in DMGP.
Figure 2 Summary of the molecular structure, mechanism of action, and outcomes of a clinical trial regarding symptoms and gastric emptying.
Abbreviations: DMV, dorsal motor nucleus of the vagus; GE, gastric emptying; RM, relamorelin; SEM, standard error of the mean.
Pharmacologic agents working as 5HT4 receptor agonists have been shown to enhance GE and small bowel and colonic transit and mediate reflexes controlling gastrointestinal motility and secretion.Citation77–Citation79 Historically, cisaprideCitation80 and tegaserodCitation81 were developed based on these mechanisms but were not sustainable due to cardiotoxicity.Citation82 Prucalopride, a selective 5-hydroxytryptamine-4 receptor agonist, has been evaluated in patients with DMGP and IDGP with improvement in GE t1/2 emptying (p<0.05) and GCSI scores, including fullness/satiety (p<0.0005), nausea/vomiting (p<0.001), and bloating/distension (p<0.00001), compared with placebo.Citation83 Velusetrag, a 5-HT4 agonist, was evaluated in patients with GP (53% diabetes) in a randomized crossover placebo-controlled study evaluating dosing regimens of 5, 10, and 30 mg/day. Improvement in GE time, as defined by a 20% reduction of GE t1/2, was achieved in 52% of patients (with both diabetic and idiopathic GP) with the 30 mg/day dose compared with placebo (p=0.002).Citation84 Pharmacologic receptor data have shown that Velusetrag is 500× more selective than cisapride without cardiotoxicity.Citation85
Basic cellular level therapies
Macrophage phenotype and loss of ICC in the gastric muscle layers have led to cellular explanations for delayed GE. In diabetic models, macrophages with the M2 phenotype show normal GE, whereas in vitro studies have shown that the M1 phenotype leads to tumor necrosis factor-α production, resulting in Kit loss and ICC injury.Citation86 Furthermore, low numbers of CD206-positive M2 macrophages in the bodyCitation17 and antrumCitation18 of human subjects have been associated with ICC loss and DMGP. Based on this notion, pioglitazone (thiazolidinedione class) could have the potential to modify these changes of depleted CD206 M2 macrophages in the stomach.
Similarly, reduction in heme oxygenase (HO1) has been associated with the loss of ICC and delayed GE. In a randomized controlled trial, hemin infusion with albumin was compared with albumin alone and its effect on HO1 levels. Patients received infusions on days 1, 3, and 8 and subsequent weekly infusions for 7 weeks. Beyond 1 week, hemin did not increase HO1 levels or improve symptoms or GE.Citation87
Drug–drug interactions
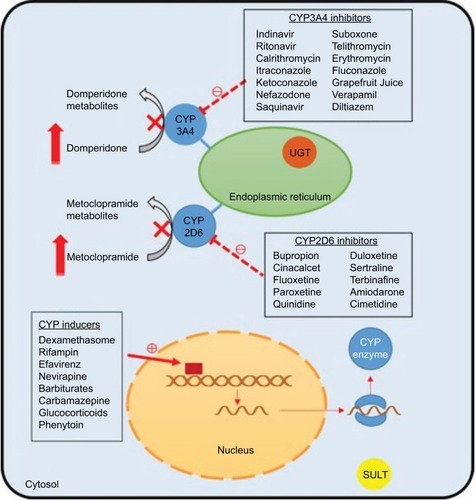
A common clinical problem encountered in the management of patients with DMGP is drug–drug interactions. Typically, patients require a combination of antiemetics, prokinetic agents, and pain modulators. Prokinetic agents, such as domperidone and metoclopramide, have been associated with QT prolongation,Citation88 and a combination of these regimens with antiemetics, such as promethazine and ondansetron,Citation89 or antidepressants could have a synergistic effect.Citation90,Citation91 Due to the high prevalence of anxiety and depression in patients with GP,Citation92 concomitant use of selective serotonin reuptake inhibitors and metoclopramide could also lead to a greater risk of side effects ().Citation93
Figure 3 Potential drug–drug interactions in gastroparesis.
Abbreviations: CYP, Cytochrome P450; UGT, glucuronosyltransferase; SULT, sulfotransferases.
The cardiotoxic effects of metoclopramide and domperidone were investigated in neonatal rat cardiomyocytes. Cardiotoxicity was deemed to be due to potent local anesthetic-like inhibition of cardiac Na channelsCitation94 and inhibition of hERG channel activity, which inhibits the rapid component of the cardiac delayed rectifier K(+) current.Citation88,Citation95 Recently, a meta-analysis of observational studies reported a 70% increase in the risk of cardiac arrhythmia and sudden cardiac death with domperidone use.Citation88 However, these data should be regarded with caution given the poor methodological quality of each composite study.Citation96 In our personal 20 years’ experience with domperidone, it has demonstrated good clinical efficacy in doses of up to 120 mg/day. Out of 64 patients treated with domperidone (DMGP 45%, IDGP 36%, and other 19%), 10 patients had prolonged QTc ranging from 453 to 509; however, no clinical cardiovascular adverse events were reported.Citation54 Baseline and routine follow-up electrocardiography testing should be performed in patients on domperidone, and more specifically, when these patients also require antiemetics, prokinetics, and antidepressants to monitor for QTc prolongation.
Glycemic control and comorbidities in DMGP
Acute and chronic hyperglycemia can delay GECitation97; however, there is limited data showing that long-term glucose control can improve GE.Citation98 In the Epidemiology of Diabetes Intensive Complications study, elevated baseline HbA1c and years of diabetes were associated with delayed GE in patients with T1DM.Citation9 In healthy people, hyperglycemia (blood glucose ≥200 mg/dL) reduces antral motility,Citation99 and severe hyperglycemia (blood glucose 290–340 mg/dL) can reduce antral motility and GE in patients with T1DM.Citation100 Furthermore, hyperglycemia (mean blood glucose 285 mg/dL) may reduce the length of the MMC cycle.Citation101 Thus, it is plausible that reduction in antral motility and MMC cycles may result in delayed GE.
Delayed GE may also be associated with hypoglycemia, and this condition has been termed “gastric” hypoglycemia.Citation102 A study involving 31 patients with diabetes (16 T1DM and 15 T2DM) with unexplained postprandial hypoglycemia and 18 insulin-dependent patients with diabetes without postprandial hypoglycemia underwent GE 13C breath tests (BreathID® system). GE was slower in hypoglycemic patients with diabetes than in nonhypoglycemic patients with diabetes with t1/2 139.9±74.1 vs. 77.8±23.3 (p<0.001).Citation103
Blood glucose excursions due to poor tolerability of oral hypoglycemic agents or fear of insulin use during episodes of poor oral intake may lead to subsequent microvascular/macrovascular complications and comorbidities. People with diabetes with GP symptoms and delayed GE have been associated with an increased risk of the development of cardiovascular disease (p<0.05), hypertension (p=0.005), and retinopathy (p<0.001) but not with overall mortality when compared with people with diabetes without GP symptoms and normal GE.Citation104 Similarly, in a 25-year follow-up study of 86 patients with DMGP, delayed GE of solid or liquid meals was not associated with increased mortality.Citation105
Nutrition and DMGP
Dietary modifications are often needed to control gastroparetic symptoms. More frequently, small portions of a diabetic diet have been effective in reducing symptoms in DMGP. Fifty-six patients with insulin-dependent diabetes and GP were randomized to a small particle diet consisting of foods that could be easily mashed or ground and a control diet that consisted of large particle foods with a low glycemic index (i.e., whole meat, seafood, cheese slices, almonds, and nuts). Symptoms of nausea/vomiting (p=0.01), postprandial fullness (p=0.02), bloating (p=0.006), and heartburn/regurgitation (p=0.02) were improved in the intervention arm, but no improvement was seen in abdominal pain.Citation106 Similarly, avoidance of high-fat solid meals, implementation of a low-fat/low-fiber diet, and increased liquid content meals led to symptomatic improvement.Citation107,Citation108 Carbonated beverages should be avoided as they can aggravate proximal gastric distension and can theoretically worsen abdominal bloating and the sensation of fullness in DMGP.Citation109
Patients with DMGP may have diets deficient in calories, vitamins, and minerals.Citation110 Poor oral tolerance of a liquid meal may be a clinical sign for the need for an alternative means of nutrition. A trial of nasojejunal tube feedings may be used to assess tolerance of feedingsCitation111 and as a bridge to placement of a surgical jejunal tube. Surgical placement of a jejunostomy tube beyond the affected stomach has been shown to improve self-reported overall health status among patients with DMGP; however, up to 54% of patients may have some complications requiring further surgery or hospitalization.Citation112 An alternative to surgical placement of a jejunostomy tube is a direct percutaneous endoscopic jejunostomy (DPEJ), which has been associated with modest technical success (68%) and minimal adverse events, including bowel perforation (2.4%), jejunal volvuli (1%), and major bleeding (1%).Citation113 Perendoscopic 20F gastrostomy tubes with extension (PEGJ) are technically easier to place than a 20F DPEJ; however, their placement is associated with a high degree of retrograde tube migration,Citation114 which may often be seen in patients with DMGP after prolonged episodes of nausea/vomiting and retching. Placement of endoscopic clips during the placement of a PEGJCitation115 and a new technique called the PEG-Pedi-PEG technique,Citation116 which involves placement of a pediatric PEG tube bumper in the small bowel, may limit retrograde tube migration; however, reports of its clinical success in patients with DMGP are limited.
QoL and biopsychosocial factors in DMGP
Patients with DMGP have an impaired QoL due to symptoms of GP, and many will have concomitant mood disorders with nearly 50% of patients reporting anxiety and 38% of them reporting depression.Citation117 Patients with mood disorders are more likely to report worse GP symptoms,Citation92,Citation118 and clinical symptoms, such as abdominal pain and nausea/vomiting, have been linked with an overall poorer QoL.Citation119,Citation120 Abdominal pain is the predominant symptom in one-fifth of patients with GP, whereas nausea/vomiting is the predominant symptom in 44% of gastroparetics.Citation120 Abdominal pain is often epigastric (43%), postprandial (72%), nocturnal (74%), and frequently associated with interference with sleep (66%).Citation119,Citation121 It is important to differentiate the entity of abdominal wall pain related to contractions of the rectus abdominis muscle during episodes of vomiting as opposed to visceral abdominal pain. Abdominal wall pain responds to anesthetic patches, heat pad applications, and breathing/relaxation techniques.Citation122 Regardless of GP subtype, abdominal pain appears to be present in both DMGP (89%) and IDGP (90%),Citation119 but moderate–severe upper abdominal pain is more prevalent in IDGP than in DMGP and correlates with other symptoms, such as nausea/vomiting, bloating, lower abdominal pain, bowel disturbances, and opiate use (p<0.05).Citation120 Prokinetics, such as relamorelinCitation76 and prucalopride,Citation83 have not been shown to improve symptoms of abdominal pain except for a metoclopramide nasal spray.Citation45 The effects of domperidone on abdominal pain have shown conflicting results with one prospective study showing no improvementCitation48 and another study reporting positive clinical outcomes.Citation56 Data on symptomatic improvement with Velusetrag are still pending.Citation84
It is conceivable that due to a lack of symptom control with traditional medications, patients with DMGP may resort to the use of narcotics for the alleviation of abdominal pain. In a recent study of 223 patients (27.8% DMGP, 54.7% IDGP, and 17.5% other) with delayed GE who were referred to a tertiary medical center, opioids were used in nearly one-third of participants, whereas 19.3% of these patients were on chronic opioids (>1 month). Symptoms of upper abdominal pain, nausea, vomiting, retching, early satiety, and postprandial fullness were worse in patients on chronic opioids than in patients who were not on opioids (p<0.05).Citation123 Patients on chronic opioids also had greater interference with sleep due to abdominal pain, lower employment rates, fewer working hours among those employed, and a greater number of hospitalizations.Citation123 Worsening symptoms of abdominal pain with opioid use may be multifactorial and due to narcotic bowel syndrome, opioid-induced constipation, worsening psychopathology, and addiction.Citation124 In addition, opioids can further delay GE in patients with GP and lead to worsening symptoms.Citation119,Citation121,Citation125
Endoscopic/surgical interventions
In DMGP, intrapyloric injection of botulinum toxin to the pylorus has resulted in unconvincing results in terms of clinical efficacy.Citation126 However, with the advent of endoscopic ultrasonography, it is postulated that injection of botulinum toxin with direct ultrasonographic visualization to the muscularis propria of the pyloric sphincter will lead to better clinical outcomes.Citation127,Citation128 Endoscopic transpyloric stent placement has also been proposed as a salvage therapy and as a bridge to more permanent therapies, such as gastric electric stimulation (GES). In a prospective cohort of 30 patients with refractory GP, 48 transpyloric stents were placed, resulting in 98% technical and 75% clinical success. Stent migration was least common (48%) when the stents were sutured to the gastric wall.Citation129
Patients with refractory GP may be offered GES if medical treatment fails. The Enterra GES has been shown to be effective in clinical practice for patients with DMGP. Clinical parameters associated with favorable clinical responses include DMGP rather than IDGP as an indication, nausea/vomiting as a primary symptom as opposed to abdominal pain, and the absence of narcotic use prior to GES implantation.Citation130 Complications of GES have included infection (3.9%), device/lead migration (2.7%), and pain at the implantation site (0.7%).Citation131 In a prospective cohort of 151 patients (72 DMGP, 73 IDGP, and 6 others), GES improved symptoms of nausea, vomiting, loss of appetite, and early satiety.Citation132 The longest follow-up study showed that GES improved symptoms by ~50%; however, 75% of patients continued to have delayed GE during a follow-up period of up to 10 years.Citation133 The addition of pyloroplasty was conceived as an adjunct to GES in order to normalize or improve GE and further reduce clinical symptoms in drug-refractory GP. A prospective cohort of 49 patients with GP (17 DMGP, 9 IDGP, and 23 postsurgical GP) showed that GE was 64% faster by 4 hours (p<0.001) in patients who underwent GES+pyloroplasty compared with 7% improvement in patients who underwent GES alone and symptoms were reduced by >70%.Citation134 Simultaneous GES implantation and Heineke–Mikulicz pyloroplasty have not shown an increase in morbidity.Citation135
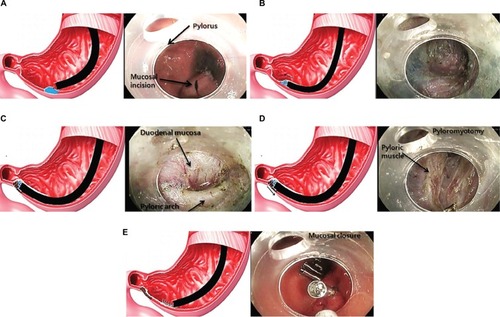
Laparoscopic pyloroplasty as a first-line treatment in refractory GP has shown improvement in GE and a reduction of clinical symptoms. In a prospective cohort of 177 patients, 105 of whom also had a fundoplication for concurrent reflux, GE improved or normalized in 90% of patients with clinical improvement in symptoms of nausea, vomiting, bloating, and abdominal pain.Citation136 Debate continues as to whether pyloroplasty alone will suffice, or a combination of GES implantation and pyloroplasty will lead to better clinical outcomes. A double-blind study is under current clinical investigation to address this question. Due to advancements of endoscopic techniques, gastric per-oral endoscopic pyloromyotomy (G-POEM) is a new nonsurgical treatment option for refractory GP and may be an alternative to laparoscopic pyloroplasty (). In a prospective cohort of 47 patients who underwent G-POEM (27 IDGP, 12 DMGP, and 8 post-surgical GP), preprocedure meal retention at 4 hours was 37% compared with 20% (p<0.03) after G-POEM.Citation137 In a separate cohort of 29 patients (15 IDGP, 7 DMGP, 5 postsurgical GP, and 2 scleroderma), clinical improvement (69%) and GE normalization (70%) were still present at a 6-month followup.Citation138 A multicenter prospective study of G-POEM evaluated 30 patients with refractory GP (11 DMGP, 12 post-surgical GP and 7 IDGP), all of whom had a technically successful intervention with an average procedure time of 72 minutes. Two adverse events were reported (one capnoperitoneum and one prepyloric ulcer), and four patients did not respond to G-POEM. Most patients (86%) had good clinical response, and GE improved or normalized in 35% and 47% of patients, respectively.Citation139 The implementation of EndoFLIP may aid in patient selection for G-POEM as it can evaluate the pyloric sphincter compliance or resistance in symptomatic patients with nausea, vomiting, and delayed GE.Citation140
Figure 4 Gastric per-oral endoscopic pyloromyotomy for gastroparesis.
Abbreviation: G-POEM, gastric per-oral endoscopic pyloromyotomy.
Synthesis of medical and surgical treatment approaches
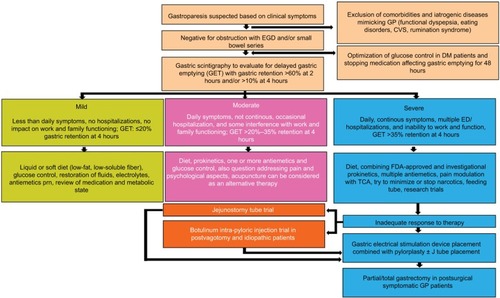
We provide a “progress report” of where we are in 2018, and this is best appreciated in the treatment algorithm (). Since the approval of metoclopramide in 1980, >35 years have passed with no major therapeutic advances in prokinetics. Symptom improvement has been achieved through aggressive antiemetic therapy and, in some cases, acupuncture. We are now starting to witness a surge in drug development. In addition, recognition of pyloric dysfunction as an integral part of the pathophysiology of GP has set the stage for simultaneous GES implantation with pyloroplasty. Advances in endoscopic techniques have also allowed for an alternative to surgical pyloroplasty via endoscopic pyloromyotomy. The next decade will see the greatest therapeutic progress that we have witnessed in GP with the possibility of prevention and cure.
Figure 5 Treatment algorithm in diabetic gastroparesis.
Expert analysis and concluding commentary
Medical therapy should initially be pursued in all patients with DMGP. New pharmacological agents are rapidly evolving and are currently approaching the FDA approval stage. This will provide a “menu” of effective prokinetics.
Antiemetic therapy is the foundation for gaining initial symptom control, and the agents available have different receptor targets in the chemoreceptor trigger zone. Strategic utilization of these agents alone and in combination is my recommendation.
We have increased our knowledge of the pathophysiology of GP where we have extended beyond the observations of antral ICC depletion to understanding the unappreciated crucial role of pyloric damage in the pathophysiology of DMGP.
Armed with this new understanding of the pathophysiology and specifically targeting the pylorus, we now have the surgical and endoscopic tools in hand as well as the clinical outcome data to confidently state that we have and can continue to successfully overcome GP.
Certainly, I continue to recommend that medical therapy should be actively initiated but with knowledge that when responses are suboptimal, there should be no hesitation in turning to what I term the “the final solution” for GP with gastric neurostimulation “combined with a pyloroplasty”.
Now, the focus is on prevention of GP by drilling down to the molecular level in the smooth muscle. New knowledge from M1 and M2 macrophages as well as inflammatory pathways has led to further understanding of how to prevent ICC depletion and damage to the enteric nervous system.
Acknowledgments
We would like to recognize how far this field has come and what has been accomplished, thanks to the National Institutes of Health and their decision to fund seven centers of academic excellence that constitute the Clinical Gastroparesis Consortium. Over the past 12 years, the consortium has made seminal contributions to advancing the field. We look forward to their continued leadership and discoveries in GP in the coming years.
Disclosure
The authors report no conflicts of interest in this work.
References
- LacyBECrowellMDMathisCBauerDHeinbergLJGastroparesis: quality of life and health care utilizationJ Clin Gastroenterol2018521202427775961
- ChoungRSLockeGR3rdSchleckCDZinsmeisterARMeltonLJ3rdTalleyNJRisk of gastroparesis in subjects with type 1 and 2 diabetes in the general populationAm J Gastroenterol20121071828822085818
- ParkmanHPHallinanEKHaslerWLNausea and vomiting in gastroparesis: similarities and differences in idiopathic and diabetic gastroparesisNeurogastroenterol Motil201628121902191427350152
- ParkmanHPYatesKHaslerWLSimilarities and differences between diabetic and idiopathic gastroparesisClin Gastroenterol Hepatol201191210561064 quiz e1133–e105421871247
- RaoSSCamilleriMHaslerWLEvaluation of gastrointestinal transit in clinical practice: position paper of the American and European Neurogastroenterology and Motility SocietiesNeurogastroenterol Motil201123182321138500
- ParkmanHPHallinanEKHaslerWLEarly satiety and postprandial fullness in gastroparesis correlate with gastroparesis severity, gastric emptying, and water load testingNeurogastroenterol Motil2017294
- KochKLHaslerWLYatesKPBaseline features and differences in 48 week clinical outcomes in patients with gastroparesis and type 1 vs. type 2 diabetesNeurogastroenterol Motil20162871001101526946489
- HomkoCSirajESParkmanHPThe impact of gastroparesis on diabetes control: patient perceptionsJ Diabetes Complications201630582682927166926
- BharuchaAEBatey-SchaeferBClearyPADelayed gastric emptying is associated with early and long-term hyperglycemia in type 1 diabetes mellitusGastroenterology2015149233033925980755
- ShenHLYangSPWangKJEvaluation of gastric blood supply in diabetic patients with gastroparesis by contrast-enhanced ultrasoundBr J Radiol20168910682016036627759430
- GroverMFarrugiaGLurkenMSCellular changes in diabetic and idiopathic gastroparesisGastroenterology2011140515751585e157821300066
- IwasakiHKajimuraMOsawaSA deficiency of gastric interstitial cells of Cajal accompanied by decreased expression of neuronal nitric oxide synthase and substance P in patients with type 2 diabetes mellitusJ Gastroenterol200641111076108717160518
- DeliGBosnyakEPuschGKomolySFeherGDiabetic neuropathies: diagnosis and managementNeuroendocrinology201398426728024458095
- GroverMBernardCEPasrichaPJClinicalhistological associations in gastroparesis: results from the Gastroparesis Clinical Research ConsortiumNeurogastroenterol Motil2012246531539.e24922339929
- KashyapPFarrugiaGOxidative stress: key player in gastrointestinal complications of diabetesNeurogastroenterol Motil201123211111421226884
- NeshatianLGibbonsSJFarrugiaGMacrophages in diabetic gastroparesis–the missing link?Neurogastroenterol Motil201527171825168158
- BernardCEGibbonsSJMannISAssociation of low numbers of CD206-positive cells with loss of ICC in the gastric body of patients with diabetic gastroparesisNeurogastroenterol Motil20142691275128425041465
- GroverMBernardCEPasrichaPJDiabetic and idiopathic gastroparesis is associated with loss of CD206-positive macrophages in the gastric antrumNeurogastroenterol Motil2017296
- HeckertJThomasRMParkmanHPGastric neuromuscular histology in patients with refractory gastroparesis: relationships to etiology, gastric emptying, and response to gastric electric stimulationNeurogastroenterol Motil2017298
- MohammadMKPepperDJKedarAMeasures of autonomic dysfunction in diabetic and idiopathic gastroparesisGastroenterol Res20169456569
- HorvathVJVittalHLorinczAReduced stem cell factor links smooth myopathy and loss of interstitial cells of cajal in murine diabetic gastroparesisGastroenterology2006130375977016530517
- MearinFCamilleriMMalageladaJRPyloric dysfunction in diabetics with recurrent nausea and vomitingGastroenterology1986906191919253699409
- MoravejiSBashashatiMElhanafiSDepleted interstitial cells of Cajal and fibrosis in the pylorus: novel features of gastroparesisNeurogastroenterol Motil20162871048105426940535
- MalikZSankineniAParkmanHPAssessing pyloric sphincter pathophysiology using EndoFLIP in patients with gastroparesisNeurogastroenterol Motil201527452453125712043
- HaslerWLMayKPWilsonLARelating gastric scintigraphy and symptoms to motility capsule transit and pressure findings in suspected gastroparesisNeurogastroenterol Motil2017302
- MidaniDParkmanHPGranisetron transdermal system for treatment of symptoms of gastroparesis: a prescription registry studyJ Neurogastroenterol Motil201622465065527400689
- FountoulakisNDunnJThomasSKarallieddeJSuccessful management of refractory diabetic gastroparesis with long-term Aprepitant treatmentDiabet Med201734101483148628636760
- PasrichaPJYatesKSarosiekIAprepitant for symptoms of gastroparesis and related disorders: the apron randomized clinical trialAm J Gastroenterol2016111S480S48127685301
- PasrichaPJYatesKPSarosiekIAprepitant has mixed effects on nausea and reduces other symptoms in patients with gastroparesis and related disordersGastroenterology201815416576.e1129111115
- ClinicalTrials.govStudy to Assess the Efficacy of VLY-686 in Relieving Symptoms of Gastroparesis2017 Available from: https://clinicaltrials.gov/ct2/show/study/NCT02970968Accessed September 16th, 2017
- HuilgolVEvansJHellmanRSSoergelKHAcute effect of clonidine on gastric emptying in patients with diabetic gastropathy and controlsAliment Pharmacol Ther200216594595011966503
- Rosa-e-SilvaLTronconLEOliveiraRBIazigiNGalloLJrFossMCTreatment of diabetic gastroparesis with oral clonidineAliment Pharmacol Ther1995921791837605859
- ThumshirnMCamilleriMChoiMGZinsmeisterARModulation of gastric sensory and motor functions by nitrergic and alpha2-adrenergic agents in humansGastroenterology1999116357358510029616
- Danielli MillerNSchiffEBen-AryeEBenefits of acupuncture for diabetic gastroparesis: a comparative preliminary studyAcupunct Med201432213914524323633
- YangMLiXLiuSMeta-analysis of acupuncture for relieving non-organic dyspeptic symptoms suggestive of diabetic gastroparesisBMC Complement Altern Med20131331124206922
- SarosiekISongGSunYCentral and peripheral effects of trans-cutaneous acupuncture treatment for nausea in patients with diabetic gastroparesisJ Neurogastroenterol Motil201723224525328163260
- McCallumRWSoykanISridharKRRicciDALangeRCPlankeyMWDelta-9-tetrahydrocannabinol delays the gastric emptying of solid food in humans: a double-blind, randomized studyAliment Pharmacol Ther19991317780
- StanghelliniVTalleyNJChanFRome IV–gastroduodenal disordersGastroenterology Epub201621526860903
- US Food and Drug AdministrationFDA Approved Drug Products1980 Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&varApplNo=017854Accessed September 16, 2017
- Metoclopramide (Reglan)Med Lett Drugs Ther19822461467697087890
- FoodUSAdministrationDrugFDA Requires Boxed Warning and Risk Mitigation Strategy for Metoclopramide-Containing Drugs2009 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/017854s052lbl.pdfAccessed September 16, 2017
- EhrenpreisEDDeepakPSifuentesHDeviRDuHLeikinJBThe metoclopramide black box warning for tardive dyskinesia: effect on clinical practice, adverse event reporting, and prescription drug lawsuitsAm J Gastroenterol2013108686687223735907
- ParkmanHPMishraAJacobsMClinical response and side effects of metoclopramide: associations with clinical, demographic, and pharmacogenetic parametersJ Clin Gastroenterol201246649450322688145
- ParkmanHPCarlsonMRGonyerDMetoclopramide nasal spray is effective in symptoms of gastroparesis in diabetics compared to conventional oral tabletNeurogastroenterol Motil201426452152824372829
- McCallumRWFassRBhandariBRCarlsonMRAlvesWSymptom severity influences drug efficacy in women with diabetic gastroparesis: results of a phase 3 study with metoclopramide nasal sprayGastroenterology20171525S1313
- BraunAPDomperidone in the treatment of symptoms of delayed gastric emptying in diabetic patientsAdv Ther1989625162
- FranzeseABorrelliOCorradoGDomperidone is more effective than cisapride in children with diabetic gastroparesisAliment Pharmacol Ther200216595195711966504
- HeckertJParkmanHPTherapeutic response to domperidone in gastroparesis: a prospective study using the GCSI-daily diaryNeurogastroenterol Motil2018301
- HeerMMuller-DuysingWBenesIDiabetic gastroparesis: treatment with domperidone – a double-blind, placebo-controlled trialDigestion19832742142176653921
- HorowitzMHardingPEChattertonBECollinsPJShearmanDJAcute and chronic effects of domperidone on gastric emptying in diabetic autonomic neuropathyDig Dis Sci1985301193965269
- KochKLSternRMStewartWRVaseyMWGastric emptying and gastric myoelectrical activity in patients with diabetic gastroparesis: effect of long-term domperidone treatmentAm J Gastroenterol1989849106910752773901
- KozarekRDomperidone for symptomatic management of diabetic gastroparesis in metoclopramide treatment failuresAdvances in Therapy1990726168
- NaglerJMiskovitzPClinical evaluation of domperidone in the treatment of chronic postprandial idiopathic upper gastrointestinal distressAm J Gastroenterol19817664954997036711
- OrtizACooperCJAlvarezAGomezYSarosiekIMcCallumRWCardiovascular safety profile and clinical experience with high-dose domperidone therapy for nausea and vomitingAm J Med Sci2015349542142425828198
- PattersonDAbellTRothsteinRKochKBarnettJA double-blind multicenter comparison of domperidone and metoclopramide in the treatment of diabetic patients with symptoms of gastroparesisAm J Gastroenterol19999451230123410235199
- ScheyRSaadiMMidaniDRobertsACParupalliRPark-manHPDomperidone to treat symptoms of gastroparesis: benefits and side effects from a large single-center cohortDig Dis Sci201661123545355127530760
- SilversDKipnesMBroadstoneVDomperidone in the management of symptoms of diabetic gastroparesis: efficacy, tolerability, and quality-of-life outcomes in a multicenter controlled trial. DOM-USA-5 Study GroupClin Ther19982034384539663360
- SoykanISarosiekIMcCallumRWThe effect of chronic oral domperidone therapy on gastrointestinal symptoms, gastric emptying, and quality of life in patients with gastroparesisAm J Gastroenterol19979269769809177513
- WattsGFArmitageMSinclairJHillRDTreatment of diabetic gastroparesis with oral domperidoneDiabet Med1985264914922951124
- SugumarASinghAPasrichaPJA systematic review of the efficacy of domperidone for the treatment of diabetic gastroparesisClin Gastroenterol Hepatol20086772673318524689
- De ColleCvan der HartMChenJRassoulpourAPasrichaPJ1079 NG101: a potent and selective dopamine D2 receptor antagonist as a potential alternative to metoclopramide and domperidone for the treatment of gastroparesisGastroenterology20161504S214
- FarrugiaGMacielagMJPeetersTLSarrMGGaldesASzurszewskiJHMotilin and OHM-11526 activate a calcium current in human and canine jejunal circular smooth muscleAm J Physiol19972732 Pt 1G404G4129277420
- MagantiKOnyemereKJonesMPOral erythromycin and symptomatic relief of gastroparesis: a systematic reviewAm J Gastroenterol200398225926312591038
- ParkmanHPPaganoAPVozzelliMARyanJPGastrokinetic effects of erythromycin: myogenic and neurogenic mechanisms of action in rabbit stomachAm J Physiol19952693 Pt 1G418G4267573453
- RichardsRDDavenportKMcCallumRWThe treatment of idiopathic and diabetic gastroparesis with acute intravenous and chronic oral erythromycinAm J Gastroenterol19938822032078424421
- TalleyNJVerlindenMGeenenDJEffects of a motilin receptor agonist (ABT-229) on upper gastrointestinal symptoms in type 1 diabetes mellitus: a randomised, double blind, placebo controlled trialGut200149339540111511562
- DhirRRichterJEErythromycin in the short- and long-term control of dyspepsia symptoms in patients with gastroparesisJ Clin Gastroenterol200438323724215128069
- ChiniPToskesPPWaseemSHouWMcDonaldRMoshireeBEffect of azithromycin on small bowel motility in patients with gastrointestinal dysmotilityScand J Gastroenterol201247442242722364597
- MoshireeBMcDonaldRHouWToskesPPComparison of the effect of azithromycin versus erythromycin on antroduodenal pressure profiles of patients with chronic functional gastrointestinal pain and gastroparesisDig Dis Sci201055367568319924535
- PotterTGSniderKRAzithromycin for the treatment of gastroparesisAnn Pharmacother201347341141523447477
- JanssenPHarrisMSJonesMThe relation between symptom improvement and gastric emptying in the treatment of diabetic and idiopathic gastroparesisAm J Gastroenterol201310891382139124005344
- ColldenGTschopMHMullerTDTherapeutic potential of targeting the ghrelin pathwayInt J Mol Sci2017184E79828398233
- ShinACamilleriMBusciglioIThe ghrelin agonist RM-131 accelerates gastric emptying of solids and reduces symptoms in patients with type 1 diabetes mellitusClin Gastroenterol Hepatol2013111114531459.e5423639598
- ShinACamilleriMBusciglioIRandomized controlled phase Ib study of ghrelin agonist, RM-131, in type 2 diabetic women with delayed gastric emptying: pharmacokinetics and pharmacodynamicsDiabetes Care2013361414822961573
- CamilleriMMcCallumRWTackJSpenceSCGottesdienerKFiedorekFTEfficacy and safety of relamorelin in diabetics with symptoms of gastroparesis: a randomized, placebo-controlled studyGastroenterology2017153512401250.e228760384
- LemboACamilleriMMcCallumRRelamorelin reduces vomiting frequency and severity and accelerates gastric emptying in adults with diabetic gastroparesisGastroenterology201615118796.e627055601
- BourasEPCamilleriMBurtonDDMcKinzieSSelective stimulation of colonic transit by the benzofuran 5HT4 agonist, prucalopride, in healthy humansGut199944568268610205205
- De SchryverAMAndriesseGISamsomMSmoutAJGooszenHGAkkermansLMThe effects of the specific 5HT(4) receptor agonist, prucalopride, on colonic motility in healthy volunteersAliment Pharmacol Ther200216360361211876716
- KessingBFSmoutAJBenninkRJKraaijpoelNOorsJMBrede-noordAJPrucalopride decreases esophageal acid exposure and accelerates gastric emptying in healthy subjectsNeurogastroenterol Motil20142681079108624891067
- BradenBEnghoferMSchaubMUsadelKHCasparyWFLembckeBLong-term cisapride treatment improves diabetic gastroparesis but not glycaemic controlAliment Pharmacol Ther20021671341134612144585
- DegenLPetrigCStuderDSchrollerSBeglingerCEffect of tegaserod on gut transit in male and female subjectsNeurogastroenterol Motil200517682182616336497
- TackJCamilleriMChangLSystematic review: cardiovascular safety profile of 5-HT(4) agonists developed for gastrointestinal disordersAliment Pharmacol Ther201235774576722356640
- CarboneFRotondoAAndrewsCOP054-LB1A controlled cross-over trial shows benefit of prucalopride for symptom control and gastric emptying in gastroparesisUEG Suppl Abstracts201536561571
- AhnABarnesCShaywitzDGrimaldiMCanafaxDMVelusetrag improves gastric emptying time in subjects with diabetic or idiopathic gastroparesisGastroenterology20151484S507
- SmithJABeattieDTMarquessDShawJPVickeryRGHumphreyPPThe in vitro pharmacological profile of TD-5108, a selective 5-HT(4) receptor agonist with high intrinsic activityNaunyn Schmiedebergs Arch Pharmacol2008378112513718415081
- EisenmanSTGibbonsSJVerhulstPJCiprianiGSaurDFarrugiaGTumor necrosis factor alpha derived from classically activated “M1”macrophages reduces interstitial cell of Cajal numbersNeurogastroenterol Motil2017294
- BharuchaAEDaleySLLowPAEffects of hemin on heme oxygenase-1, gastric emptying, and symptoms in diabetic gastroparesisNeurogastroenterol Motil201628111731174027283929
- LeelakanokNHolcombeASchweizerMLDomperidone and risk of ventricular arrhythmia and cardiac death: a systematic review and meta-analysisClin Drug Investig201636297107
- BaguleyWAHayWTMackieKPCheneyFWCullenBFCardiac dysrhythmias associated with the intravenous administration of ondansetron and metoclopramideAnesth Analg1997846138013819174325
- DigbyGMachaalanyJMalikPMultifactorial QT interval prolongationCardiol J201017218418820544619
- DigbyGCPerez RieraARBarbosa BarrosRAcquired long QT interval: a case series of multifactorial QT prolongationClin Cardiol201134957758221887689
- HaslerWLParkmanHPWilsonLAPsychological dysfunction is associated with symptom severity but not disease etiology or degree of gastric retention in patients with gastroparesisAm J Gastroenterol2010105112357236720588262
- YoussefASParkmanHPNagarSDrug-drug interactions in pharmacologic management of gastroparesisNeurogastroenterol Motil201527111528154126059917
- StoetzerCVoelkerMDollTHeinekeJWegnerFLefflerACardiotoxic antiemetics metoclopramide and domperidone block cardiac voltage-gated Na+ channelsAnesth Analg20171241526027861438
- RossiMGiorgiGDomperidone and long QT syndromeCurr Drug Saf20105325726220394569
- BashashatiMSarosiekISiddiquiTMcCallumRWAdverse effects of domperidone: prolonged QuesT for knowledge?Dig Dis Sci201661123384338627714509
- HallandMBharuchaAERelationship between control of glycemia and gastric emptying disturbances in diabetes mellitusClin Gastroenterol Hepatol201614792993626717862
- JonesKLRussoABerryMKStevensJEWishartJMHorowitzMA longitudinal study of gastric emptying and upper gastrointestinal symptoms in patients with diabetes mellitusAm J Med2002113644945512427492
- HaslerWLSoudahHCDulaiGOwyangCMediation of hyperglycemia-evoked gastric slow-wave dysrhythmias by endogenous prostaglandinsGastroenterology199510837277367875475
- SamsomMAkkermansLMJebbinkRJvan IsseltHvanBerge-HenegouwenGPSmoutAJGastrointestinal motor mechanisms in hyperglycaemia induced delayed gastric emptying in type I diabetes mellitusGut19974056416469203944
- Oster-JorgensenEQvistNPedersenSARasmussenLHovendalCPThe influence of induced hyperglycaemia on the characteristics of intestinal motility and bile kinetics in healthy menScand J Gastroenterol19922742852881589706
- HorowitzMJonesKLRaynerCKReadNW‘Gastric’ hypoglycaemia – an important concept in diabetes managementNeurogastroenterol Motil200618640540716700718
- LysyJIsraeliEStrauss-LiviatanNGoldinERelationships between hypoglycaemia and gastric emptying abnormalities in insulin-treated diabetic patientsNeurogastroenterol Motil200618643344016700722
- HyettBMartinezFJGillBMDelayed radionucleotide gastric emptying studies predict morbidity in diabetics with symptoms of gastroparesisGastroenterology2009137244545219410575
- ChangJRaynerCKJonesKLHorowitzMPrognosis of diabetic gastroparesis – a 25-year evaluationDiabet Med2013305e185e18823350946
- OlaussonEAStorsrudSGrundinHIsakssonMAttvallSSimrenMA small particle size diet reduces upper gastrointestinal symptoms in patients with diabetic gastroparesis: a randomized controlled trialAm J Gastroenterol2014109337538524419482
- HomkoCJDuffyFFriedenbergFKBodenGParkmanHPEffect of dietary fat and food consistency on gastroparesis symptoms in patients with gastroparesisNeurogastroenterol Motil201527450150825600163
- YuKKeMYLiWHZhangSQFangXCThe impact of soluble dietary fibre on gastric emptying, postprandial blood glucose and insulin in patients with type 2 diabetesAsia Pac J Clin Nutr201423221021824901089
- PouderouxPFriedmanNShiraziPRingelsteinJGKeshavarzianAEffect of carbonated water on gastric emptying and intragastric meal distributionDig Dis Sci199742134399009113
- ParkmanHPYatesKPHaslerWLDietary intake and nutritional deficiencies in patients with diabetic or idiopathic gastroparesisGastroenterology20111412486498498e1e721684286
- CamilleriMParkmanHPShafiMAAbellTLGersonLAmerican College of GastroenterologyClinical guideline: management of gastroparesisAm J Gastroenterol201310811837 quiz 3823147521
- FontanaRJBarnettJLJejunostomy tube placement in refractory diabetic gastroparesis: a retrospective reviewAm J Gastroenterol19969110217421788855743
- MapleJTPetersenBTBaronTHGostoutCJWong Kee SongLMButtarNSDirect percutaneous endoscopic jejunostomy: outcomes in 307 consecutive attemptsAm J Gastroenterol2005100122681268816393220
- FanACBaronTHRumallaAHarewoodGCComparison of direct percutaneous endoscopic jejunostomy and PEG with jejunal extensionGastrointest Endosc200256689089412447304
- UdorahMOFleischmanMWBalaVCaiQEndoscopic clips prevent displacement of intestinal feeding tubes: a long-term follow-up studyDig Dis Sci201055237137419242799
- SamarasenaJBKwakNHChangKJLeeJGThe PEG-Pedi-PEG technique: a novel method for percutaneous endoscopic gastrojejunostomy tube placement (with video)Gastrointest Endosc20168461030103327329090
- TeiglandTIversenMMSangnesDADimcevskiGSoftelandEA longitudinal study on patients with diabetes and symptoms of gastroparesis – associations with impaired quality of life and increased depressive and anxiety symptomsJ Diabetes Complications2018321899429153755
- PasrichaPJYatesKPNguyenLOutcomes and factors associated with reduced symptoms in patients with gastroparesisGastroenterology2015149717621774.e426299414
- CherianDSachdevaPFisherRSParkmanHPAbdominal pain is a frequent symptom of gastroparesisClin Gastroenterol Hepatol20108867668120472097
- HaslerWLWilsonLAParkmanHPFactors related to abdominal pain in gastroparesis: contrast to patients with predominant nausea and vomitingNeurogastroenterol Motil2013255427438e300e30123414452
- HoogerwerfWAPasrichaPJKallooANSchusterMMPain: the overlooked symptom in gastroparesisAm J Gastroenterol19999441029103310201478
- BoelensOBScheltingaMRHoutermanSRoumenRMRandomized clinical trial of trigger point infiltration with lidocaine to diagnose anterior cutaneous nerve entrapment syndromeBr J Surg2013100221722123180371
- JehangirAParkmanHPChronic opioids in gastroparesis: relationship with gastrointestinal symptoms, healthcare utilization and employmentWorld J Gastroenterol201723407310732029142478
- SzigethyEKniselyMDrossmanDOpioid misuse in gastroenterology and non-opioid management of abdominal painNat Rev Gastroenterol Hepatol201815316818029139482
- JeongIDCamilleriMShinAA randomised, placebo-controlled trial comparing the effects of tapentadol and oxycodone on gastrointestinal and colonic transit in healthy humansAliment Pharmacol Ther20123591088109622348605
- UklejaATandonKShahKAlvarezAEndoscopic botox injections in therapy of refractory gastroparesisWorld J Gastrointest Endosc20157879079826191343
- YinGTanWHuDEndoscopic ultrasonography-guided intrapyloric injection of botulinum toxin to treat diabetic gastroparesisDig Endosc201628775927556873
- GuoHFangCHuangYTreatment of diabetic gastroparesis with botulinum toxin injection guided by endoscopic ultrasound in a patient with type 1 diabetes: the first reportActa Diabetol201754550951127696071
- KhashabMABesharatiSNgamruengphongSRefractory gastroparesis can be successfully managed with endoscopic transpyloric stent placement and fixation (with video)Gastrointest Endosc20158261106110926253017
- MarankiJLLytesVMeilahnJEPredictive factors for clinical improvement with Enterra gastric electric stimulation treatment for refractory gastroparesisDig Dis Sci20085382072207818080765
- ChuHLinZZhongLMcCallumRWHouXTreatment of high-frequency gastric electrical stimulation for gastroparesisJ Gastroenterol Hepatol20122761017102622128901
- HeckertJSankineniAHughesWBHarbisonSParkmanHGastric electric stimulation for refractory gastroparesis: a prospective analysis of 151 patients at a single centerDig Dis Sci201661116817526280084
- McCallumRWLinZForsterJRoeserKHouQSarosiekIGastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 yearsClin Gastroenterol Hepatol201194314319.e121185396
- SarosiekIForsterJLinZCherrySSarosiekJMcCallumRThe addition of pyloroplasty as a new surgical approach to enhance effectiveness of gastric electrical stimulation therapy in patients with gastroparesisNeurogastroenterol Motil2013252e134e180
- DavisBRSarosiekIBashashatiMAlvaradoBMcCallumRWThe long-term efficacy and safety of pyloroplasty combined with gastric electrical stimulation therapy in gastroparesisJ Gastrointest Surg201721222222727896652
- ShadaALDunstCMPescarusRLaparoscopic pyloroplasty is a safe and effective first-line surgical therapy for refractory gastroparesisSurg Endosc20163041326133226293794
- RodriguezJHHaskinsINStrongATPer oral endoscopic pyloromyotomy for refractory gastroparesis: initial results from a single institutionSurg Endosc201731125381538828567693
- GonzalezJMBenezechAVittonVBarthetMG-POEM with antropyloromyotomy for the treatment of refractory gastroparesis: mid-term follow-up and factors predicting outcomeAliment Pharmacol Ther201746336437028504312
- KhashabMANgamruengphongSCarr-LockeDGastric peroral endoscopic myotomy for refractory gastroparesis: results from the first multicenter study on endoscopic pyloromyotomy (with video)Gastrointest Endosc201785112312827354102
- SnapeWJLinMSAgarwalNShawREEvaluation of the pylorus with concurrent intraluminal pressure and EndoFLIP in patients with nausea and vomitingNeurogastroenterol Motil201628575876426813266
- OwyangCPhenotypic switching in diabetic gastroparesis: mechanism directs therapyGastroenterology201114141134113721875587
- GonzalezJMLestelleVBenezechAGastric peroral endoscopic myotomy with antropyloromyotomy in the treatment of refractory gastroparesis: clinical experience with follow-up and scintigraphic evaluation (with video)Gastrointest Endosc201785113213927478028
