Abstract
Background:
Chronic constipation is an important clinical condition which can result in serious discomfort and even require hospitalization. Powder and liquid lactulose are designated as clinically equivalent for the treatment of constipation, but there are significant differences in the taste, consistency, and portability of the products, which may affect patient compliance and therefore clinical outcome.
Aim:
To evaluate patient preference between powder and liquid lactulose in terms of overall preference, taste, consistency, and portability, and safety in terms of adverse events.
Methods:
Three sites randomized patients (total n = 50) to powder or liquid lactulose for seven days with crossover. Patient preference was assessed by a questionnaire, and the occurrence of adverse events was monitored.
Results:
Of those expressing a preference, 44% and 57% more patients preferred the taste and consistency, respectively, of powder over liquid lactulose. More than six times as many patients preferred the portability of powder compared with liquid lactulose and, overall, 77% more patients preferred powder over liquid lactulose. There was no difference between treatment groups in terms of adverse events (P = 0.635).
Conclusions:
More patients preferred powder compared with liquid lactulose and the products were equally safe. These findings may impact patient compliance, and therefore may affect clinical outcome.
Keywords:
Introduction
Chronic constipation is an important clinical condition which can result in serious discomfort and decreased quality of life, and can even require hospitalization.Citation1,Citation2 Chronic constipation affects an estimated 15% of the North American population and has a wide range of underlying causes, from dehydration, to opioid use, to medical conditions such as endocrine, gastrointestinal, and neurologic disorders.Citation2 Many pharmacologic, both over-the-counter and prescription, and herbal laxatives are available for the treatment of chronic constipation.Citation2 However, a recent systematic review of the literature found that only lactulose and polyethylene glycol consistently and repeatedly loosened stools and thereby relieved constipation.Citation3 Therefore, a prescription osmotic laxative like lactulose is a common therapeutic option for the treatment of chronic constipation.Citation2,Citation4
Lactulose is available in a dry, powder form (Kristalose®, lactose for oral solution) to be dissolved in water and a liquid/syrup form. While the products are designated as clinically equivalent, there are notable differences in the taste, consistency, and portability between the products. We hypothesized that these differences could result in a difference in patient preference between powder and liquid lactulose. Increased patient preference can correlate with increased patient compliance. It is well known that decreased patient compliance results in poor patient outcomes.Citation5,Citation6 Additionally, a significant number of patients with hepatic encephalopathy require daily treatment with large amounts of liquid lactulose (more than 50 mL per day) and these patients are often noncompliant because they are unable to ingest and/or keep down this large amount of lactulose syrup due to its taste and consistency.Citation7–Citation9 Therefore, we designed and conducted a study to determine whether patients prefer powder or liquid lactulose in terms of overall preference, taste, consistency, and portability. The safety of the products was also assessed.
Methods
Patients
This clinical trial was a prospective, randomized, open-label, multicenter, seven-day, crossover study. Patients seen at the outpatient clinics of Wake Research Associates (Raleigh, NC, USA), Rapid Medical Research (Cleveland, OH, USA), and Arya Gastroenterology (Brooklyn, NY, USA) with a recent diagnosis of chronic constipation (within the last 90 days) were eligible for enrollment in this study. Patients provided written informed consent before enrollment, and the study protocol was approved by the Western Institutional Review Board and the study was registered on ClinicalTrials.gov (NCT00712543). Inclusion criteria included a recent diagnosis of chronic constipation (within the last 90 days). Exclusion criteria included patients with galactosemia (galactose-sensitive diet), patients younger than 18 years of age, patients currently on lactulose therapy, and patients unable to understand the requirements of the study or unwilling to provide written informed consent and agreement to abide by the study restrictions.
Study randomization, design, and medications
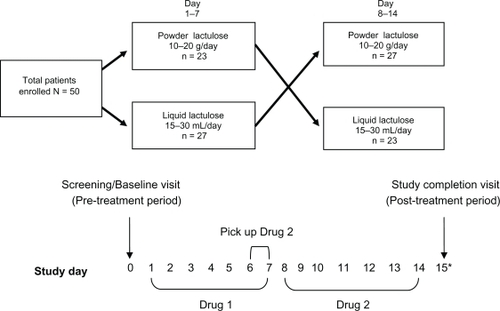
Patients were randomized in a 1:1 ratio to receive powder or liquid lactulose first for seven days (dose determined by treating physician, range for powder = 10–20 g/day, range for liquid = 15–30 mL/day), and then the patients crossed over to the alternative treatment for the following seven days (dose again determined by treating physician, ). Patients returned to the study site on study day 6 or 7 to pick up the formulation of lactulose which they were scheduled to cross over to for the remainder of the study (). Sealed envelopes containing the patient randomization scheme were provided to the study sites. Powder lactulose (Kristalose®, Cumberland Pharmaceuticals Inc., Nashville, TN, USA) was provided in 10 g pouches and liquid lactulose syrup (Generlac, Morton Grove Pharmaceuticals Inc., Morton Grove, IL, USA) was provided in a 473 mL (pint) bottle. Patient preference was assessed on a questionnaire administered by study staffin the days following completion of the study. Both treatment groups received both drugs in a crossover design (drug sequence determined by randomization), and then preference was evaluated after patients had been exposed to both treatments in this prospective study. The study sponsor developed the patient questionnaire and two versions were developed and utilized equally to avoid bias. One version of the questionnaire listed powder first as an answer selection for all of the questions, while the other listed liquid first as an answer selection for all of the questions. Each question on the questionnaire had three possible answers, ie, liquid, powder, or no preference. Spanish translations of the questionnaire and other study documents were made available at the request of the Brooklyn, NY site. Upon completion of the study, patients were allowed a seven-day window (total study duration including questionnaire visit = 21 days) to return to the study site and complete the questionnaire, and adverse events were monitored throughout the entire study period ().
Study objectives
The primary objective of this study was to determine overall patient preference for the powder or liquid form of lactulose. Secondary objectives included determination of patient preference for the powder or liquid form of lactulose in terms of taste, consistency, and portability, as well as safety in terms of the incidence of adverse events.
Statistical analysis
The responses on the questionnaire were summarized for each treatment sequence and then combined for an overall assessment. For each question, the Mainland–Gart test was used to test whether subjects preferred one product over the other product. The Mainland–Gart test excludes patients who show “no preference” (P value 1, ).Citation10,Citation11 A second analysis method was performed for each question on the questionnaire using the Prescott test to determine whether subjects preferred one product over the other product.Citation12 The Prescott test includes patients who show “no preference” (P value 2, ).Citation12 The Mainland–Gart test and the Prescott test were performed side by side to assess the robustness of the data, more specifically in terms of how the inclusion of the subjects who had “no preference” affected the inference regarding which drug is preferred. Adverse events were summarized based on the treatment (powder or liquid lactulose) the subject was taking at the time of the onset of the event (). Fisher’s Exact test was used to test whether there was a significant difference in the number of adverse events between the two treatment groups ().
Table 2 Patient preference data in terms of overall preference and preference of taste, consistency, and portability
Table 3 Adverse events experienced by patients while taking liquid or powder lactulose
Results
Patient characteristics
A total of 50 patients with a recent diagnosis of chronic constipation were enrolled in this study. Twenty-three of the 50 patients received powder lactulose for the first seven days followed by liquid lactulose on the following seven days, while 27 of the 50 patients first received liquid lactulose for seven days followed by powder lactulose on the following seven days. Two of the patients failed to return to the study site to complete the questionnaire, but limited safety data were still available for these patients. The study group demographics are shown in . The mean (standard deviation, SD) age of the patients was 49 (15) years, with a female-to-male ratio of 2.85 to 1 (). The majority of the patients were African-American, with Caucasians, Hispanics, and Asians making up smaller portions of the study population (). The mean height of the study population was 167 (10.4) cm and the mean weight was 87.4 (21.0) kg ().
Table 1 Patient demographics
Of the 50 patients enrolled in the study, seven protocol deviations were recorded in six (12%) patients. Protocol deviations included patients who received the study drug outside the treatment window or patients who made a clinical visit on the incorrect day.
Preference data
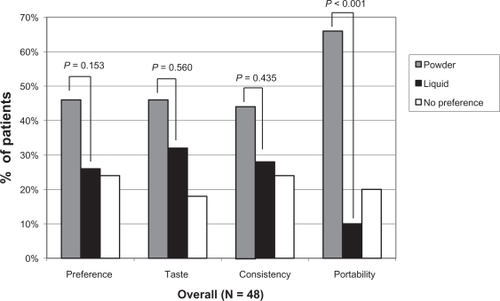
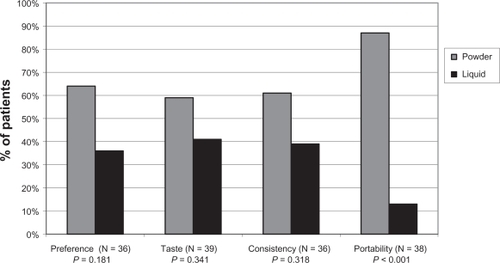
Of the 48 patients for whom preference data were available, no significant difference in terms of overall preference, taste, or consistency between powder and liquid lactulose was found, likely due to the small sample size ( and ). However, significantly more patients preferred powder lactulose in terms of portability (P < 0.001, and ). In addition, of those patients expressing a preference, 23/39 (59%) and 22/36 (61%) patients preferred the taste and consistency of powder over liquid lactulose, respectively, and overall 23/36 (64%) patients preferred powder over liquid lactulose (not significant, and ). A comparison of these patient ratios revealed that of those patients expressing a preference, 44% and 57% more patients preferred the taste and consistency of powder over liquid lactulose, respectively, and overall 77% more patients preferred powder over liquid lactulose. In addition, more than six times as many patients whom expressed a preference preferred the portability of powder over liquid lactulose (P < 0.001, and ).
Safety data
Adverse events were of mild to moderate intensity, and ranged from gastrointestinal disorders, such as flatulence and diarrhea, to muscle spasms and pharyngolaryngeal pain. There were no statistically significant differences between treatment groups in terms of adverse event occurrence, given that 13 (26%) patients experienced 21 adverse events while taking powder lactulose and 10 (20%) patients experienced 18 adverse events while taking liquid lactulose (P = 0.635, for the number of patients experiencing one or more adverse events, ). Additionally, there were no significant differences between treatment groups for any adverse event subtype ().
Discussion
The main objective of this study was to determine whether patients prefer to take powder or liquid lactulose. Although the study was somewhat underpowered, the data showed a numeric difference in favor of powder lactulose being preferred considerably more than liquid lactulose in terms of overall preference and preference of taste and consistency. In addition, significantly more patients preferred the portability of powder lactulose compared with liquid lactulose. Powder and liquid lactulose were also found to be equally safe, because there was not a significant difference in the incidence of adverse events between treatment groups.
This study had several limitations. As was already mentioned, the study was underpowered due to the small sample size and due to three possible outcomes on the preference questionnaire (preference of powder, preference of liquid, or no preference). In addition, the study was limited geographically to the eastern US, and a broader involvement of sites may have affected the outcome of the study. The study may have also been too short in duration, as a longer dosing regimen may have altered the outcome of the study. Patients may have also had a pre-existing preference for one formulation or the other based on being previously treated with either or both of the products, but this was not factored into the study. Lastly, this study did not use standardized dosing, because the study doses were instead determined by the treating physician. For example, one patient may have been prescribed 20 g of powder lactulose per day compared with 15 mL of liquid lactulose (10 g powder lactulose = 15 mL liquid lactulose), which may have affected patient preference.
Overall, more patients with chronic constipation preferred powder lactulose compared with liquid lactulose and the products were equally safe. Most importantly, these findings may impact patient compliance, and therefore may effect clinical outcome and thereby determine whether additional medical attention may be required.
Disclosure
This study was funded in full by Cumberland Pharmaceuticals Inc. BV and BK are employees of Cumberland Pharmaceuticals Inc. CFB has no financial interests regarding this work.
References
- JohansonJFKralsteinJChronic constipation: A survey of the patient perspectiveAliment Pharmacol Ther200725559960817305761
- GallagherPO’MahonyDConstipation in old ageBest Pract Res Clin Gastroenterol200923687588719942165
- American College of Gastroenterology Chronic Constipation Task ForceAn evidence-based approach to the management of chronic constipation in North AmericaAm J Gastroenterol2005100Suppl 1S1S416008640
- PetticrewMRodgersMBoothAEffectiveness of laxatives in adultsQual Health Care200110426827311743157
- SwartzMLCitrucel (methylcellulose/bulk-forming laxative)Gastroenterol Nurs198912150522487811
- Loening-BauckeVPolyethylene glycol without electrolytes for children with constipation and encopresisJ Pediatr Gastroenterol Nutr200234437237711930092
- LeevyCBPhillipsJAHospitalizations during the use of rifaximin versus lactulose for the treatment of hepatic encephalopathyDig Dis Sci200752373774117245628
- SundaramVShaikhOSHepatic encephalopathy: Pathophysiology and emerging therapiesMed Clin North Am2009934819836vii19577116
- Generlac (lactulose for oral solution) [Product labeling]Morton Grove, ILMorton Grove Pharmaceuticals Inc2004
- MainlandDElementary Medical StatisticsPhiladelphia, PAWB Saunders1963
- GartJJAn exact test for comparing matched proportions in cross-over designsBiometrika19695617580
- PrescottRJThe comparison of success rates in cross-over trials in the presence of an order effectAppl Stat1981301915