Abstract
Purpose:
Biliary atresia (BA) is the most common indication of liver transplantation in children. Pathogenesis of hepatic fibrosis, which is a prominent feature of BA, remains obscure. The purpose of this work was to determine the cellular sources of transforming growth factor beta-1 (TGFβ1) and establish the relationship between TGFβ1-producing cells and extracellular matrix producing myofibroblasts (MFBs) in advanced BA.
Methods:
Trichrome staining and immunohistochemistry were carried out to determine the expression pattern of collagen and TGFβ1 protein in explant liver specimens from patients with BA. The intensities of portal and lobular TGFβ1 expressions were compared. Immunofluorescence technique was carried out to determine the relationship between α-smooth muscle actin (α-SMA)-positive-MFB and TGFβ1-positve cells.
Results:
Lobular TGFβ1 protein expression was significantly higher than portal (89 ± 6 versus 10 ± 1 arbitrary units, P ≤ 0.05), whereas no difference was noted in livers used as control (10 ± 1.6 versus 19 ± 5 arbitrary units, P = 0.11). TGFβ1 expression was more in the center of nodules versus MFB in surrounding fibrous septa. Contrary to TGFβ1 expression, α1-SMA was mostly expressed in the portal structures and the adjacent fibrous septa enacting lobulation of the parenchyma. The results obtained by coimmunofluorescence staining showed no colocalization of α-SMA and TGFβ1.
Conclusions:
TGFβ1 protein expression is mostly localized to hepatocytes in advanced BA. These findings suggest a paracrine mechanisms of TGFβ1-driven fibrogenesis in advanced BA.
Introduction
Untreated biliary atresia (BA) is universally fatal, mandating timely diagnosis and expedited portoenterostomy operation. This procedure, despite its appeal at the time of initial diagnosis, permits long-term survival in only about 20% of the affected infants. Liver transplantation (LT) becomes necessary for the long-term survival of most of the affected children due to the complications of cirrhosis, such as portal hypertension and poor growth. Although the exact pathogenesis of BA remains elusive, it is generally agreed that BA phenotype can result from several distinct mechanisms of liver injury, provoking a stereotypic response comprising inflammation, bile duct proliferation, apoptosis, and fibrogenesis.Citation1 In this context, it is not surprising that the extent of liver fibrosis is the most important predictor of the outcome of portoenterostomy procedure.Citation2–Citation4 However, despite its prime significance, the assessment and management of liver fibrosis have not yet been incorporated in routine postportoenterostomy patient care, mostly due to poor understanding of the underlying mechanisms of progressive liver fibrosis. Conversely, significant progress has been made in understanding the pathogenesis of liver fibrosis due to diseases afflicting adult populations. As such, contrary to conventional dogma, liver fibrosis is now considered to be a reversible pathologic event, provided that underlying mechanisms are addressed in a timely manner.Citation5–Citation8 Similarly, a consensus has emerged for the pivotal role of hepatic stellate cells (HSCs) in liver fibrosis.Citation9–Citation12
Transforming growth factor β-1 (TGFβ1) is the master fibrogenic cytokine that is well known to be involved in liver fibrosis in a variety of liver diseases.Citation13–Citation15 Through its receptors on myofibroblasts (MFBs) and other cells, TGFβ1 orchestrates key fibrogenic events, most notably expansion of the MFB pool, induction of extracellular matrix proteins, and inhibition of matrix metalloproteinases.Citation16–Citation20 However, disease-specific mechanisms driving TGFβ1 upregulation are not well understood. Given that downstream fibrogenic actions of TGFβ1 are relatively well understood,Citation10,Citation21 elucidation of its cellular sources is a critical initial step not only for understanding possible triggers but also to delineate the march of molecular events linking ongoing liver injury to fibrosis. For example, as with any other chronic liver disease, it is quite conceivable that the cellular sources of TGFβ1 will vary according to the triggers determined by site, underlying mechanisms, and the extent of liver injury.
Reconstruction of the topology of events linking injury to repair (fibrosis) therefore requires successive examinations, which is an enterprise that is hampered by ethical restrictions on liver biopsies after portoenterostomy, thus limiting the scope of fibrosis assessment to explant specimens only. In this work, using explant specimens, we sought to determine whether TGFβ1 is predominantly produced in portal and periportal structures, which are the main sites of liver injury or hepatic lobular cells, situated distant from the site of injury, thereby indicating paracrine mechanisms of TGFβ1-driven fibrogenesis in advanced BA.
Materials and methods
To accomplish our goal, using explant specimens, we carried out trichrome staining and immunohistochemistry to determine the hepatic expressions of collagen and TGFβ1 proteins. To determine whether TGFβ1 is expressed by collagen-producing MFB, immunofluorescence was performed to determine colocalization of TGFβ1 and α1 smooth muscle actin (α-SMA). α-SMA is commonly used as a marker of collagen-producing MFB, the main fibrogenic cell type with diverse origins, including HSCs.
Patient population and liver samples
After approval from the Institutional Review Board of the University of Florida (UF), FL, USA, patients with BA who required LT were identified from the UF liver transplant database. To determine the expression of markers of fibrosis in BA, liver sections were obtained from explant specimens archived from LT done between the years 2000 and 2008 (N = 30; 16 males, 14 females). The mean age at the time of LT was 1.8 ± 0.369 years (range 2–17 years). Liver sections from archived sections of living related liver donors were used as controls. The alternative approach, using margins from hepatoblastoma resections as controls, was not pursued because our preliminary work indicated extensive and inconsistent expression of fibrogenic cytokines in these ‘healthy margins’. Therefore, albeit age-related limitations, healthy human liver sections were used as controls in this study. All biopsied specimens were 4 μm thick, fixed in phosphate-buffered formalin, and embedded in paraffin. Serial adjacent sections were used for hematoxylin and eosin staining, trichrome staining, immunohistochemistry, and immunofluorescence.
Expression and distribution of collagen
Excessive collagen deposition in the extracellular matrix is a hallmark of liver fibrosis. Collagen distribution was determined by trichrome staining using a kit from ScyTek Laboratories (TGG500, West Logan, UT, USA) according to the directions of the manufacturer. The stage of liver fibrosis was determined in a blinded fashion by using trichrome-stained slides according to the METAVIR scoring system.Citation22 We used the METAVIR system because, through extensive intraobserver variation studies, this system was designed to be simple, consistent, and reproducible.Citation23 Similarly, this system has been applied to a variety of chronic liver diseases,Citation24 including biliary disorders such as primary biliary cirrhosisCitation25 and primary sclerosing cholangitis.Citation26 Moreover, it is noteworthy that the staging components of the most commonly employed scoring systems, especially when applied to advanced liver disease, yield similar results.
TGFβ1 immunohistochemistry
After rehydration, 4-μm paraffin-embedded sections on glass slides were incubated in 3% hydrogen peroxide to block the endogenous peroxidase activity. Sections were then blocked by incubation in 3% bovine serum albumin in phosphate-buffered saline (PBS). A monoclonal mouse antihuman TGFβ1 antibody (Abcam 27969, Cambridge, MA, USA) that reacts to both monomeric and dimeric forms of TGFβ1 was applied at a dilution of 1:500 (diluted in 3% bovine serum albumin) and incubated at 4°C overnight. After a 5-min wash in PBS, the slides were treated with goat antimouse IgG horseradish peroxidase-labeled secondary antibodies (Bio-Rad, Cat#17065, Hercules, CA, USA) at a dilution of 1:500 (diluted in 3% bovine serum albumin) for 30 min at room temperature. Bound antibodies were detected with an ABC kit (Vector Laboratories, Burlingame, CA, USA). The slides were stained with diaminobenzidine, washed, counterstained with Mayer’s hematoxylin, dehydrated, treated with xylene, and mounted. As a control to immunostaining, separate sections from both BA and normal human liver tissues were incubated with and without primary and appropriate isotyped antibodies.
Intensity of TGFβ1 immunostaining
The intensity of the TGFβ1 immunostaining was determined by using ImageJ software (http://rsb.info.nih.gov/ij). From each slide, five random portal and lobular areas were analyzed with a color histogram using a color deconvolution plug-in routinely used for diaminobenzidine staining.Citation27
Immunofluorescence
After rehydration, 4-μm sections of paraffin-embedded tissue on glass slides were blocked by incubation in 4% bovine serum albumin in PBS. A monoclonal TGFβ1 mouse antihuman antibody (Abcam 27969) and a polyclonal rabbit anti-α-SMA (Abcam 5694) were applied to the slides at the dilution of 1:100 (diluted in 4% bovine serum albumin) and incubated at 4°C overnight. After being washed for 5 min in PBS, the slides were treated with fluorescently labeled secondary antibodies at a dilution of 1:500 (diluted in 4% bovine serum albumin) for 45 min at room temperature. Secondary antibodies used included donkey antimouse IgG (Alexa Fluor® 488, Invitrogen A21202, Carlsbad, CA, USA) and donkey antirabbit IgG (Alexa Fluor 594, Invitrogen A21207). Afterward, the tissue sections were washed with PBS and mounted with UltraCruz® medium (sc-24941) containing 4′,6-diamidino-2-phenylindole (DAPI) for nuclear staining. As a control for the technique and nonspecific Fc receptor binding, BA and normal human liver tissue sections were incubated with and without primary or secondary antibody as well as isotyped rabbit (ab27478) and mouse (ab18447) antibodies.
Data analysis
Student t-test was applied by using GraphPad software (La Jolla, CA, USA) to compare the means of color pixel histograms (representing the intensity of TGFβ1 expression) and METAVIR scores. Values are presented as mean ± standard error of the mean. A P value of <0.05 was considered statistically significant.
Results
Expression and distribution of collagen in advanced BA
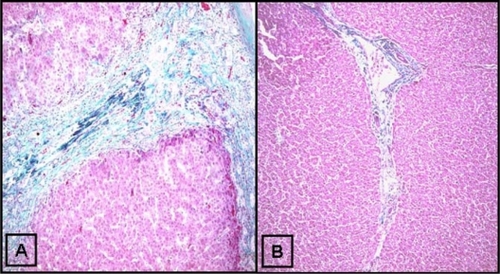
The purpose of trichrome staining was two-fold: 1) to determine the distribution of collagen to serve as a reference for the distribution of TGFβ1 and 2) to determine the extent of fibrosis by the METAVIR scoring system. In this system, F0 represents no fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis and few septa; F3, numerous septa extending to adjacent portal tracts or terminal hepatic venules; and F4, cirrhosis. The mean METAVIR score was 3.8 ± 0.8 (N = 30, minimum 3, maximum 4), consistent with advanced cirrhosis, a typical feature of end-stage liver disease due to BA. A representative trichrome-stained slide is shown in showing thick collagenous bands interspersed between nodular liver tissues, thus producing portal-to-portal bridging fibrosis, a histological pattern typical for biliary cirrhosis. In contrast, healthy control livers () had only minimal collagen staining in portal areas, consistent with noted F0 METAVIR scores.
Figure 1 Trichrome staining for collagen in human biliary atresia. Trichrome staining was done as described in the Materials and methods section. A) Thick bands of collagen septa (green) separating nodular liver tissue. B) Trichrome staining of a healthy control liver (original magnification ×10).
Hepatic distribution of TGFβ1 and α1-SMA in advanced BA
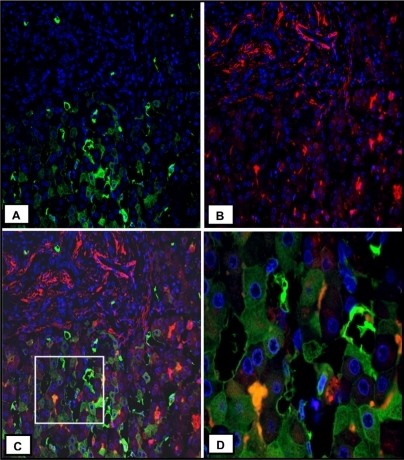
In explant liver specimens, as shown in , heterogeneous overexpression of TGFβ1 is evident in the hepatic parenchyma, more in the center of the liver nodules, although hepatocytes close to collagen in fibrous septa are also noted to express a low level of TGFβ1 protein. Conversely, in livers used as control, the distribution of TGFβ1 is virtually all interstitial and quite homogeneous throughout the hepatic parenchyma (). Contrary to TGFβ1 expression, α-SMA is expressed mostly in the portal structures and the adjacent fibrous septa enacting lobulation of the parenchyma (). As expected, in liver specimens used as control, α-SMA expression is restricted to portal structures (). Consistent with the TGFβ1 expression in hepatic parenchyma, quantitative analysis of TGFβ1 protein content was significantly higher in lobular versus portal areas (89 ± 6 versus 10 ± 1 arbitrary units, P ≤ 0.05) in BA (). In contrast, in livers used as control, lobular versus portal TGFβ1 expression was not significantly different, although a trend of more portal expression was noted (10 ± 1.6 versus 19 ± 5 arbitrary units, P = 0.11; ). These results were in agreement with the coimmunofluorescence staining, which showed no significant localization of α-SMA and TGFβ1 (). Once again, most of the TGFβ1 expression is seen in parenchymal cells (), whereas α-SMA expression is mostly restricted to the area of fibrous septa (). The summary of these findings is that in advanced BA, TGFβ1 is mainly expressed by hepatic parenchymal cells.
Figure 2 Immunohistochemical localization of TGFβ1 protein in human BA. Specific staining of TGFβ1 protein is represented by brown coloration. A) The expression of TGFβ1 in BA, which is mostly in lobular areas. B) TGFβ1 expression in a liver used as control. C) Expression of α1-SMA in BA. D) α1-SMA from a liver used as control (original magnification ×10).
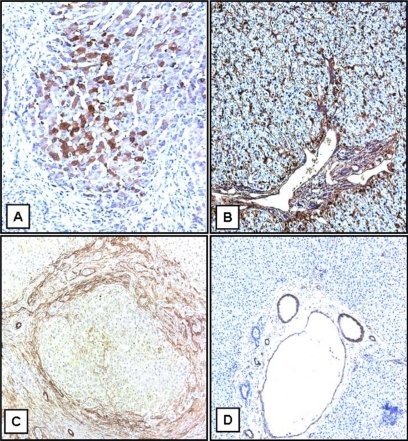
Figure 3 Pattern of hepatic distribution of TGFβ1 protein in human BA. Liver sections were obtained from archived explant specimens from patients who required LT due to BA (N = 9). The control sections were procured from the biopsies of living related liver donors. The expression of TGFβ1 was performed by immunostaining, which was quantified by using ImageJ software. From each slide, five random portal and lobular areas were analyzed. GraphPad software was used to analyze the data using t-test to compare the means of color pixel histograms from portal and lobular areas. A P value ≤ 0.05 was considered significant.
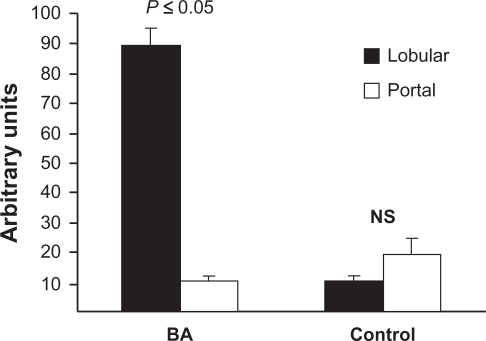
Figure 4 Characterization of hepatic cellular sources of TGFβ1 in human BA. A) Immunofluorescence staining for TGFβ1 (green, original magnification ×20). B) Immunofluorescence staining for α-SMA (red, original magnification ×20). C) Merge image of A and B. D) The original magnification ×60 of the box in C. Heterogeneous expression of TGFβ1 is seen in hepatocytes.
Discussion
The present report documents the novel observation that hepatic TGFβ1 is principally immune-localized to parenchymal cells in advanced liver disease due to BA. Additional novel observations are as follows: 1) TGFβ1 expression is homogenous, interstitial, and quantitatively more portal than lobular in normal human liver; 2) in contrast to normal human liver, heterogeneous lobular expression of TGFβ1 becomes more significant in advanced BA; and 3) more TGFβ1 is expressed in the center of nodules, away from fibrous septa, suggesting the existence of paracrine interactions between parenchymal cells and fibrogenic MFB in advanced cirrhosis due to BA.
This report unveils the significance of parenchymal cells in the injury-repair interactions at advanced stages of BA. In progressive cholestatic disorders such as BA, extracellular matrix deposition typically starts in periportal areas, extends to produce bridging, and ultimately leads to frank cirrhosis. Primary events executing fibrogenesis entail recruitment and proliferation of specialized collagen-producing MFBs. Because of their well-known phenotypic evolution to become MFBs, HSCs are known as prototypic profibrogenic cells in the hepatic parenchyma. Depending on the type of liver injury, other cell types such as resident portal fibroblasts,Citation28 biliary duct cells,Citation29 and even hepatocytesCitation30 can also contribute to the pool of extracellular matrix-producing MFBs. TGFβ1 plays an overarching role orchestrating key fibrogenic events such as MFB recruitment and proliferation as well as upregulation of extracellular matrix protein synthesis. Fundamental questions emerging from the main finding of this work remain: what are the triggers for TGFβ1 expression in hepatocyte; what are the roles of HSC and Kupffer in TGFβ1 expression from hepatocyte; and what are molecular mechanisms of fibrogenic interactions between hepatocytes and other parenchymal and nonparenchymal cells? Given that cholestasis and oxidative stress are the most important pathophysiologic mechanisms of liver injury in BA,Citation23 elucidation of the fibrogenic role of bile acids and oxidative stress will be the next most logical step in the investigation of hepatic overexpression of TGFβ1. Hepatic retention of hydrophobic bile acids due to biliary obstruction plays an important role in the pathogenesis of cholestatic liver injury.Citation31–Citation33 This is exemplified by bile acid-induced liver injury in children with progressive familial intrahepatic cholestasis type 2. In this disorder, homozygous mutations in the gene encoding the canalicular membrane bile salt export pump impair hepatocellular secretion of conjugated bile acids, resulting in elevated hepatic bile acid concentrations and progressive cholestasis, fibrosis, and liver failure.Citation34 Similarly, hydrophobic bile acids are known to cause oxidative stressCitation35 and apoptosis,Citation36 causing perpetual hepatocyte loss and fibrosis.Citation37
TGFβ1 is best known as a fibrogenic cytokine, a role well documented in both human diseases and transgenic mouse models of hepatic TGFβ1 overexpression.Citation38,Citation39 However, further work is required to determine the precise biological or pathological significance of the observations presented in this report. Several biological traits peculiar to TGFβ1 need careful consideration for the appropriate interpretation of these results. First, apart from its well-known profibrogenic actions, TGFβ1 also controls diverse cellular functions such as proliferation, differentiation, adhesion, migration, matrix expression, matrix degradation, and immune surveillance. Determined by the context, a stringent balance of these actions is required for normal tissue homeostasis. Second, although TGFβ1 transcription occurs in many cell types, its translation and secretion appear to be under strict regulation, a phenomenon explaining the well-documented discrepancy between TGFβ1 protein levels and corresponding mRNA expression.Citation40,Citation41 Therefore, in addition to knowledge of pathophysiological context, temporally stacked examinations are necessary to determine which pathological events underpin parenchymal TGFβ1 expression in BA. A major impediment in this regard is the ethical restriction on interval liver biopsies after Kasai operation. Therefore, the interpretation of single point examinations (mRNA or protein-based) such as those adopted in this and earlier reportsCitation42,Citation43 is somewhat limited. Nonetheless, this work provides a framework for the synthesis of testable hypotheses.
For example, although parenchymal cells can produce extracellular matrix proteins,Citation44 the lack of pericellular extracellular matrix in these livers and the presence of characteristic portoportal bridging () strongly support paracrine fibrogenic interactions between hepatocytes and MFBs in advanced BA. Similarly, because cholestasis and oxidative stress are common pathophysiologic mechanisms of liver injury in advanced BA, it is reasonable to postulate that coupled actions of toxic bile acids and mediators of oxidative stress could be the underlying triggers for hepatic upregulation of TGFβ1. Our unpublished data from tissue culture studies support this hypothesis. Similarly, Kupffer cell activation, a frequent source of oxidative stress, is known to amplify the TGFβ1 signal pathway,Citation45 supporting the hypothesis that Kupffer cells may have a role in hepatocyte overexpression of TGFβ1. These hypotheses are the focus of our ongoing investigations. Similarly, questions pending resolution through further research concern the impact of oxidative stress and toxic bile acids on expression of noncollagenous matrix proteins and matrix metalloproteinases; whether noncanonical TGFβ1 signaling pathways intersect with canonical pathway; and, if so, what the pathological and molecular determinants of these intersections are.
Acknowledgements
This work was supported by funds from the Children’s Miracle Network. Publication of this article was funded in part by the University of Florida Open-Access Publishing Fund. The authors thank Dr Bryon E Petersen for his expert input in the analysis of immunofluorescence and for reviewing the manuscript. We also thank Marda Jorgensen for technical advice on immunohistochemistry.
Disclosure
The authors report no conflicts of interest in this work.
References
- NarkewiczMRBiliary atresia: an update on our understanding of the disorderCurr Opin Pediatr200113543544011801889
- DavenportMHowardERMacroscopic appearance at portoenterostomy – a prognostic variable in biliary atresiaJ Pediatr Surg19963110138713908906668
- ShteyerERammGAXuCWhiteFVShepherdRWOutcome after portoenterostomy in biliary atresia: pivotal role of degree of liver fibrosis and intensity of stellate cell activationJ Pediatr Gastroenterol Nutr2006421939916385261
- WeerasooriyaVSWhiteFVShepherdRWHepatic fibrosis and survival in biliary atresiaJ Pediatr2004144112312514722530
- DienstagJLGoldinRDHeathcoteEJHistological outcome during long-term lamivudine therapyGastroenterology2003124110511712512035
- HadziyannisSJTassopoulosNCHeathcoteEJAdefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis BN Engl J Med2003348980080712606734
- LaiCLChienRNLeungNWA one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study GroupN Engl J Med1998339261689654535
- PoynardTMcHutchisonJMannsMImpact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis CGastroenterology200212251303131311984517
- AdrianJEKampsJAScherphofGLA novel lipid-based drug carrier targeted to the non-parenchymal cells, including hepatic stellate cells, in the fibrotic livers of bile duct ligated ratsBiochim Biophys Acta2007176861430143917493581
- BolkeniusUHahnDGressnerAMBreitkopfKDooleySWickertLGlucocorticoids decrease the bioavailability of TGF-beta which leads to a reduced TGF-beta signaling in hepatic stellate cellsBiochem Biophys Res Commun200432541264127015555563
- CaligiuriAde FrancoRMRomanelliRGAntifibrogenic effects of canrenone, an antialdosteronic drug, on human hepatic stellate cellsGastroenterology2003124250452012557155
- ChenABenoDWDavisBHSuppression of stellate cell type I collagen gene expression involves AP-2 transmodulation of nuclear factor-1-dependent gene transcriptionJ Biol Chem19962714225994259988824237
- KanzlerSBaumannMSchirmacherPPrediction of progressive liver fibrosis in hepatitis C infection by serum and tissue levels of transforming growth factor-betaJ Viral Hepat20018643043711703574
- NelsonDRGonzalez-PeraltaRPQianKTransforming growth factor-beta 1 in chronic hepatitis CJ Viral Hepat19974129359031062
- WilliamsEJGacaMDBrigstockDRArthurMJBenyonRCIncreased expression of connective tissue growth factor in fibrotic human liver and in activated hepatic stellate cellsJ Hepatol200032575476110845662
- van Obberghen-SchillingERocheNSFlandersKCSpornMBRobertsABTransforming growth factor beta 1 positively regulates its own expression in normal and transformed cellsJ Biol Chem198826316774177463259578
- HinzBPhanSHThannickalVJGalliABochaton-PiallatMLGabbianiGThe myofibroblast: one function, multiple originsAm J Pathol200717061807181617525249
- LiZDranoffJAChanEPUemuraMSevignyJWellsRGTransforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in cultureHepatology20074641246125617625791
- FriedmanSLMolecular regulation of hepatic fibrosis, an integrated cellular response to tissue injuryJ Biol Chem200027542247225010644669
- BatallerRBrennerDALiver fibrosisJ Clin Invest2005115220921815690074
- CassimanDLibbrechtLDesmetVDenefCRoskamsTHepatic stellate cell/myofibroblast subpopulations in fibrotic human and rat liversJ Hepatol200236220020911830331
- Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study GroupHepatology1994201 Pt 115208020885
- HaafizABLiver fibrosis in biliary atresiaExpert Rev Gastroenterol Hepatol20104333534320528120
- FoucherJChanteloupEVergniolJDiagnosis of cirrhosis by transient elastography (FibroScan): a prospective studyGut200655340340816020491
- Gomez-DominguezEMendozaJGarcia-BueyLTransient elastography to assess hepatic fibrosis in primary biliary cirrhosisAliment Pharmacol Ther200827544144718081731
- StaubFTournoux-FaconCRoumyJLiver fibrosis staging with contrast-enhanced ultrasonography: prospective multicenter study compared with METAVIR scoringEur Radiol20091981991199719259683
- GrewalDJainRBrarGSGrewalSPPentacam tomograms: a novel method for quantification of posterior capsule opacificationInvest Ophthalmol Vis Sci20084952004200818436833
- MoreiraRKHepatic stellate cells and liver fibrosisArch Pathol Lab Med2007131111728173417979495
- GressnerOAWeiskirchenRGressnerAMEvolving concepts of liver fibrogenesis provide new diagnostic and therapeutic optionsComp Hepatol20076717663771
- ZeisbergMYangCMartinoMFibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transitionJ Biol Chem200728232233372334717562716
- ArmstrongMJCareyMCThe hydrophobic-hydrophilic balance of bile salts. Inverse correlation between reverse-phase high performance liquid chromatographic mobilities and micellar cholesterol-solubilizing capacitiesJ Lipid Res198223170807057113
- AttiliAFAngelicoMCantaforaAAlvaroDCapocacciaLBile acid-induced liver toxicity: relation to the hydrophobic-hydrophilic balance of bile acidsMed Hypotheses198619157692871479
- GreimHCzyganPSchaffnerFPopperHDetermination of bile acids in needle biopsies of human liverBiochem Med1973822802864753210
- JansenPLStrautnieksSSJacqueminEHepatocanalicular bile salt export pump deficiency in patients with progressive familial intra-hepatic cholestasisGastroenterology199911761370137910579978
- PastorAColladoPSAlmarMGonzalez-GallegoJAntioxidant enzyme status in biliary obstructed rats: effects of N-acetylcysteineJ Hepatol19972723633709288612
- JonesBARaoYPStravitzRTGoresGJBile salt-induced apoptosis of hepatocytes involves activation of protein kinase CAm J Physiol19972725 Pt 1G1109G11159176220
- Svegliati-BaroniGRidolfiFHannivoortRBile acids induce hepatic stellate cell proliferation via activation of the epidermal growth factor receptorGastroenterology200512841042105515825085
- SandersonNFactorVNagyPHepatic expression of mature transforming growth factor beta 1 in transgenic mice results in multiple tissue lesionsProc Natl Acad Sci U S A1995927257225767708687
- KanzlerSLohseAWKeilATGF-beta1 in liver fibrosis: an inducible transgenic mouse model to study liver fibrogenesisAm J Physiol19992764 Pt 1G1059G106810198351
- ThompsonNLFlandersKCSmithJMEllingsworthLRRobertsABSpornMBExpression of transforming growth factor-beta 1 in specific cells and tissues of adult and neonatal miceJ Cell Biol198910826616692645303
- AssoianRKFleurdelysBEStevensonHCExpression and secretion of type beta transforming growth factor by activated human macrophagesProc Natl Acad Sci U S A19878417602060242888109
- LamireauTLe BailBBoussarieLExpression of collagens type I and IV, osteonectin and transforming growth factor beta-1 (TGFbeta1) in biliary atresia and paucity of intrahepatic bile ducts during infancyJ Hepatol199931224825510453937
- RammGANairVGBridleKRShepherdRWCrawfordDHContribution of hepatic parenchymal and nonparenchymal cells to hepatic fibrogenesis in biliary atresiaAm J Pathol199815325275359708812
- BedossaPParadisVLiver extracellular matrix in health and diseaseJ Pathol2003200450451512845618
- UrushiharaNIwagakiHYagiTElevation of serum interleukin-18 levels and activation of Kupffer cells in biliary atresiaJ Pediatr Surg200035344644910726686