Abstract
Background
In the US, neither the prevalence nor the gastrointestinal (GI) diagnosis/symptoms associated with Helicobacter pylori (HP) have been examined in different racial/ethnic groups.
Aim
To determine the racial/ethnic differences in HP infection associated with GI diagnoses/symptoms using the Cerner Health Facts® database.
Methods
This cross-sectional study collected data during the period of 2000–2015 from the following ethnic/racial groups: 8,236,317 white, 2,085,389 black, 426,622 Hispanic, 293,156 Asian Pacific/Islander (APIs), and 89,179 Native American/Alaskan Native (NA/AN) patients aged 21–65 years old; the data were then analyzed. The primary dependent variable was a diagnosis of HP (ICD-9-Clinical Modification/ICD-10 classification). SAS version 9.4 was used for the statistical analysis. The statistical analysis was performed on 11,130,663 patients with GI symptoms, and of these, 152,086 patients were positive for the infection.
Results
Hispanics and NA/ANs had the highest prevalence of HP associated with upper GI symptoms/diagnosis. Nevertheless, blacks and APIs presented the highest relative risk (RR) of HP associated with dyspepsia (RR [95% CI] =11.2 [10.7–11.9] and 14.2 [12.8–15.6]), peptic ulcer (RR =13.8 [13.3–14.5] and 10.7 [9.3–12.3]), and atrophic gastritis (RR =9 [8.5–9.6] and 7.4 [6.4–8.5]), respectively. In all racial/ethnic groups, HP was also associated with inflammatory bowel diseases, liver diseases, and celiac diseases.
Conclusion
Black and API populations had the highest risk of HP associated with upper GI symptoms/diagnosis. Black patients also had the highest risk for HP associated with GI cancer.
Introduction
Patients positive for Helicobacter pylori (HP) infection have an estimated 10%–20% lifetime risk of developing ulcer disease and a 1%–2% risk of developing distal gastric cancer.Citation1 In 1991, Graham et al demonstrated that among asymptomatic persons, HP infection was found to be twice as frequent in blacks as in whites.Citation2 At that time, these investigators suggested that the apparent increase in susceptibility to HP infection in blacks could have a genetic basis.Citation2 Furthermore, Hispanics, Native Americans/Alaskan Natives (NA/ANs), and Asians have much higher rates of HP seropositivity than the white population.Citation3–Citation5
It is unclear whether upper gastrointestinal (GI) symptoms are more frequent in patients with HP infection than in a comparable uninfected population. Budzyński and KłopockaCitation6 established that HP infection is the cause of the most common form of chronic gastritis and contributes to the pathogenesis of functional dyspepsia and gastritis symptoms, particularly in patients who live in stressful conditions (mental stress can affect the mucosal immune response, increasing HP colonization).Citation7 In addition, patients with acute GI infection during psychological or physical stress may evoke GI symptoms and lead to increased mucosal infiltration.Citation6,Citation7 Vinagre et alCitation8 demonstrated that heavy and prolonged smoking and inadequate dietary habits were associated with a higher degree of gastric mucosa inflammation and intense neutrophilia. Dixon considers that HP infection is at least the triggering factor for, if not the direct cause of, atrophy and intestinal metaplasia.Citation9
In 2015, Darko et alCitation10 evaluated patients who underwent endoscopy and reported that HP infection was high in patients with upper GI symptoms and was even higher in patients with gastritis and duodenal ulcers. However, the authors demonstrated that HP infection declined among patients during this period from 69.7% in 1999 to 45.2% in 2012.
The rates of stomach cancer reported by these investigators were less frequent (0.7% in 1999 and 0.4% in 2012). Other symptoms associated with HP infection were esophageal reflux (9.9%), hematemesis (3.1%), melena stools (3.2%), vomiting (1.5%), and belching (2.1%).Citation7 Obayo et alCitation11 also reported that abdominal pain was associated with HP infection in 51.6% of 176 patients from southwestern Uganda with HP infection. Demma et alCitation4 reported an average annual age-adjusted rate of hospitalizations per 100,000 NA/ANs of 232.4 for ulcer-associated conditions, 14.2 for gastric cancer, and 6.1 for mucosa-associated lymphoid tissue (MALT) lymphoma. Conversely, the rate of HP discharge diagnosis was 28.2 per 100,000 persons.
In the US population, neither the prevalence nor the GI diagnoses/symptoms associated with the risk of HP infection have been examined in a large population of different racial/ethnic groups.Citation12–Citation14 Therefore, the objectives of this investigation were 1) to estimate the association of diagnosed HP infections overall and among patients with GI diagnoses and symptoms by race/ethnicity and 2) to determine the relative risk (RR) of HP infection and GI diagnoses and symptoms by race/ethnicity.
Methods
Electronic health record data
The analyzed data were obtained from the Cerner Health Facts® database (Cerner Corporation, North Kansas, MO, USA), which uses an automated electronic medical record (EMR) system to capture hospital procedures, laboratory testing and results, diagnostic information (eg, diagnosis of HP infection), demographics, medical history, and inpatient and outpatient stays over time. Data in Health Facts were extracted from the EMRs of hospitals with which Cerner Corporation has a data use agreement. Encounters may include pharmacy, clinical and microbiology laboratory, admission, and billing information from affiliated patient care locations. All admissions, medication orders and dispersals, laboratory orders, and specimens are date and time stamped, providing a temporal relationship between treatment patterns and clinical information. Cerner Corporation has established Health Insurance Portability and Accountability Act-compliant operating policies to establish de-identification for Health Facts. Health Facts research has been reviewed and approved by the Institutional Review Board at the University of Missouri, Kansas City.
Patient consent for use of the data was deemed unnecessary by the Institutional Review Board because the data were de-identified.
Patients
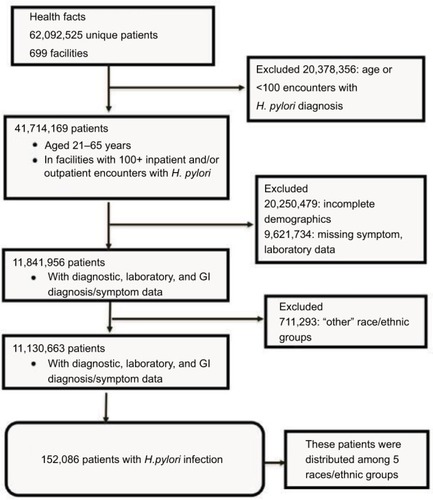
presents a flowchart of the selection process of patients diagnosed with HP infection. Health Facts include EMR data of >62 million patients from >699 facilities between January 1, 2001 and December 2015. The current analysis examined the data from facilities that reported at least 100 patients with a medical diagnosis of HP.
Figure 1 Flow diagram of the selection of patients analyzed in the study.
In this investigation, we included patients with the following criteria: 1) adults between the ages of 21 and 65 years, 2) patients with GI ICD-9- Clinical Modification (CM)/ICD-10 recorded symptoms or medical diagnosis, 3) those with an ICD-9-CM/ICD-10 clinical diagnosis of HP, 4) patients with a positive laboratory test for HP, and 5) no missing data for race/ethnicity, sex, and age.
Patients were excluded when they had the following characteristics: 1) children and adolescents under 21 years of age, 2) patients over 65 years, 3) those with duplicate inpatient and/or outpatient encounters, and 4) missing data for race/ethnicity, sex, and age.
also demonstrates that from the >62 million patients, we excluded 20,378,386 subjects under 21 or over 65 years and those who came from facilities with <100 encounters with HP. From 41,714,169 patients who met the eligibility criteria, we excluded 20,250,479 patients with incomplete demographic data and 9,621,434 patients with missing symptoms or missing laboratory data. shows that 11.8 million patients met the inclusion criteria, and an additional 164,821 had a confirmed diagnosis of HP infection (20,807 by the ICD-9-CM or ICD-10 criteria and 144,733 through a positive laboratory test).Citation15–Citation20 In this investigation, we did not present the results of 711,293 patients because they reported the “others” category for the race/ethnic variable. Therefore, statistical analysis was performed on 11,130,663 patients with GI symptoms, and of these, 152,086 patients were positive for the infection.
Study variables
The study variables included the following:
HP diagnosis via laboratory test (Logical Observation Identifiers Names and Codes: L-32637-1, L-32637-1, L-32637-1, L-32637-1, L-17780-8, L-31843-6, L-29891-9, L-5176-3, L-5174-8, and L-7900-4)Citation15–Citation20 or diagnosis code (ICD-9-CM/ICD-10 classification: 041.86 and B96.81), following the practice parameters of the Committee of the American College of Gastroenterology;Citation21
clinical history and self-reported symptoms: the ICD-9-CM and ICD-10 classification codes that we used to identify all the GI diagnoses and symptoms evaluated were reflux =530.81, K21.9; heartburn =787.1, R12.x; epigastric pain =789.06, R10.13; gas pain, FE =787.3, R14; abdominal pain =789.0, R10.x; nausea/vomiting =787.0, R11.x; diarrhea/vomiting = self-reported symptom; hematemesis =578.0, K92.0; GI bleeding =430.82, 456.0, 456.20, 569.3, 569.85, 569.86, 578.0, 578.1, 578.9, K92.2; blood in stool =578.1, K92.1; acute gastritis =535.x, K29.x; esophagitis =530.1, K20.9; dyspepsia =536.8, K30.x; peptic ulcer =531.x–534.x, K25.x–K28.x; gastric ulcer =531.x, K25.x; duodenal ulcer =532.x, K26.x; atrophic gastritis with bleeding =535.11, K29.41; atrophic gastritis without bleeding/metaplasia =535.10, K29.40; GI neoplasm =211.0–211.5, 230.x, 239.0, 235.2–235.5, D37.x; and GI cancer =150.x–159.x, 196.2, 197.4–197.8, C15.x–C26.x;
demographic characteristics: age (21–30, 31–40, 41–50, and 51–65 years), sex (male and female), and racial/ethnic group (white, black, Hispanic, Asian Pacific/Islander [API], and NA/AN populations); and
other GI diagnoses, with the following ICD-9-CM/ICD-10 codes: GI infection (001.x–009.x, A00.x–A09.x), appendicitis (540.x–543.x, K35.x), other functional intestinal diseases (564.x, K59.x), inflammatory bowel disease (555.x, K50.x), diverticula of the intestine (562.x, K57.x), celiac disease (579.0, K90.0), liver disease (570.x–573.x, K70.x–K72.x, K74–K77.x), other malignant lymphomas (202.8, C85.99), stomatitis (528.2, K12.x), abnormal weight gain (783.1, R63.5), and abnormal weight loss (783.21, R63.4).
Statistical analyses
Statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA). Descriptive and inferential statistics were performed on the primary dependent variable. The prevalence of HP infection among patients with GI diagnoses/symptoms was estimated and stratified by race/ethnicity. We also determined the RR and 95% CI of HP infection with GI clinical diagnoses/symptoms. For each race/ethnic group, we determined the risk of HP infection associated with belonging to an age- or gender-specific group or having antecedents of GI symptoms. Using the demographic variables, the prevalence ratios give the risk of the infection in reference to a determined demographic characteristic. For example, for the gender variable, the risk of HP infection when the patients were females (using the male gender as the reference group) was determined. Using the variable “age,” the risk of infection associated with belonging to some age group was determined, using the younger age group (21–30 years) as a reference group.
The calculation of the RRs was performed as the ratio of the probability of an event occurring and the probability of it not occurring; for example, the probability of developing an HP infection in a group with a GI symptom compared to the probability of developing the infection in the group without that symptom.
Using the chi-square test, we determined the association of HP infection with GI diagnoses/symptoms. In all cases, a p-value ≤0.05 was considered statistically significant.
Results
The distribution of the sample by race/ethnicity was as follows: 8,236,317 white, 2,085,389 black, 426,622 Hispanic, 293,156 API, and 89,179 NA/AN unique patients. Among them, 164,821 had a confirmed diagnosis of HP infection (20,807 by the ICD-9-CM or ICD-10 criteria and 144,733 through a positive laboratory test).Citation15–Citation20 In this study, we present the results of 152,086 HP patients (12,735 subjects were excluded because they belonged to the “others” category for the race/ethnicity variable).
presents the RR of HP infection based on sex and age among the ethnic/race populations studied. The risk of HP infection was higher in females than in males among the white, black, and Hispanic groups. Females also had a higher frequency of the infection compared to males (63.8% vs 36.2%; 66.0% vs 33.99%; 63.8% vs 36.2%; 62.5% vs 37.5%; and 56.8% vs 43.2% for white, black, Hispanic, API, and AN/NA, populations, respectively).
Table 1 Prevalence ratio of Helicobacter pylori infection by race/ethnicity (N=11,130,663 patients with GI symptoms and 152,086 positive for H. pylori infection)
The distribution of patients by age showed that except for Hispanics (21.2%), patients who were 51–65 years old had the highest frequency of HP (35.3%, 33.01%, 30.94%, and 27.4% for white, black, API, and NA/AN patients, respectively). The frequencies for the groups of 41–50 and 31–40 years old were 23.5%, 26.8%, 23.63%, 24.3%, and 22% and 19.3%, 20.4%, 28.11%, 25.7%, and 23.96% for white, black, Hispanic, APIs, and NA/ANs, respectively. Young adults aged 21–30 years had the lowest frequencies of infection (21.9%, 19.81%, 27.1%, 19.1%, and 26.7% for white, black, Hispanic, API, and NA/AN populations, respectively). also shows that compared to patients from the other age groups, black, Hispanic, and API patients above the age of 40 had the highest RR of HP infection.
GI symptoms and HP infection by race/ethnicity
The prevalence of HP infection among patients with GI symptoms was higher in Hispanic, API, and NA/AN populations and was the highest for reflux, heartburn, epigastric pain, gas pain, and diarrhea/vomiting (). HP infection associated with complications of peptic ulcers was more prevalent in Hispanic and NA/AN populations as follows: hematemesis (12.6% and 23.01%), GI bleeding (8.7% and 13.7%), and blood in the stool (9.8% and 13.7%), respectively.
Table 2 Prevalence of Helicobacter pylori infection by GI symptoms and diagnoses stratified by race/ethnicity (N=11,130,663 patients with GI symptoms and 152,086 positive for H. pylori infection)
Black and API populations with a clinical diagnosis of reflux, heartburn, gas pain, GI bleeding, and blood in the stool had the highest risk of HP infection compared to individuals without these diagnoses. However, API populations with a diagnosis of epigastric pain had the highest RR of HP infection (RR 9.8; 95% CI 9.04–10.7), followed by the white population (RR 8.1; 95% CI 7. 96–8.21). The white and black populations also had the highest risk of HP infection when they presented with a diagnosis of peptic ulcer complications, with a risk for hematemesis of 6.4 (95% CI 6.1–6.7) and 7.3 (95% CI 6.7–8.0), respectively ().
Table 3 Relative risk of Helicobacter pylori associated with gastrointestinal symptoms and diagnoses stratified by race/ethnicity (N=11,130,663 and N=152,086 positive for H. pylori infection)
GI diagnosis and HP infection by race/ethnicity
also presents the prevalence of HP infection associated with the GI clinical diagnosis for all racial/ethnic groups. This again draws attention to the finding that the highest prevalence of HP infection among patients with upper GI diagnosis was observed in Hispanic and NA/AN patients. These two populations had the highest prevalence of HP infection associated with acute gastritis and esophagitis. In the case of HP infection and dyspepsia, we observed a high prevalence in all racial/ethnic groups, as follows: 12.4%, 9.4%, 19.9%, 16.1%, and 18.1% for the white, black, His-panic, API, and NA/AN populations, respectively. Gastric and duodenal ulcers are commonly referred to as peptic ulcers. In this investigation, HP infection associated with peptic ulcers had the highest prevalence compared to the other GI diagnoses evaluated (). In Western countries, duodenal ulcers are ~4-fold more common than gastric ulcers.Citation1 We confirmed that the diagnosis of HP infection associated with duodenal ulcers had a higher prevalence than HP infection associated with gastric ulcers among the white (15.1% vs 14.8%), black (13.2% vs 12.7%), Hispanic (18.4% vs 15.8%), and API (13.7% vs 12.96%) populations. Conversely, the NA/AN populations had a higher prevalence of HP infection associated with gastric ulcers than with duodenal ulcers (29.3% vs 23.2%, respectively).
HP infection associated with atrophic gastritis without bleeding/intestinal metaplasia was highly prevalent in all study groups, with rates of 9.4%, 7.7%, 13.8%, 8.8%, and 15.1% for the white, black, Hispanic, API, and NA/AN groups, respectively. In the case of HP infection associated with neoplasm and GI cancer, these two diagnoses were 2–3 times more frequent in the Hispanic and NA/AN populations than in the other groups as follows: 7.9% and 11.0%, respectively, for neoplasm, and 4.6% and 6.5%, respectively, for cancer ().
We found that the black and API populations had a more than 7 times higher risk of HP infection when they presented with gastritis, dyspepsia, peptic, gastric, duodenal ulcer, and neoplasm (). The black population also had the highest risk of HP infection associated with GI cancer (RR 2.3; 95% CI 2.1–2.5) compared to the other racial/ethnic groups ().
Prevalence and RR of HP infection among patients with other GI clinical diagnoses by racial/ethnic groups
The prevalence of HP infection associated with other GI clinical symptoms was 5.4%, 5.3%, 9.3%, 4.3%, and 6.4% for abnormal weight gain and 6.2%, 5.0%, 10.5%, 6.0%, and 10.8% for abnormal weight loss for the white, black, Hispanic, API, and NA/AN populations, respectively (). It is striking to note that HP infection associated with celiac disease had an important prevalence, according to the rates of 6.0%, 8.0%, 14.8%, 1.3%, and 8.1% for the white, black, Hispanic, API, and NA/AN populations, respectively ().
Table 4 Prevalence of Helicobacter pylori among patients with other GI diagnoses stratified by race/ethnicity (N=11,130,663 and N=152,086 positive for H. pylori infection)
By analyzing the RR of HP infection associated with other GI clinical diagnoses, we found that the black and API populations had a 2–4 times higher risk when they presented with GI infection, other functional intestinal diseases, and inflammatory bowel diseases (). In all population groups, patients who had a clinical diagnosis of liver diseases also had a 2–3 times higher risk of HP infection. The risk of HP infection associated with celiac disease was significantly higher in blacks (RR 8.9; 95% CI 6.6–12.1) and Hispanics (RR 6.2; 95% CI 4.3–8.97) than in whites (RR 4.3; 95% CI 4.0–4.6), APIs (RR 1.04; 95% CI 0.15–7.3), and NA/ANs (RR 1.9; 95% CI 0.64–5.6) ().
Table 5 Relative risk of Helicobacter pylori infection associated with other clinical GI diagnoses stratified by race/ethnicity (N=11,130,663 and N=152,086 positive for H. pylori infection)
Discussion
Race/ethnicity and the risk of HP infection
To our knowledge, this is the first naturalistic cross-sectional study showing the risk of HP infection associated with GI symptoms and diagnoses in patients from different racial/ethnic groups in the US obtained from Health Facts. In this investigation, among the total population studied (11,130,663), we found that black and API populations presented the highest RR of HP infection associated with almost all GI clinical symptoms and diagnoses evaluated. In agreement with our results, in 1991, Graham et alCitation2 demonstrated that in asymptomatic persons, HP infection was twice as frequent in blacks than in whites. At that time, these investigators suggested that the apparent increase in susceptibility to HP infection in blacks could have a genetic basis.Citation2 This assumption was confirmed in the study of Epplein et alCitation22 who showed that compared to whites, African–Americans of low, medium, and high African ancestry had 1.6-, 4.1-, and 5.2-fold increased odds of seropositivity to HP, primarily related to CagA seropositive strains, for which increasing African ancestry led to 2.5-, 9.6-, and 13.1-fold increased odds, respectively. The investigators also demonstrated that among African–Americans alone, compared to African ancestry, African–Americans of mixed and high ancestry had 2.5- and 3.4-fold increased odds of seropositivity to HP and 3.5- and 4.9-fold increased odds of CagA seropositive HP strains, respectively. Racial disparity begins at an early age, as demonstrated by Malaty, who observed that African–American children aged 5–9 years had an HP infection prevalence of ~30% compared to 12% for the overall child infection rate in the US.Citation23 In another longitudinal study over a 12-year follow-up period, African–American children had a 4-fold greater rate than whites, and only 4% of African–American children proceeded to lose the infection compared to 50% of the white children.Citation24
A review by Guevara et alCitation25 showed that 61.5% of the studies analyzed genetic diversity by typing the multilocus sequence typing sequences of seven conserved genes (atpA, efp, mutY, ppa, trpC, ureI, and yphC) and genes associated with virulence in different ethnic groups (cow, cagA, hspA, and oipA). Guevara et alCitation25 noted that humans have coevolved with HP bacteria due to their adaptation to the gastric environment through the modification of genes involved in the modulation of adaptive host immunity and the evolution of different mechanisms of adaptation to hosts of diverse human ethnic groups, which have allowed the development of a largely innocuous and potentially symbiotic infection.
Most studies in different racial/ethnic groups show that the populations most susceptible to HP infection are blacks and Hispanics.Citation22,Citation26–Citation28 Although the risk of HP infection in Hispanic immigrants seems to decrease with each successive generation born in the US,Citation3 it has been suggested that in Western countries, the prevalence of HP infection is higher among first- and second-generation immigrants from developing countries.Citation1 In the case of Asians, many reports demonstrated that this population has a much higher rate of HP seropositivity than their white peers.Citation2,Citation9,Citation22,Citation26 Azuma et alCitation29 reported that the severity of gastritis in HP-infected Japanese subjects is dependent on the human leukocyte antigen class II; moreover, there are few reports about GI symptoms and clinical diagnoses associated with HP infection in the NA/AN populations. Demma et alCitation4 reported an average annual age-adjusted rate of hospitalizations per 100,000 NA/ANs of 232.4 for ulcer-associated conditions, 14.2 for gastric cancer, and 6.1 for MALT lymphoma. The rate of HP discharge diagnosis was 28.2 per 100,000 persons. In our investigation, the prevalence of HP infection associated with a GI clinical diagnosis was the highest in the NA/AN populations among all the evaluated racial/ethnic groups as follows: 24.6% for peptic ulcers, 29.3% for gastric ulcers, 23.2% for duodenal ulcers, and 6.6% for GI cancer.
The aforementioned antecedents demonstrate that the environmental factor most consistently associated with HP infection is low socioeconomic status; however, a study by Epplein et alCitation22 found a very minor influence of education, income, and other demographic factors on the risk of HP infection in the black/white populations studied. Educational level was the only socioeconomic status variable adjusted for, as it was the only one significantly associated with both race/African ancestry and HP status. These investigators adjusted for household income and household size, but the researchers demonstrated that those factors had little impact on the risk of the infection. Therefore, the following different factors from the socioeconomic level could be related to the increased risk of HP infection in certain racial and/or ethnic groups: 1) the type of proteins, including the cancer-associated virulence constituents CagA and VacA, in the HP strains; 2) the susceptibility of the host, for example, ancestry informative genetic markers among race/ethnic groups alone, compared to those of low ancestry, medium ancestry, and high ancestry; and 3) the habits and customs that are transmitted from generation to generation through different racial and ethnic groups that could play an important role in the transmission of infection from parents to children. The latter aspect is of great interest and merits further research among different races and ethnic groups.
Acute gastritis and HP infection
The most common GI symptoms associated with HP infection are vomiting, burning sensation, passing gas, bloating, bad breath, nausea, belching, loss of appetite, gnawing pain, heartburn, indigestion, stomach growls, gurgling, grumbling and groaning, indigestion (dyspepsia), and gastritis.Citation8,Citation30–Citation32 Kusters et al established that the first phase of HP colonization might be associated with dyspeptic symptoms such as fullness, nausea, vomiting, inflammation of the proximal and distal mucosa, or pan-gastritis.Citation1
To our knowledge, this is the first study to demonstrate that black and API patients had the highest RR for dyspepsia, reflux, heartburn, gas pain, acute gastritis, peptic ulcers, atrophic gastritis, and neoplasm compared to the other groups (). These findings suggest that the black and API populations are more susceptible to HP colonization than the white population. Hispanic and NA/AN populations may be able to avoid HP colonization or be cleared of an established infection.Citation1 However, more longitudinal studies on the natural history of HP infection in different racial groups are required.
Dyspepsia and HP infection
In this investigation, we found that the prevalence of HP infection in patients with a clinical diagnosis of functional dyspepsia was 19.9% in Hispanics and 18.1% in NA/ANs; it was lower in the black population (9.4%), followed by whites (12.4%) and APIs (16.1%). Dyspeptic symptoms may have a reflux-like character, with heartburn and regurgitation; patients may also show dysmotility-like symptoms, with early satiety and nausea; or they may be ulcer-like, with pain and vomiting.Citation1,Citation32,Citation33 In this investigation, the prevalence of HP infection associated with reflux and heartburn was higher for Hispanics (10.3% and 14.3%) and NA/ANs (12% and 15%) than for whites (5.5% and 8.7%), respectively. Similar prevalence rates were reported for the Western adult population.Citation1,Citation34–Citation36 By analyzing the RR of HP infection in patients with dyspepsia, we found that the black and API populations had the highest risk. The interaction among genetic factors and the virulence of HP infection may be the reasons for this higher risk of HP infection among API patients with dyspepsia.Citation12,Citation36–Citation38
Peptic ulcer disease and HP infection
Thirty years ago, ~95% of duodenal ulcers and 85% of gastric ulcers occurred in the presence of HP.Citation1 Nomura et alCitation39 demonstrated that the lifetime risk of developing peptic ulcers in patients with HP infection was 3–10 times higher than in those without infection. In this investigation, we found that HP infection associated with peptic ulcers was markedly more frequent in the Hispanic (18.7%) and NA/AN (24.6%) populations than in the white population (14%). These prevalence rates were similar to those reported by other investigators.Citation7 In Western countries, duodenal ulcers are 4 times more frequent than gastric ulcers.Citation1 In accordance with this argument, in our investigation, we demonstrated that except for the NA/AN population, the prevalence of HP infection associated with duodenal ulcers was more frequent than that with gastric ulcers in all racial/ethnic groups. Duodenal ulcers have been shown to be more common in young adult patients, whereas gastric ulcers predominantly arise in subjects aged >40 years.Citation1 In our study, the highest risk for HP infection associated with age was found in the age groups of 41–50 and 51–65 years, for all racial/ethnic populations. In the US, HP infection mainly affects older adults (~50% of those aged >60 years compared to 20% of those aged <40 years), particularly those with low economic resources.Citation40,Citation41 However, other factors that may influence peptic ulcer risk in infected subjects are the amount of gastric acid production, the presence of gastric metaplasia in the duodenal bulb, smoking, and genetic factors.Citation1,Citation8,Citation29,Citation38 The most common ulcer-associated symptoms are nausea, epigastric discomfort, and ulcer pain.Citation42 In this investigation, we found that the prevalence of HP infection associated with nausea and epigastric discomfort was higher in the NA/AN populations than in the other groups. Nevertheless, the white population had the highest risk of HP infection associated with nausea/vomiting (RR 3.02) and epigastric pain (RR 8.1).
GI complications and symptoms associated with peptic ulcers
The most common complications of ulcer disease include bleeding, perforation, and stricture formation; in particular, bleeding occurs in 15%–20% of ulcers.Citation1 In this investigation, we found a similar prevalence of HP infection associated with GI bleeding for Hispanic and NA/AN patients (8.7% and 13.7%, respectively). However, the white, black, and API populations presented lower prevalences than the Hispanic and NA/AN populations (). The prevalence rates of hematemesis and blood in the stool were also higher in Hispanics (12.6% and 9.8%, respectively) and NA/ANs (23% and 13.7%, respectively) than in the other groups (). The risk of GI complications associated with HP infection depends on the severity of gastritis.Citation1,Citation39 GI complications are determined by host- and bacteria-related factors.Citation12,Citation26,Citation38,Citation42 Among the bacterial factors is the presence of cytotoxin-associated gene A (cagA), which is related to severe gastritis, peptic ulceration, atrophic gastritis, and cancer.Citation38 Among the host factors is acid production in response to HP infection.Citation1,Citation39,Citation43 CagA is a component of a type IV bacterial secretion system termed the cag island, and cagA-positive strains of HP inject CagA into host cells, altering host cell physiology and the adaptive immune response in a manner that permits HP persistence.Citation22
GI cancer and HP infection
HP infection increases the risk of cancer.Citation43–Citation45 Nevertheless, compared to infection with a cagA-negative strain, infection with a cagA-positive strain may increase the risk of cancer by ~2–3 times.Citation1,Citation39,Citation40 In this investigation, the prevalence of HP infection associated with GI cancer was 2.4%, 2.0%, 4.6%, 1.9%, and 6.5% for the white, black, Hispanic, API, and NA/AN populations, respectively. However, the prevalence of GI cancer in patients with HP infection varies across studies.Citation1,Citation38,Citation39,Citation46 For example, in Africa, Obayo et alCitation11 evaluated 176 patients who underwent biopsy and found that 13.6% had esophageal cancer, 7.6% had gastric ulcers, and 7.1% had gastric cancer. These authors demonstrated that patients aged >40 years had a significantly higher prevalence of these pathologies. Darko et alCitation10 studied patients who underwent endoscopy and reported that gastritis had a frequency of 8.1% in 1,128 patients evaluated in 1999 and 50.2% in 1,273 patients evaluated in 2002. The rates of stomach cancer (0.7% in 1999 and 0.4% in 2012) reported by these investigators were lower than those found in this investigation.Citation10 Hansson et alCitation47 demonstrated that patients with gastric ulcers have a 3-fold higher chance of developing gastric cancer than patients with duodenal ulcers.
Emerging immunoproteomics studies have identified additional HP antigens,Citation21 and new epidemiologic research on 15 distinct human HP antibodies has revealed important implications for gastric cancer risk.Citation22,Citation26 The simultaneous presence of the HP vacuolating toxin (VacA), Helicobacter cysteine-rich protein C, and the chaperonin GroEL, in addition to CagA, increases the risk of chronic atrophic gastritis (a precursor lesion to gastric cancer) 18-fold,Citation26 and GroEL may be a new independent risk marker for gastric cancer.Citation22
Other GI conditions and HP infection
For more than two decades, HP infection has been implicated in a multitude of other conditions, such as iron deficiency anemia, immune thrombocytopenic purpura, gastroesophageal reflux disease, and functional dyspepsia.Citation6,Citation35,Citation37 In this study, HP infection was associated with the diagnosis of GI infection, appendicitis, inflammatory bowel disease, diverticular disease, and celiac disease ( and ). Kusters et alCitation1 and other investigatorsCitation46 established that colonization with HP is not a disease in itself but a condition that affects the risk of developing various clinical disorders of the upper GI tract and possibly of the hepatobiliary tract. In this investigation, we demonstrated that the risk of HP infection was 2–3 times higher in patients with liver diseases. Budzyński and Kłopocka clearly described a bidirectional relationship between HP infection and the brain–gut axis. They considered that both the contagion process and the host’s neuroendocrine–immunological reaction to it have an effect, resulting in disturbances in the upper and lower digestive tract permeability, motility, and secretion, mainly as a form of irritable bowel syndrome.Citation6
In conclusion, in the US, NA/ANs and Hispanic patients had the highest rate of HP infection associated with GI symptoms and clinical diagnosis. A higher prevalence of HP infection associated with gastric ulcers, rather than duodenal ulcers, was observed in the NA/AN population, different from that expected for Western populations. However, the black and API populations had the highest risk of HP infection associated with gastritis, dyspepsia, peptic ulcer, atrophic gastritis/metaplasia, and neoplasm. Black patients also had the highest risk for HP infection associated with GI cancer. Therefore, it is of paramount importance that physicians recognize and more deeply investigate how race and ethnicity predispose patients to infection. It is important to develop appropriate guidelines for different racial/ethnic groups, taking into consideration the high prevalence of this infection and its relationship with other GI diagnoses in all family members.
Acknowledgments
This research was supported, in part, by the University of Guanajuato, Mexico.
Disclosure
The authors report no conflicts of interest in this work.
References
- KustersJGvan VlietAHMKuiperEJPathogenesis of Helicobacter pylori infectionClin Microbiol Rev20061944949016847081
- GrahamDYMalatyHMEvansDGEvansDJJrKleinPDAdamEEpidemiology of Helicobacter pylori in an asymptomatic population in the United States: effect of age, race and socioeconomic statusGastroenterology1991100149515012019355
- TsaiCJPerrySSanchezLParsonnetJHelicobacter pylori infection in different generations of Hispanics in the San Francisco Bay AreaAm J Epidemiol200516235135716014772
- DemmaLJHolmanRCSobelJEpidemiology of hospitalization with ulcers, gastric cancer, and Helicobacter pylori infection among American Indian and Alaska native personsAm J Trop Med Hyg20087881181818458318
- JiangJXLiuQMaoXYZhangHHZhangGXXuSFDownward trend in the prevalence of Helicobacter pylori infections and corresponding frequent upper gastrointestinal diseases profile changes in Southeastern China between 2003 and 2012Springerplus20165160127652174
- BudzyńskiJKłopockaMBrain–gut axis in the pathogenesis of Helicobacter pylori infectionWorld J Gastroenterol2014205212522524833851
- JiaKAnLWangFAggravation of Helicobacter pylori stomach infection in stressed military recruitsJ Int Med Res20164436737626800706
- VinagreRMVilar-e-SilvaAFecuryAAMartinsLCRole of Helicobacter pylori infection and lifestyle habits in the development of gastroduodenal diseases in a population from the Brazilian AmazonArq Gastroenterol20135017017424322186
- DixonMFPathology of gastritis and peptic ulcerationMobleyHLTMendzGLHazellSLHelicobacter pylori: Physiology and GeneticsWashington, DCASM Press200138
- DarkoRYawsonAEOseiVOwusu-AnsahJAluze-EleSChanging patterns of the prevalence of Helicobacter pylori among patients at a corporate hospital in GhanaGhana Med J20154914715326693189
- ObayoSMuzooraCOcamaPCooneyMMWilsonTProbertCSUpper gastrointestinal diseases in patients for endoscopy in South Western UgandaAfr Health Sci20151595996626957987
- ChenYLMoXQHuangGRGene polymorphisms of pathogenic Helicobacter pylori in patients with different types of gastrointestinal diseasesWorld J Gastroenterol2016229718972627956795
- KawakuboHTanakaHTsuruokaNUpper gastrointestinal symptoms are more frequent in female than male young healthy Japanese volunteers as evaluated by questionnaireJ Neurogastroenterol Motil20162224825326755685
- MujawarPDhirajBNikumbhBSuryawanshiKHPagarePSSuranaAHelicobacter pylori associated gastritis in northern Maharashtra, India: a histopathological study of gastric mucosal biopsiesJ Clin Diagn Res20159E04EC06
- SheRCWilsonARLitwinCMEvaluation of Helicobacter pylori immunoglobulin G (IgG), IgA, and IgM serologic testing compared to stool antigen testingClin Vaccine Immunol2009161253125519515865
- KazemiSTavakkoliHHabizadehMREmamiMHDiagnostic values of Helicobacter pylori diagnostic tests: stool antigen test, urea breath test, rapid urease test, serology and histologyJ Res Med Sci2011161097110422973378
- El ZimaityHMWuJAkamatsuTGrahamDYA reliable method for the simultaneous identification of H. pylori and gastric metaplasia in the duodenumJ Clin Pathol19995291491610711255
- LageAPGodfroidEFauconnierADiagnosis of Helicobacter pylori infection by PCR: comparison with other invasive techniques and detection of cagA gene in gastric biopsy specimensJ Clin Microbiol199533275227568567918
- GentaRMGrahamDYComparison of biopsy sites for the histopathologic diagnosis of Helicobacter pylori: a topographic study of H. pylori density and distributionGastrointest Endosc1994403423457794303
- FaigelDOMagaretNCorlessCLiebermanDAFennertyMBEvaluation of rapid antibody tests for the diagnosis of Helicobacter pylori infectionAm J Gastroenterol200095727710638562
- CheyWDWongBCPractice Parameters Committee of the American College of GastroenterologyAmerican College of Gastroenterology guideline on the management of Helicobacter pylori infectionAm J Gastroenterol20071021808182517608775
- EppleinMSignorelloLBZhengWRace, African ancestry, and Helicobacter pylori infection in a low-income United States populationCancer Epidemiol Biomarkers Prev20112082683421357376
- MalatyHMEpidemiology of Helicobacter pylori infectionBest Pract Res Clin Gastroenterol200721220521417382273
- MalatyHMGrahamDYWattigneyWASrinivasanSROsatoMBerensonGSNatural history of Helicobacter pylori infection in childhood: 12-year follow-up cohort study in a biracial communityClin Infect Dis199928227928210064244
- GuevaraTAlixACriolloRCoevolución genética Homo sapiens-Helicobacter pylori y sus implicaciones en el desarrollo del cáncer gástrico: una revisión sistemática. [A systematic review of genetic coevolution of Homo sapiens and Helicobacter pylori: implications for the development of gastric cancer]Rev Col Gastroenterol201631376390 Spanish
- SonnenbergALashRHGentaRMA national study of Helicobactor pylori infection in gastric biopsy specimensGastroenterology20101391894190120727889
- MorrisLHelicobacter pylori: epidemiology and routes of transmissionEpidemiol Rev20002228329711218379
- GohKLChanWKShiotaSYamaokaYEpidemiology of Helicobacter pylori infection and public health implicationsHelicobacter20111619
- AzumaTKonishiJTanakaYContribution of HLA-DQA gene to host’s response to Helicobacter pyloriLancet1994343542543
- LeeJYKimNDiagnosis of Helicobacter pylori by invasive test: histologyAnn Transl Med201531025705642
- Sánchez-CuénJAIrineo-CabralesABBernal-MagañaGPeraza-GarayFJRegression of gastric intestinal metaplasia after the eradication of Helicobacter pylori infection in a hospital in MexicoRev Esp Enferm Dig201610877077527804306
- MitchellHMEpidemiology of infectionMobleyHLTMendzGLHazellSLHelicobacter pylori: Physiology and GeneticsWashington, DCASM Press2001
- UchidaTMiftahussururMPittayanonRHelicobacter pylori infection in Thailand: a nationwide study of the CagA phenotypePLoS One201510e013677526355839
- LeowAHLimYYLiewWCGohKLTime trends in upper gastrointestinal diseases and Helicobacter pylori infection in a multiracial Asian population – a 20 year experience over three time periodsAliment Pharmacol Ther20164383183726847417
- MiwaHWhy dyspepsia can occur without organic disease: pathogenesis and management of functional dyspepsiaJ Gastroenterol20124786287122766746
- MarshallBJEpidemiology of H pylori in Western countriesHuntRHTytgatGNJHelicobacter pylori Basic Mechanisms to Clinical CureDordrechtKluwer Academic Publishers1994123138
- HongSJKimSWHelicobacter pylori infection in gastroesophageal reflux disease in the Asian countriesGastroenterol Res Pract2015201598524925642246
- AzumaTYamazakiSYamakawaAAssociation between diversity in the Src homology 2 domain-containing tyrosine phosphatase binding site of Helicobacter pylori CagA protein and gastric atrophy and cancerJ Infect Dis200418982082714976598
- NomuraAStemmermannGNChyouPHPerez-PerezGIBlaserMJHelicobacter pylori infection and the risk for duodenal and gastric ulcerationAnn Intern Med19941209779817741826
- ManuelDCutlerAGoldsteinJFennertyMBBrownKDecreasing prevalence combined with increasing eradication of Helicobacter pylori infection in the United States has not resulted in fewer hospital admissions for peptic ulcer disease-related complicationsAliment Pharmacol Ther20071214231427
- MalatyHMEvansDGEvansDJJrGrahamDYHelicobacter pylori in Hispanics: comparison with blacks and whites of similar age and socioeconomic classGastroenterology19921038138181499931
- PetersonWLFendrickAMCaveDRPeuraDAGarabedian-RuffaloSMLaineLHelicobacter pylori-related disease: guidelines for testing and treatmentArch Intern Med20001601285129110809031
- WaldumHLKlevelandPMSørdalØFHelicobacter pylori and gastric acid: an intimate and reciprocal relationshipTherap Adv Gastroenterol20169836844
- YaoYWuJGuTChengYLiGComparative analysis of the interaction of HSPs in dendritic cells, macrophages, RGM-1 cells infected by Helicobacter pyloriAm J Transl Res20161041844194
- SuYLHuangHLHuangBSCombination of OipA, BabA, and SabA as candidate biomarkers for predicting Helicobacter pylori related gastric cancerSci Rep2016611228442746
- GrahamDYOsatoMSOlsonCAZhangJFiguraNEffect of H. pylori infection and CagA status on leukocyte counts and liver function tests: extra-gastric manifestations of H. pylori infectionHelicobacter199831741789731987
- HanssonLENyrénOHsingAWThe risk of stomach cancer in patients with gastric or duodenal ulcer diseaseN Engl J Med19963352422498657240
