Abstract
Background:
Infliximab has shown benefit in Crohn’s disease (CD) and ulcerative colitis (UC).
Objective:
Evaluation of long-term outcome of therapy for both diseases.
Methods:
We analyzed retrospectively patients treated at infusion centers from one institution. Demographic, laboratory parameters leading up to biologic therapy and the subsequent pattern of outcomes in either disease were established as a database. Initial failure, subsequent need to change therapy, or need to adjust therapy were evaluated. Kruskal–Wallis (nonparametric) tests to compare two groups and Kaplan–Meier survival curve analysis were used to compare outcomes.
Results:
Over approximately 6 years, 71 CD and 26 UC patients received 999 and 215 infusions, respectively, for a median of 62 months. Of these, 17% for CD and 19% for UC patients were primary failures. Following the start of infliximab, 18% of CD and 11% of UC patients required stoppage and switching to another type of therapy. In either CD or UC patients, 54% or 62%, respectively, continued therapy without the need to change to other treatments. Few serious side effects were noted. No important statistically significant differences in treatment patterns or outcome were observed between the groups.
Discussion:
Long-term treatment of both inflammatory bowel diseases reflects outcomes of clinical trials.
Conclusions:
This study emphasizes similarities between CD and UC and reports therapeutic success for an extended time.
Keywords:
Introduction
The description of cell signaling inflammatory cytokine cascades in the past two decades has led to the development of novel biological therapies, especially in the inflammatory bowel diseases (IBDs) Crohn’s disease (CD) and idiopathic ulcerative colitis (UC). Initially, CD was considered to be a predominantly type 1 T helper (Th1) and UC a Th2 cytokine cascade-mediated disease.Citation1–Citation4 Therefore, infliximab (IFX), which is an immunoglobulin G1 (IgG1)-based chimeric mouse–human monoclonal antitumour necrosis factor alpha (TNFα) antibody, was primarily approved for targeting CD. However, TNFα was found in significant quantities in serum, stool, and colonic mucosa of patients with active UC.Citation5–Citation8 Although a number of smaller clinical studies reported conflicting results in UC,Citation9–Citation12 analysis of a number of studies confirmed the role of IFX in this disease as well.Citation13
ACCENT (A Crohn’s Disease Clinical Trial Evaluating Infliximab in a New Long-term Treatment Regimen) I and II established the use of IFX for active maintenance, and fistulizing CD.Citation14,Citation15 The Active Ulcerative Colitis Trial (ACT I) and ACT II also established its use in UC.Citation16 The overall use of IFX for CD has now passed beyond a decade of experience. In Canada, approval for UC of this agent is now over 4 years old. Previous utilization of IFX in UC required special requests for humanitarian purposes.
Although randomized trials are important instruments in establishing the use of any agent in diseases, it is also very important to evaluate whether agents in use achieve expectations in clinical practice. In an effort to compare clinical outcomes, we evaluated the effects of IFX on CD and UC over a period of 6 years from a single center.
Methods
We retrospectively evaluated patients from infusion clinics that serve outpatients referred from hospitals. We included only those patients from our institution who were treated at these centers between January 2002 and April 2008. Some patients during this period were started in the previous 2 years at the hospital itself and then transferred to infusion clinics.
Ethics Review Board approval from the Jewish General Hospital was obtained, and the charts of patients were reviewed by two of the authors (RA and PM). A database was established using information abstracted from charts. Diagnoses of CD and UC conformed to accepted criteria.Citation17
Relevant information was obtained, including type of disease, age, sex, time of disease prior to IFX therapy, and location of CD (terminal ileum and colon, colon only, or ileum/small bowel only) or UC (left or total colitis). Smoking history was poorly recorded and therefore omitted.
Laboratory data were recorded for three periods to assess changes: after diagnosis up to 1 month prior to IFX therapy (general period), within 1 month prior to IFX therapy, and anytime after receiving IFX therapy. Parameters recorded include hemoglobin, white blood cells, platelet count, mean platelet volume, lymphocyte number and percentage, polymorphonuclear leukocytes, C-reactive protein, albumin, total iron-binding capacity, percent saturation, and ferritin.
Relevant therapeutic data before IFX were recorded for two periods: general period and 1 month prior to infusion. We listed therapy in categories of increasing order. In this scheme, 0 = no therapy, 1 = 5-acetylsalicylic acid, 2 = prednisone, 3 = first-line immunomodulators azathioprine/6-mercaptopurine or methotrexate, 4 = second-line immunomodulators cyclosporine A or mycophenolate mofetil, and 5 = previous IFX more than 1 year earlier within the limits of the study period. Information on the intake of medications other than IFX after the start of infusion was recorded inconsistently and was therefore omitted from analysis. However, previous surgical interventions and the presence of external fistulae were recorded. Characteristics of IFX therapy were tabulated separately, including results of a purified protein derivative test, the starting dose, physician’s indication for IFX, and side effects.
Definitions
Indications for IFX treatment included severity of disease defined by physician’s opinion as stated on the request for IFX application. Other indications were control of fistulae with or without aggravated clinical disease or other reasons.
Therapeutic markers and follow-up times in the study were defined by the length of treatment. Primary nonresponders were defined as any patient receiving three or fewer infusions, excluding those with an episodic form of treatment. These patients remained symptomatic with diarrhea, pain, or continued blood loss.
The median length of follow-up was also defined by the median length of treatment. Duration of response was reflected in the time between the first dose (induction phase) and the last dose and is based on similar definitions used elsewhere.Citation18 Those patients continuing therapy were less symptomatic and were considered to be in remission by their physicians. Thus, continued therapy at the end of the observation period was chosen as a surrogate marker of successful maintenance or failure to relapse. No attempt was made to evaluate endoscopic outcome.
Outcome of therapy failure was divided into two types. Grade A failure included a loss of response with increased symptoms resulting in an altered form of therapy, such as surgery, switch to other biologics in the same class, switch to other nonbiologic therapy, or side effects. Grade B failure indicates a loss of response with a requirement for changing frequency or dose of IFX.
Some patients stopped IFX because of miscellaneous reasons. These are included in the results and are not considered a failure of therapy.
Statistical analysis
Analyses were conducted using Intercooled Stata 8.2 statistical software (StataCorp, College Station, TX, USA). A Chi-square test was used to examine the association in two-way tables between disease type and another categorical variable, such as line of treatment; Fisher’s exact test was used if any expected cell count was less than 5. Comparisons of continuous numerical variables by disease type were calculated using a two-group t-test, assuming equal or unequal variances as appropriate. When continuous variables appeared to be non-normally distributed, a nonparametric Kruskal–Wallis (K–W) test was used to test for differences between disease groups.
Length of time, such as the length of disease prior to IFX therapy, was observed for all patients and analyzed using a K–W test for differences in medians. Length of time, such as the time to treatment deterioration and length of treatment, was not observed for all patients (because the events of deterioration and end of treatment could occur after the observed study period), so these values were handled as either observed or censored and survival analysis methods were applied. Nonparametric Kaplan–Meier survival curves were plotted for time until treatment deterioration and length of treatment by disease groups. Differences between the survival curves were examined using a log-rank test. All tests were two-tailed and alpha was set at P = 0.05 for statistical significance.
Three patients with indeterminate colitis were treated as having CD for the analysis.
Results
Ninety-seven patients (71 patients with CD [50% male] and 26 with diagnosis of UC [43% male]) were infused during the observation period. Demographic features of the patients are displayed in . The distribution of CD across disease sites followed expectations, and the majority of patients with UC had pancolitis. Seventeen (24%) CD and two (8%) UC patients underwent surgery anytime prior to IFX. Two patients with previous colectomy for UC had pouchitis, and one of these developed a resistant rectovaginal fistula. Of the CD patients, 15 (21%) had a variety of fistulae that failed medical or surgical therapy. Two CD patients who tested positive for tuberculosis were treated prophylactically.
Table 1 Demographic features of 71 patients with Crohn’s disease (CD) and 26 with idiopathic ulcerative colitis (UC). Data are listed for time-dependent variables as prior to infliximab (IFX) infusion. “General” refers to any time up to 1 month pre-IFX. Marginally significant differences were noted for fistulae by disease type (exact P = 0.06) and surgery before IFX by disease type (exact P = 0.09). No other differences were statistically significant
In each group, over 40% received corticosteroids generally. By 1 month prior to IFX, almost two-thirds of CD and 90% of UC patients received corticosteroids. Overall, in CD, nine (16%) did not respond (corticosteroid resistant) and six (11%) could not discontinue corticosteroids (corticosteroid dependent). In the case of UC, the outcome was seven (33%) and one (5%), respectively. None of the comparisons was significant.
Azathioprine, 6-mercaptopurine, or methotrexate (11/71 in CD, 15.5%) were used in about a third in either CD or UC patients in general. However, by 1 month prior to IFX use, over 80% were using immunomodulators in both diseases. Immunomodulators were continued throughout with episodic IFX use.
outlines a summary of laboratory data obtained for CD and UC patients generally, 1 month before, and at some point within about 1 year after starting IFX therapy. Only two laboratory variables were found to be significantly different between CD and UC. One month prior to IFX infusion, hemoglobin was lower in UC than in CD patients (120.03 ± 18.3 vs 129.06 ± 18.1 g/L, respectively, P = 0.017). After infusions began, lymphocytes were lower in the CD group (CD < UC, 16.1 ± 14.3 vs 24.1 ± 16.5, P = 0.02).
Table 2 Laboratory values for patients with Crohn’s disease (CD) or idiopathic ulcerative colitis (UC). Values are listed as during a general period, 1 month prior to infliximab (IFX) infusion, and general after IFX infusion. Sample sizes are noted for each statistic. Mean values are provided. Bracketed values represent SD
Few C-reactive protein values were available, but, as expected, levels dropped in the period after IFX therapy. Only seven in both groups had C-reactive protein measured 1 month before and at variable time intervals after. As an exercise, a nonparametric sign test showed a significant drop in these values after IFX had been started in patients continued on therapy (P = 0.016). This suggests that larger numbers of patients could have shown significant changes within each disease group as well.
In both groups, virtually all patients were started on IFX 5 mg/kg (See ). In CD patients, 83% were initiated because of severity of disease. Fistulae with or without activity were the indication in 16%, and other reasons were given in the rest (one patient). Indications were also severity of disease in UC for 81% of the cases. One UC patient with a rectovaginal fistula as the primary indication was otherwise well.
Table 3 Characteristics of infliximab treatment for patients with Crohn’s disease (CD) and idiopathic ulcerative colitis (UC)
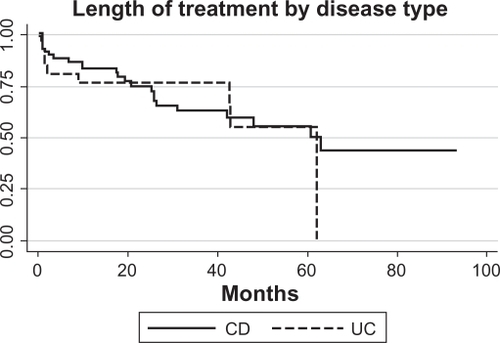
The yearly distribution of IFX initiation between 2000 and 2008 is shown in . There were 1214 infusions (999 for CD and 215 for UC). The median infusions per patient was 12 (range 2–48) for CD and seven (range 2–32) for UC. For the entire group, the median time of follow-up based on length of treatment was 62 months. This is derived from the survival curve in .
Figure 1 The distribution of the number of infusions (by year) and year of start of IFX treatment by disease group are shown (N = 97). Some patients who were tracked at infusion centers during the period of interest of the study began therapy earlier (also described in Methods).
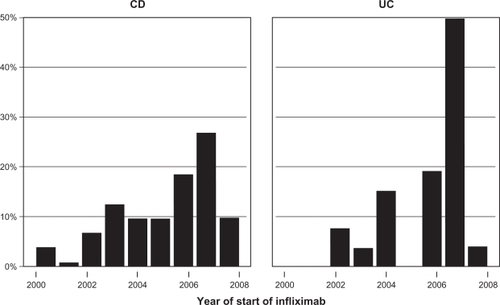
Figure 2 Length of treatment for CD and/or UC is shown for the duration of the observational period of 76 months (log-rank P = 0.5; n = 97).
Although there were more infusions per CD patient than for UC (mean ± standard deviation [SD] 14.1 ± 11.2 vs 8.6 ± 7.1 units, respectively, K–W P = 0.03), the yearly rate was similar (mean ± SD 11.2 ± 11.8 vs 9.9 ± 9.3 infusions/year, K–W P = 0.9).
In the CD group, 55 (78%) had known inductive infusions at 0, 2, and 6 weeks and 96% were infused on a regular basis. In this group, only two patients (3%) received episodic infusions. In the UC group, 81% received triple induction infusions and 96% were regularly maintained. One patient (4%) received episodic infusion during the period of observation. In both groups, 16% of the patient records were not clear as to scheduling of infusions.
There were no deaths during the observation period. In the entire group of 97 patients, only four (4%) serious side effects were listed. One UC patient was recorded as having a delayed lupus syndrome-like reaction. Other side effects included a combination of headaches, nausea, leg swelling, and sinusitis-like symptoms and led to discontinuation of IFX therapy. A higher rate of side effects in UC showed only a marginal significance (P = 0.061).
Characteristics of IFX treatment between CD and UC patients are shown in . Of the entire group, there were 17 primary failures (12 CD and five UC). Missing data resulted in failure to classify six patients (four CD and two UC). As a result, an overall response rate of 76% (77% CD and 73% UC) was observed.
Table 4 Summary of therapeutic failures (primary and Grade A), adjustment requirements (Grade B), or ongoing therapy without primary or Grade A failure
Of the entire group, 54 (56%) patients met our definition of durable response (ongoing therapy without primary or Grade A failure). Of these, only one patient was started within 3 months of the end period. Therefore, 54% of the CD group and 62% of the UC group had durable responses. The summary of failures and responses are outlined in .
Of those patients who were continued on IFX (total 74: 55 CD and 19 UC), a total of 12 (10 CD and two UC) required alternative forms of therapy. These included surgery (five CD patients), switching to adalimumab (four CD patients and one UC patient), and two other unspecified treatments (one CD patient and one UC patient). An additional six patients stopped IFX without meeting definitions of primary failure. The reasons included personal choice, fear of potential IFX side effects, or loss of financial coverage. For the entire group, 23 patients required frequency or dose adjustment (15 CD and eight UC).
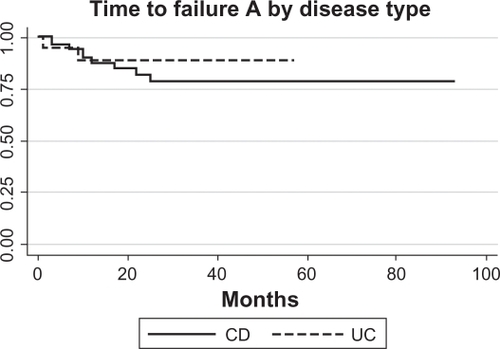
shows survival analysis for Grade A failure (need to change therapy) for the patients without primary failure and with three patients excluded for missing data (log-rank P-value for the difference between groups = 0.86).
Figure 3 Survival analysis of CD (N = 9/52) and UC (N = 2/19) patients who developed Grade A failure (need to stop infliximab for medical reasons and alter therapy). Only patients who did not have primary failure were included, and three patients were missing sufficient data.
We conducted further analysis using logistic regression, with Grade A failure as the dichotomous outcome, among patients with CD who did not have primary failure. The variables that we considered as predictive factors were age, time to disease, gender, and location (described in ); laboratory values 1 month pre-IFX (described in ); and any fistula and any severe disease (described in ). The sample contained 53 CD patients without primary failure and known Grade A failure outcome, with 10 patients who had Grade A failure. The sample sizes for the logistic regressions ranged from seven to 53 patients because of missing covariate data. When predictor factors were entered separately in logistic regression models, total iron-binding capacity at 1 month pre-IFX was the only significant predictor of Grade A failure (P = 0.028). Models were not run for the UC patients, because only two UC patients had Grade A failure among those without primary failure.
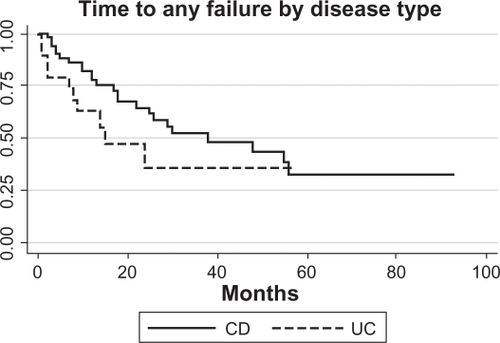
shows survival analysis for any failure defined as either Grade A and/or Grade B (need to adjust therapy). The graph shows patients without primary failure and excludes three with missing data. Five patients (all CD) with Grade A failure also had preceding Grade B failure. There was no significant difference between the survival curves for CD and UC patients (log-rank P-value = 0.14).
Figure 4 Survival analysis CD (N = 24/52) and UC (N = 10/19) patients who developed Grade A failure and/or Grade B failure (need to alter frequency or dose of infliximab therapy). Only patients who did not have primary failure were included, and three patients were missing data.
As an exercise, we also did an ad hoc log-rank analysis to determine whether the consumption of immunomodulators anytime before and continued postinitiation of IFX impacted on durability of response. The addition of neither azathioprine/6-mercaptopurine (P = 0.87) nor methotrexate (P = 0.69) affected this parameter.
Discussion
This long-term retrospective comparison using IFX for mainly luminal CD and either moderately severe CD or UC highlights similarities between the two diseases. These are manifested by patterns of clinical and laboratory variables leading up to the start of infusion, response, and durability rates for longer time periods than currently published in controlled trials. Few serious side effects occurred leading to altering therapy with IFX.
We intended to evaluate patterns of medication use and laboratory values evolving toward biologic therapy and course after the start of treatment. There is some controversy as to whether the addition of immmunomodulators alters response to IFX. The original ACCENT I and II and ACT I and II trials did not establish the benefits of concomitant therapy.Citation14–Citation16,Citation19 Similarly, the COMMIT (Combination of Maintenance Methotrexate-Infliximab Trial) evaluating methotrexate additional therapy with IFX for CD failed to find benefit.Citation20 Others did observe that the addition of immunomodulators prior to IFX does help to maintain duration of response, especially if started more than 3 months before.Citation18 Furthermore, the SONIC (Study of Biologic and Immunomodulator Naive Patients in Crohn’s Disease) suggests that azathioprine/6-mercaptopurine and IFX may induce steroid-free remission better than IFX alone.Citation21 Although we did not find that continuation of these drugs altered duration of response in this study, it was underpowered. Therefore, the relatively high percentage observed in our report may still be explained by the addition of these drugs prior to IFX infusion.
Among laboratory parameters, it was of interest that albumin, total iron-binding capacity, and ferritin levels remained relatively stable in all three periods. Although perhaps of interest, total iron-binding capacity in the month before IFX infusion was the only univariate predictor of need to change therapy. However, no plausible explanation for this finding, other than perhaps chance, could be offered as to why it would be predictive. C-reactive protein was reduced following IFX. However, too few values were available for reliable statistical comparisons. Elevated CRP levels prior to infusion improved by 4 weeks after therapy in another study.Citation22
Outcome of therapy reflects published reports with some improvement. The ACCENT I study for luminally active CD showed a single infusion-induced remission in 58% of patients, and 39% and 45% were still in remission at the end of 46 weeks with either the 5 mg/kg or 10 mg/kg doses, respectively.Citation14 The placebo response was 21%. Similarly, the ACCENT II trial for treatment of fistulae in CD showed a sustained 36% response compared with 19% of placebo recipients after 54 weeks.Citation15 A subsequent subanalysis reported short-term efficacy in rectovaginal fistulae as well.Citation23 Treatment with IFX for CD reduced the need for hospitalizations and surgery.Citation20,Citation24
In the case of UC, early reports indicated conflicting outcomes, especially for rescue treatment to prevent colectomy. Jarnerot et al reported that IFX significantly reduced risk of colectomy compared with 20% of placebo-treated patients.Citation25 A more recent retrospective study suggested that colectomy rates are moderately better with IFX,Citation26 but another such study showed no salvage benefit to IFX.Citation10 In the ACT I and II trials, 69% and 64% of UC patients, respectively, responded, and roughly similar outcomes as the ACCENT trials were reported at 54 weeks. The 5 mg/kg remission rate was 45% and the 10 mg/kg was 44% compared with placebo at 20%.Citation16
In the current study, 76% of either group of patients responded and were higher than reported in randomized trials. In the two largest observational trials of CD patients, the Danish national survey and a Belgian single-center trial, 82.7% and 89.2% responded, respectively.Citation22,Citation27 The reason for these higher response rates is not entirely clear. The randomized trials had a more restricted definition of response. More received triple induction and more patients were on immunomodulators. These maneuvers may have helped to increase response rates.
In CD, a number of studies now have reported durability of response beyond the original 52 weeks of randomized trials. The follow-up times ranged from 15 to 72 months with continued maintenance rates of 37%–66.4%.Citation20,Citation22,Citation28,Citation29 Our study fits into the middle range of durability of these studies.
There are fewer reports on the long-term effects of IFX in UC. The largest number of cases was published by Ferrante et al from the group in Leuven, Belgium.Citation30 Most of these patients were severe or moderately severe, and this study also covered a duration of 6 years. A response rate of 65% was reported, with two-thirds achieving a durable response. The concomitant use of immunomodulators did not influence outcome. The Danish national survey also included 17 UC patients; however, these were not analyzed separately.Citation27 Our limited data suggest similar outcomes for patients with UC as those with CD.
The frequency of side effects was low and similar to the DanishCitation27 report and the study by Rudolph et al.Citation20 Rates were higher in the study from Belgium.Citation22 A possible explanation for our low severe side effect rate may be explained by the fact that the majority of both groups of patients were already taking immunomodulators before starting IFX. This might limit infusion reactions and protect against side effects, as was noted in the Danish study.Citation27 Similarly, regular infusions likely reduced anti-TNFα chimeric antibodies and reactions.Citation24 Finally, the relative youth of both groups may have been protective.
Several weaknesses in our report need to be addressed. The first is that few patients were included in the cohort. The effect of this is that any subtle differences in outcome of treatment between CD and UC may be missed. However, with very few exceptions (like side effects), there were no trends observed, and the differences found between groups were negligible. Second, pouchitis is sometimes classified as a separate entity, but its pathogenesis is putatively a continuation of the overall process in UC,Citation31,Citation32 and it is doubtful that its inclusion (about 8% of total UC) would impact on outcome. Thirdly, we had difficulty ascertaining some variables, especially after IFX. For example, classifying severity of disease before was based on the referring physician’s opinion and, following therapy, assessment was also limited. Similarly, evaluation of other therapy after IFX was severely limited. As a result, we are unable to specifically comment on the outcome of those patients who were steroid nonresponsive or dependent and clearly delineate withdrawal of other immunomodulators. Finally, minor infusion reactions and need for premedication were not clearly defined. However, knowledge of these facts would not appreciably alter overall outcome.
In summary, we report a retrospective review of the pattern of use and outcome of IFX therapy over a 6-year period in a group of moderately severe CD and UC patients. The study suggests similar effects of IFX in both diseases and shows durability and relative safety over an extended time period.
Acknowledgements
We would like to thank Marie-Luce Bernier RN and Stefania D’Aleo BSc RN for invaluable help in collecting data on patients.
Disclosure
Both Drs A Cohen and A Szilagyi have served on advisory board meetings for Schering-Plough. Dr A Cohen participates in clinical trials sponsored by Schering-Plough. The other authors have no conflicts of interest to declare.
A donation of $1999 (Canadian) was received from Schering-Plough.
An abstract of this work was presented in Banff, Alberta, Canada, in February 2009. A publication of the abstract appears in Can J Gastroenterol. 2009;23 Suppl. A:132A.
References
- XavierRJPodolskyDKUnraveling the pathogenesis of inflammatory bowl diseaseNature2007448715242743417653185
- ShiDDasJDasGInflammatory bowel disease requires the interplay between innate and adaptive immune signalsCell Res2006161707416467877
- FussIJHellerFBoirivantMNonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitisJ Clin Invest2004113101490149715146247
- LakatosLImmunology of inflammatory bowel diseasesActa Physiol Hung200087435537211732889
- TsukadaYNakamuraTIimuraMCytokine profile in colonic mucosa of ulcerative colitis correlates with diseases activity and response to granulocytopharesisAm J Gastroenterol200297112820282812425554
- MurchSHLamkinVASavageMOSerum concentrations of tumor necrosis factor alpha in childhood chronic inflammatory bowel diseaseGut19913289139171885073
- MurchSHBraeggerCPWalker-SmithJAMacDonaldTTLocation of tumor necrosis factor α by immunohistochemistry in chronic inflammatory bowel diseaseGut19933412170517098031350
- BraeggerCPNichollsSMurchSHTumor necrosis factor alpha in stool as a marker of intestinal inflammationLancet1992339878589911345871
- ProbertCSHearingSDSchreiberSInfliximab in moderately severe glucocorticoid resistant ulcerative colitis: a randomized controlled trialGut2003527998100212801957
- JakobovitsSLJewellDPTravisSPLInfliximab for the treatment of ulcerative colitis: outcomes in Oxford from 2000 to 2006Aliment Pharmcol Ther200725910551060
- SandsBETremaineWJSandbornWJInfliximab in the treatment of severe, steroid-refractory ulcerative colitis: a pilot studyInflamm Bowel Dis200172838811383595
- OschenskuhnTSackmannMGokeBInfliximab for acute, not steroid refractory ulcerative colitis: a randomized pilot studyEur J Gastroenterol Hepatol200416111167117115489577
- GisbertJPGonzalez-LamaYMateJSystematic review: infliximab therapy in ulcerative colitisAliment Pharmacol Ther2006251193717229218
- HanauerSBFeaganBFLichtensteinGRMaintenance infliximab for Crohn’s disease: the ACCENT I randomized trialLancet200235993171541154912047962
- SandsBEAndersonFHBernsteinCNInfliximab maintenance therapy for fistulizing Crohn’s diseaseN Engl J Med2004350987688514985485
- RutgeertsPSandbornWJFeaganBFInfliximab for induction and maintenance therapy for ulcerative colitisN Engl J Med2005353232462247616339095
- Lennard-JonesJEClassification of inflammatory bowel diseaseScand J Gastroenterol Suppl1989170262617184
- RudolphSJWeinbergDLMcCabeRPLong-term durability of Crohn’s disease treatment with infliximabDig Dis Sci20085341033104117934827
- LichtensteinGRDiamondRHWagnerAInfliximab administration as a 3-dose induction followed by scheduled maintenance therapy in IBD: comparable clinical outcomes with or without concomitant immunomodulatorsGastroenterology20071325A146
- FeaganBMcDonaldJWPanaccioneRA randomized trial of methotrexate in combination with infliximab for the treatment of Crohn’s diseaseGastroenterology20081351294295
- ColombelJFSandbornWJReinishHInfliximab, azathioprine or combination therapy for Crohn’s diseaseN Engl J Med2010362151383139520393175
- SchnitzlerFFidderHFerranteMLong-term outcome of treatment with infliximab in 614 Crohn’s disease patients: results from a single centre cohortGut200958449250018832518
- SandsBEBlankMAPatelKvan DeventerSJLong-term treatmnet of rectovaginal fistulas in Crohn’s disease: response to infliximab in the ACCENT II studyClin Gastroenterol Hepatol200421091292015476155
- RutgeertsPFeaganBGLichtensteinGRComparison of scheduled and episodic treatment strategies of infliximab in Crohn’s diseaseGastroenterology2004126240241314762776
- JarnerotGHertervigEFriis-LibyIInfliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled studyGastroenterology200512871805181115940615
- LeesCDHeysDHoGTA retrospective analysis of the efficacy and safety of infliximab as rescue therapy in acute severe ulcerative colitisAliment Pharmacol Ther200726341141917635376
- CaspersenSElkjaerMRiisLInfliximab for inflammatory bowel disease in Denmark 1999–2005: Clinical outcome and follow-up evaluation of malignancy and mortalityClin Gastroenterol Hepatol20086111212121718848503
- PoupardinCLemannMGendreJPEfficacy of infliximab in Crohn’s disease. Result of a retrospective multicenter study with a 15-month follow-upGastroenterol Clin Biol200630224725216565658
- TeshimaCWThompsonADhanoaLLong- term response rates to infliximab therapy for Crohn’s disease in an outpatient cohortCan J Gastroenterol200923534835219440565
- FerranteMVermeireSKonstantinatosHPredictors of early response to infliximab in patients with ulcerative colitisInflamm Bowel Dis200713212312817206703
- StocchiLPembertonJHPouch and pouchitisGastroenterol Clin North Am200130122324111394032
- ViscidoAHabibFIKohnAInfliximab in refractory pouchitis complicated by fistulae following ileo-anal pouch for ulcerative colitisAliment Phamacol Ther2003171012631271