Abstract
Chromosomal instability is a major pathway of sporadic colon carcinogenesis. Chromosome arm 1p appears to be one of the “hot spots” in the non-neoplastic mucosa that, when deleted, is associated with the initiation of carcinogenesis. Chromosome arm 1p contains genes associated with DNA repair, spindle checkpoint function, apoptosis, multiple microRNAs, the Wnt signaling pathway, tumor suppression, antioxidant activities, and defense against environmental toxins. Loss of 1p is dangerous since it would likely contribute to genomic instability leading to tumorigenesis. The 1p deletion-associated colon carcinogenesis pathways are reviewed at the molecular and cellular levels. Sporadic colon cancer is strongly linked to a high-fat/low-vegetable/low-micronutrient, Western-style diet. We also consider how selected dietary-related compounds (eg, excess hydrophobic bile acids, and low levels of folic acid, niacin, plant-derived antioxidants, and other modulatory compounds) might affect processes leading to chromosomal deletions, and to the molecular and cellular pathways specifically altered by chromosome 1p loss.
Introduction
Chromosomal instability is a major feature of sporadic colon carcinogenesis.Citation1–Citation11 Eighty-five percent of colorectal cancers are aneuploid, the remaining 15% being diploid.Citation5 Chromosome 1p deletions in colon tumors have been reported by laboratories from at least 15 countries around the world.Citation12–Citation49 Chromosome 1p deletions occur at an early stage of colon carcinogenesis,Citation21,Citation24,Citation26–Citation28,Citation30,Citation31,Citation33,Citation37,Citation39,Citation41–Citation45 and are strongly linked to karyotypic evolution during colon cancer development.Citation43
Many reports in the literature indicate that the macroscopically normal mucosa proximal or distal to a colon cancer exhibit aneuploidy (loss or gain of chromosomes or parts of chromosomes). Relevant to this review, Cianciulla et alCitation44 reported that deletions of chromosome 1p were simultaneously found in both the distant normal-appearing mucosa of 76% of patients who also harbored 1p deletions in their cancer. These findings indicate that the loss of chromosome 1p may be one of the “hot spots” among the numerous defects in the non-neoplastic mucosa associated with the possible initiation of colon carcinogenesis.Citation50–Citation70
The pioneering work of Paraskeva et alCitation71–Citation75 indicated the likely involvement of chromosome 1p loss in in vitro immortalizationCitation72,Citation74 and in the progression of adenomas to carcinomas.Citation75 The functional importance of loss of distal 1p in colon tumorigenesis was demonstrated in 1993 by Tanaka et alCitation76 who introduced chromosomal band 1p36 into colon carcinoma cells and found that their tumorigenicity was suppressed.
Chromosome 1p deletions can affect distinct pathways of sporadic colon carcinogenesis, including both chromosomal instability and chromosomal instability-negative pathways. The underlying mechanisms associated with the loss of chromosome 1p that may contribute to genomic instabilty and drive colon carcinogenesis are loss of genes associated with DNA repair, spindle checkpoint function, apoptosis, multiple microRNAs (miRNAs), the Wnt signaling pathway, tumor suppression, antioxidant activities, and defense against environmental toxins.Citation77,Citation78 Since centromeric instability and resulting telomeric fusions have been proposed as a mechanism for the loss of chromosome 1p,Citation79 the loss of genes located on chromosome 1p that function to ensure centromeric stability and telomere integrity, in turn, can exacerbate chromosomal instability throughout the genome. These 1p deletion-associated pathways that may lead to colon carcinogenesis will be reviewed at the molecular and cellular levels, and dietary factors that affect these pathways (eg, excess hydrophobic bile acids, and low levels of folic acid, niacin, plant-derived antioxidants, and other modulatory compounds) will be explored. It is likely that certain dietary factors prevent, initiate, or exacerbate genomic instability in colon epithelial cells and thus have importance for colon carcinogenesis.
Mechanisms of carcinogenesis associated with the loss of key genes on chromosome 1p
Chromosome 1, the longest human chromosome, is gene-dense with 3141 genes.Citation80 The genes located on chromosome 1 were identified with the assistance of the Weizmann Institute of Science websites:
GeneLoc (www.genecards.weizmann.ac.il/geneloc/index.shtml) and GeneCards – The Human Gene Compendium (www.genecards.org). Genes located on the p arm of chromosome 1 that are associated with protection against oxidative stress, DNA damage, mitotic perturbations, excessive cellular proliferation, development of apoptosis resistance, aberrant colonic cell differentiation, and environmental toxicity have been tabulated and the function of the gene products described (–). Since many of these genes have tumor suppressive capabilities, the simultaneous loss caused by a 1p deletion could initiate the formation of neoplastic clones and enhance tumorigenesis through Darwinian selection.Citation8
Table 1 DNA repair and DNA damage response genes
Table 8 Genes associated with protection against environmental and metabolic toxicity
Mechanisms protective against genomic instability
Cells with DNA damage, spindle damage, and dysfunctional telomeres signal DNA damage responses.Citation81–Citation84 These DNA damage responses include the activation of numerous checkpoints that arrest the damaged cells in the G1, S, G2, or M-phase of the cell cycle, depending upon the nature of the damage or dysfunction and the stage of the cell cycle of the target cell. DNA-damage checkpoints are activated following direct damage to DNA.Citation85–Citation91 Spindle assembly checkpoints are activated following damage to the mitotic machinery,Citation85,Citation92–Citation98 or as a result of DNA damage during mitosis.Citation99 Telomere checkpoints are activated by defective telomeres.Citation100–Citation106 These checkpoints prevent the damaged cell from completing DNA replication and mitosis until all damage is repaired (), and thus prevent 1) mutations that could be formed by replicating a damaged DNA template, 2) aneuploidy that could result from chromosome mis-segregation, and 3) telomere fusions that result in anaphase bridges, broken chromosomes, and translocations as a consequence of the well-known breakage–fusion–bridge cycles.Citation107–Citation114
Figure 1 The damaging effects of dietary factors and inflammatory conditions on the colonic epithelium. Damage to DNA, the mitotic spindle, and to telomeres is mediated through the generation of ROS (reactive oxygen species) and/or RNS (reactive nitrogen species). This damage results in the activation of spindle and DNA damage checkpoints, which delay mitosis until repairs are made.
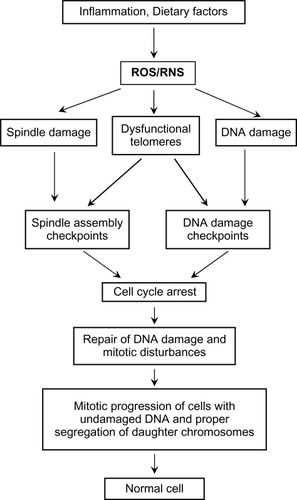
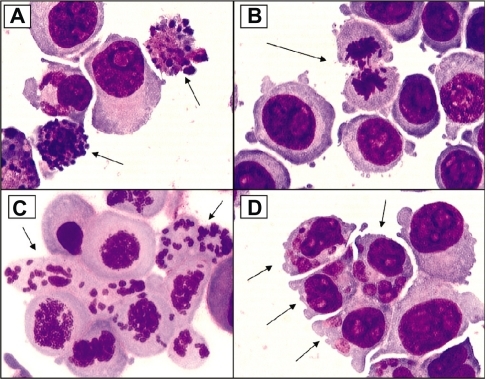
However, cells with excessive direct DNA damage,Citation115–Citation122 massive chromosome loss or chromosomal imbalances,Citation123 prolonged activation or inhibition of the spindle checkpoint pathways,Citation122–Citation127 or excessively shortened or dysfunctional telomeres,Citation128–Citation140 initiate a cascade of molecular events that ultimately leads to either caspase-dependent cell death,Citation141–Citation143 caspase-independent cell death,Citation144 or a special form of apoptosis referred to as mitotic catastropheCitation145–Citation148 (). (Brightfield micrographs are shown in illustrating the cellular alterations that accompany apoptosis [], mitotic perturbation [], mitotic catastrophe [], and micronuclei formation [associated with aneuploidy] []). The cell-destructive and cell-protective pathways are downstream of a common signal transduction network that responds to DNA damage.Citation149 The repair/survival and non-repair/cell death pathways are probably activated simultaneously.Citation149 The repair, checkpoint, and cell death response to DNA damage are, however, well co-ordinated,Citation150 the interplay of positive and negative regulatory loops resulting in a delayed death response to DNA damage.Citation149
Figure 2 Excessive spindle damage, dysfunctional telomeres, or DNA damage can result in a prolonged cell cycle arrest which activates pro-cell death pathways. This activation of pro-cell death pathways leads to removal of cells with unrepaired damage to the mitotic spindle, the chromosome ends, and DNA and prevents the potential propagation of cells with many types of genomic instability.
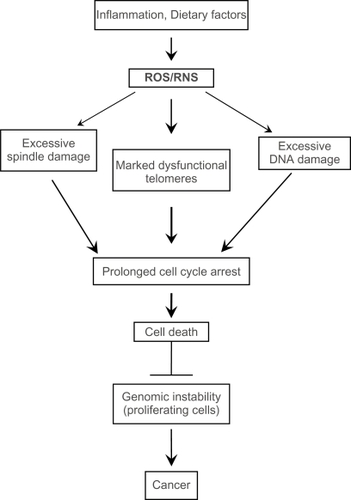
Figure 3 Examples of cellular alterations that accompany apoptosis (A), mitotic perturbation during anaphase (B), mitotic catastrophe with complete chromosome/spindle disruption (C), and abundant micronuclei formation associated with aneuploidy (D). Panels A, B, and D are examples of HCT-116 cells treated with 10 μM camptothecin. Panel C represents cells treated with 5 μM phenstatin (drug obtained through courtesy of Dr GR. Pettit, Arizona State University) (cytospin preparations of Giemsa-stained cells; ×100 oil objective lens)
DNA repair and the DNA damage response (DDR) ()
The genes on chromosome 1p associated with DNA repair or the DNA damage response (DDR) include CLSN, DCL-RE1B (APOLLO), DDI2, GADD45α, MSH4, MUTYH, RAD54L, and TP73. The functions of these gene products are described in . The pathways that lead to the prevention of genomic instability are diagrammatically shown in . DNA damage elicits a well orchestrated and highly interactive series of events called the DDR, which causes cells to undergo growth arrest so that DNA damage can be adequately repaired. Although p53 mutation or loss of heterozygosity (LOH) is a late event in colon carcinogenesis,Citation151 the loss of p73 (found on chromosome 1p) through chromosomal deletion events may act early in colon carcinogenesis. P73 is an important isoform of the p53 family, since it performs many of the transcriptional functions of p53, and may even target the same genes as p53 during the DDR. In addition, TP73 has distinct transcriptional targets and harmonizes with p53 and p63 to maintain genomic stability.Citation152–Citation158 In addition to its role in growth arrest after DNA damage to allow DNA repair to take place, p73 plays an active role in spindle dynamics, mitotic exit and chromosomal stability. The PSRC1 (proline/serine-rich coiled-coil 1) gene found on chromosome 1p (see ) encodes a protein which is a direct transcriptional target of both p53 and p73.Citation159 PSRC1 functions as a microtubule destabilizing protein that controls spindle dynamics and mitotic progression by recruiting and regulating microtubule depolymerases.Citation160 Through its transcriptional activity, p73 is important for the M-to-G1 transition during mitosis.Citation161 Functional knock-out of p73 gene expression by small interfering RNAs alters mitotic progression, resulting in an increase of ana-telophase cells, the accumulation of aberrant late mitotic figures, and the appearance of abnormalities in the subsequent interphase.Citation161 This novel pathway involves the p73-mediated transcription of Kip2/p57, a cyclin-dependent kinase inhibitor, and the coordination of mitotic exit and transition to G1.Citation161,Citation162 Like p53, p73 has been confirmed to be a tumor suppressor.Citation163–Citation167 Therefore, a loss of p73 should have a major impact in the development of genomic instability during carcinogenesis.
Figure 4 DNA damage causes several downstream molecular and cellular events. The DNA damage response involves several DNA repair proteins and transcription factors that allow the cell cycle to be arrested at several points to enhance genomic stability. All of the genes associated with these damage response pathways that are also found on chromosome 1p are highlighted in red, and reference to the appropriate tables (contain functions of gene products) in the text is provided below. The large number of molecular and cellular events affected by the loss of chromosome 1p is apparent.
Abbreviations: DDR, DNA damage response; ROS, reactive oxygen species; RNS, reactive nitrogen species.
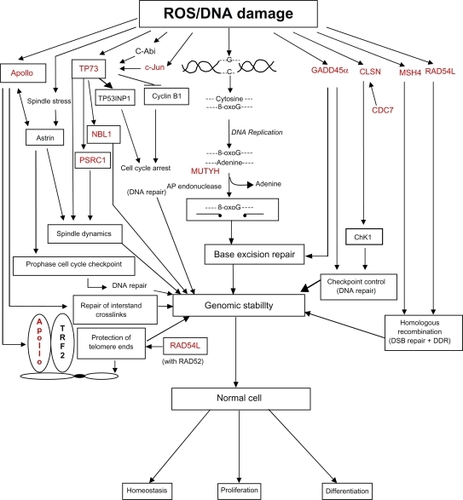
Table 2 Mitosis-related and spindle checkpoint genes
Table 6 Tumor suppressor genes
Table 7 Genes associated with antioxidant function
Since base excision repair (BER) removes damage that would otherwise be mutagenic in mammalian cells,Citation168–Citation170 BER is one of the most important DNA repair pathways in the gastrointestinal tract. BER ameliorates environmentally induced DNA damage in addition to the alkylation, oxidation, and deamination events that occur during normal metabolic processes.Citation171,Citation172 A critical enzyme in the base excision repair pathway is MUTYH (MutY homolog or A/G-specific adenine DNA glycosylase), whose germline mutation is a known cause of MAP (MutYH-associated polyposis), a recently described autosomal recessive colorectal adenoma predisposition syndrome with a very high risk of colorectal cancer.Citation173 Myh deficiency enhances intestinal tumorigenesis in multiple intestinal neoplasia (ApcMin/+) mice.Citation174 Interestingly, Myh deficiency in mice has a larger effect on tumor initiation than on progression in the small bowel.Citation174 Since 1p deletions are observed in the human non-neoplastic mucosa of patients with colon cancer,Citation44 it is possible that Myh-deficient field defects may initiate the process of colon carcinogenesis in humans as it does in the mouse model. Since MUTYH-null mouse embryonic stem cells exhibit a mutator phenotype,Citation175 the loss of MUTYH can affect multiple pathways associated with colon carcinogenesis. The role of MUTYH in the repair of oxidative DNA damage begins with the formation of 8-oxo-guanine (8-oxoG) (see ), which then causes a mispairing of the oxidized guanine base with adenine upon DNA replication. Mismatch repair processes are activated and MUTYH excises adenine leaving an apurinic (AP) site resulting, after AP endonuclease action, in a DNA single strand (ss) break.Citation176–Citation180 The activity of MUTYH, in conjunction with other glycosylases and the spontaneous generation of AP sites, may be quite extensive, since about 9000 AP sites/cell occur daily.Citation168 The AP site is then correctly repaired by the sequential action of several enzymes which catalyze template-directed insertion of one or a few nucleotides at the previously damaged site.Citation172
In addition to their role in DNA repair or the DDR, MUTYH and p73 play important roles in the death of cells that experience either excessive oxidative DNA damage or chromosomal instability. The MUTYH-mediated cell death pathway is described in the next section followed by a section on the p73-mediated cell death pathway, which utilizes part of the MUTYH pathway in its mediation of cell death in response to excessive mitotic perturbation.
MUTYH/PARP/AIF pathway of cell death
MUTYH-mediated cell death has, as a central player, the activation of PARP-1 [poly(ADP-ribose) polymerase-1] (). Excessive DNA ss breaks caused by the action of MUTYH and AP endonuclease in the nucleus results in the activation of PARP-1, which attaches polymers of ADP-ribose to proteins, thereby opening up the chromatin to allow access of DNA repair proteins.Citation181,Citation182 PARP initially serves as a survival protein facilitating the rapid repair of DNA strand breaks, and also prevents DNA degradation, in part, by inhibiting the activity of deoxyribonucleases through the process of poly(ADP) ribosylation.Citation183 Since the synthesis of ADP-ribose polymers consumes nicotinamide adenine dinucleotide (NAD+),Citation184 and NAD+ is largely found in mitochondria where it participates in the production of ATP (bottom right side of ), sustained PARP activation will consume energy reserves, resulting in cell death, usually through the process of necrosis.Citation185–Citation188 A marked deficiency in energy reserves may cause the ATP-dependent Na+/K+ transport proteins, which maintain ionic balance, to fail, resulting in cell swelling and lysis of the cell,Citation189 one of the hallmarks of necrosis.Citation190
Figure 5 The mechanisms by which excessive activity of MUTYH and AP endonucleases can lead to cell death through the activation of PARP and the generation of toxic poly(ADP)ribose (PAR) polymers and mitochondrial DNA (mtDNA) damage (see text for detailed description).
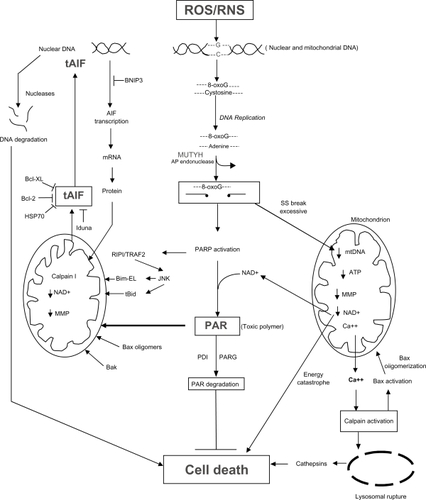
In addition to the above energy catastrophe caused by excessive PARP activity in the nucleus, persistent single-stranded gaps in newly replicated DNA initiated by the action of MUTYH in mitochondria can result in the fragmentation and depletion of mitochondrial DNA (mtDNA)Citation191,Citation192 accompanied by the loss of mitochondrial function culminating in cell deathCitation191,Citation193 (bottom right side of ). Dysfunctional mitochondria can release Ca++ into the cytosol which can activate calpains, causing Bax activation, lysosomal rupture, and the release of cathepsins into the cytosolCitation191,Citation194 resulting in a caspase-independent mode of cell death. Calpain activation can also result in Bax activation, followed by Bax oligomerization and mitochondrial damage, resulting in the loss of the mitochondrial membrane potential.
There is another unique mechanism that can lead to PARP-mediated cell death after excessive MUTYH activity, in addition to the fragmentation of mtDNA, energy catastrophe and calpain/lysosomal rupture/cathepsin pathways of mitochondrial failure described above. The main product of PARP-1 activity is the generation of polymers of ADP-ribose (PAR). Although these polymers are usually covalently bound to proteins, free PAR polymers are themselves toxicCitation195–Citation197 and function as a death signal.Citation197–Citation199 The PAR polymers bind to mitochondria and induce the release of tAIF (truncated apoptosis-inducing factor) from the mitochondria into the cytosolCitation199 (lower left side of ). tAIF is then translocated to the nucleus where it binds to DNA,Citation200–Citation202 causes DNA condensationCitation203 and recruits DNA degrading factors (eg, endogenous endo- and exo-nucleases) resulting in DNA degradationCitation198,Citation204 (upper left side of ). This series of events is part of an intricate program of caspase-independent cell death,Citation203–Citation213 and is currently an active area of research.
Several mechanisms have been proposed to explain how tAIF is released from the mitochondria into the cytosol.Citation210,Citation214 Prior to truncation, AIF is embedded in the inner mitochondrial membrane,Citation215 and the release of AIF requires its cleavageCitation215,Citation216 from a 62 kDa AIF mitochondrial form to a truncated 57 kDa soluble AIF form (tAIF).Citation217,Citation218 Calpain-I, which is activated by Ca++,Citation219 and Ca++-independent cathepsins B, L, and SCitation218,Citation220 can cleave intramitochondrial AIF.Citation221–Citation223 The calpains and cathepsins can truncate AIF in the same position at Gly102/Leu103.Citation218 Calpain-I, however, appears to be the critical enzyme regulating AIF processing in which the AIF pathway is important for cell death.Citation219 Oxidative modifcation of AIF markedly increases the susceptibility of AIF to calpain-I-mediated processing, most probably through the exposure of a normally hidden calpain cleavage site.Citation219 Since the PAR polymer is a highly negatively charged molecule, it could depolarize mitochondria leading to opening of the mitochondrial membrane permeability transition pore (MPTP) followed by the release of tAIF.Citation197,Citation199 PAR polymers of increasing complexity and molecular weight are more toxic than simple PAR polymers of low molecular weight.Citation197 The PAR polymer could also bind to PAR polymer binding proteins associated with mitochondria, which then release AIF.Citation199,Citation224–Citation226 This results in AIF cleavage producing a tAIF, which is soluble and enters the cytosol. The release of tAIF may also be caused by a significant but not excessive decrease in NAD+ (as a result of PARP activity), ATP, and the mitochondrial membrane potential, resulting in the opening of the MPTP (mitochondrial permeability transition pore).Citation186,Citation196,Citation211 The release of tAIF may also be caused by other caspase-independent pathways involving molecules that are often found in the downstream execution phase of apoptosis, such as tBid (truncated Bid),Citation227–Citation229 Bax oligomers (formed after activation of Bax by Ca++-dependent calpains),Citation211,Citation217 Bak,Citation230 and Bim-EL.Citation231,Citation232 The activation of PARP also activates other stress-response pathways such as the RIP/TRAF2/JNK pathway,Citation233–Citation235 which may be responsible, in part, for generation of tBidCitation228 and the phosphorylation of Bim-EL. The phosphorylation of Bim-EL releases Bim-EL from sequestration by the microtubular dynein motor complex,Citation236 allowing it to bind to bcl-2,Citation231 thereby enhancing the cell death process.
Mechanisms that interfere with tAIF release include the 1) degradation of the PAR polymer by PARG (PAR glycohydrolase),Citation237 2) inhibition of tAIF translocation to the nucleus by Bcl-2, Bcl-xl, HSP70, or Iduna, and 3) interference of transcription of the AIF gene by BNIP3.Citation238 PARG, Bcl-2, Bcl-xl, HSP70, Iduna, and BNIP3 have been shown to be upregulated during carcinogenesis, consistent with the development of tumor cell resistance to cell death. In addition, pro-cell death molecules involved in this MUTYH/PARP/AIF pathway, such as AIF, Bid, Bax, Bak, and Bim-EL, have been reported to be downregulated during carcinogenesis. Thus, overall, MUTYH likely has an important role in the death of cells exposed to excessive reactive oxygen species/reactive nitrogen species (ROS/RNS)-induced DNA damage, and interference with the MUTYH cell death pathway is associated with carcinogenesis.
P73 and caspase-dependent cell death
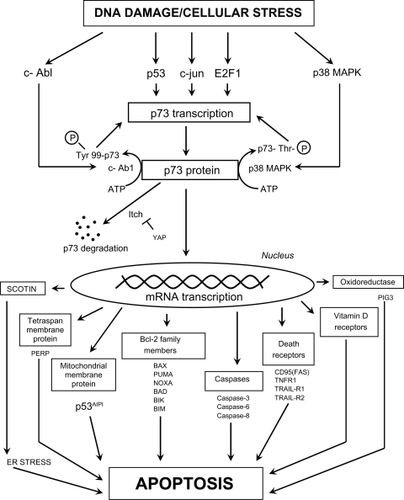
Like p53, p73 is responsible for the induction of apoptosis in response to excessive DNA damage that cannot be repaired.Citation239 P73 has the ability to upregulate the transcription of numerous classic apoptosis-related genes such as caspases 3, 6, and 8, Bcl-2 family members, and death receptors (). In order for p73 to function as a transcription factor, it must be phosphorylated. The c-Abl kinase, activated by DNA damage, phosphorylates and activates p73 on tyrosine 99.Citation240 The stress-induced mitogen-activated protein kinase, p38 MAPK, phosphorylates and activates p73 on threonine residues.Citation239 The degradation of p73 by the E3 ubiquitin-like protein, Itch, is prevented by the Yes-associated protein, YAP. E2F1, p53, and c-jun (located on chromosome 1p; and ) may also have a role in p73 activation in different cell types.Citation241,Citation242 One mechanism by which p73 induces apoptosis includes the transcription of PUMA (p53 upregulated modulator of apoptosis), which in turn causes Bax translocation to the mitochondria with the release of cytochrome c.Citation243 A second mechanism involves the transcription of scotin, which causes endoplasmic reticulum (ER) stress and subsequent apoptosis.Citation244,Citation245 Unlike p53, a direct role of p73 in the apoptotic process (eg, mitochondrial translocation and perturbation) has not been verified. The role of p73 in the regulation of the miRNA processing complex will be discussed in the section “MiRNAs and miRNA processing”. As noted above, loss of p73 through chromosome 1p deletion occurs early in colon carcinogenesis, contrary to the loss of p53 which is a late event.
Figure 6 The possible mechanisms by which p73 transcription and activation can lead to cell death through classic apoptotic mechanisms. Definitions of proteins not included in the main text: PERP (p53 apoptosis effector related to PMP22; tetraspan membrane protein and component of intercellular desmosome junctions); p53AIPI (p53 apoptosis-inducing protein 1; promoter activated by acetylated p73); FAS (CD95) (member 6 of the TNF receptor superfamily which contains a death domain); TNFR1 (member 1A of the TNF receptor superfamily); TRAIL-R1 (member 10A of the TNF receptor superfamily); TRAIL-R2 (member 10B of the TNF receptor superfamily; death receptor 5); PIG3 (p53-induced gene 3 protein; quinone oxidoreductase involved in the generation of ROS and cell death).
Mitosis-related and spindle checkpoint function ()
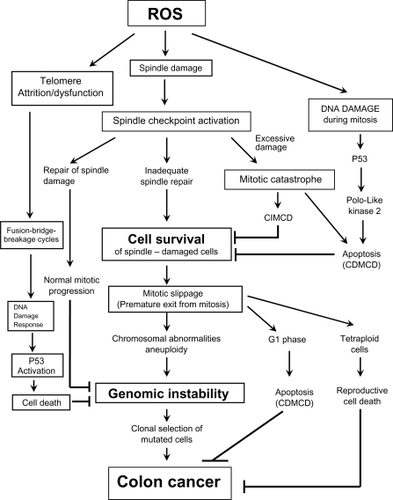
There are 24 genes on chromosome 1p whose gene products affect many different aspects of the mitotic process, and include kinases, phosphatases, centromere proteins, centrosome proteins, cyclins, regulatory mitotic proteins, motor spindle proteins, regulators of chromosomal condensation, a mitosis-related transcription factor, a deacetylase, and a major spindle checkpoint protein (). The large number of mitosis-related genes that are lost if there is a chromosome 1p deletion could potentially be responsible for colon cancer initiation and progression, since cancer epidemiology studies show that abnormal expression of mitosis-related genes is frequent in different tumor types.Citation246,Citation247 Mitotic checkpoints, and specifically the spindle assembly checkpoint, are major targets for tumor-associated alterations.Citation247 The mitotic spindle assembly checkpoint is essential for ensuring that all chromosomes are properly aligned on the metaphase plate, with every chromosome attached to a spindle microtubule by its kinetochore to prevent aneuploidy.Citation97 If these processes fail to occur and the cell undergoes a prolonged mitotic arrest (), the cell may be eliminated through caspase-dependent or caspase-independent cell death mechanismsCitation147 to ensure genomic stability ().
Figure 7 The different cellular fate following spindle, telomere and DNA damage during mitosis. Cells with excessive genomic damage can undergo caspase-dependent cell death (CDMCD) or caspase-independent mitotic cell death (CIMCD). DNA-damaged cells may, however, exit from mitosis by defying cell death pathways through a process referred to as mitotic slippage. These preneoplastic cells with DNA damage and chromosomal abnormalities can then be clonally expanded to produce a tumor and eventually develop into a malignancy through continued cycles of damage to the genome.
Oxidative stress is a major factor that can induce disturbances in spindle organization,Citation248,Citation249 induce centrosome amplification, cause proteolysis of the anaphase inhibitor securin and mitotic cyclins,Citation250 affect components of the anaphase-promoting complex,Citation251 and override the spindle checkpoint,Citation250 thereby affecting chromosomal stability. During the process of mitosis, direct oxidative damage to chromosomes resulting in double-strand breaks, or oxidative damage to telomeres can activate p53 () or p73 (), major DNA damage response proteins that elicit apoptosis through multiple caspase-dependent mechanisms. In addition, caspase-independent mitotic cell death can also occur during a mitotic catastrophe (, ), which is a prestage to distinct modes of cell death that may be caspase-dependent or caspase-independent.Citation148
The length of time that a spindle is destabilized may determine the mode and timing of cell death after mitotic exit.Citation123,Citation124,Citation126 It has been suggested that prolonged mitotic delay can lead to the decay of anti-apoptotic messenger RNAs (mRNAs)Citation252,Citation253 and/or the gradual accumulation of pro-apoptotic signals.Citation252,Citation254 Of the 24 mitosis-related genes (), the products of 7 genes have dual-role mitosis/pro-apoptotic functions. These dual-role mitosis/pro-apoptotic genes include APITD1, CCNL2, CDC2L2, CDC42, E2F2, KIF1B, and PLK3 (). Cells may become genomically unstable if they evade mitotic checkpoints through a process referred to as mitotic slippage, mitotic arrest slippage, or mitotic checkpoint slippageCitation255–Citation263 (). With mitotic slippage, the cell exits mitosis prematurely, carrying broken chromosomes, abnormal numbers of chromosomes, and unrepaired DNA damage into the daughter cells. In addition to loss of pro-apoptotic proteins, it has been reported that the gradual loss of the checkpoint effector, cyclin B, releases the mitotic arrest induced by spindle disruptive agents, despite the continued presence of spindle damage and upstream checkpoint proteins.Citation14,Citation258,Citation260 In order for a DNA-damaged cell to survive after mitotic slippage, it must evade both apoptosis in the subsequent G1 phase of the cell cycleCitation124 () and reproductive cell death that can follow centrosome amplification and the generation of tetraploid cellsCitation264 ().
Thus, a decrease in pro-apoptotic mitotic/cell cycle-related genes located on chromosome 1p (APITD1, CCNL2, CDC2L2, CDC42, E2F2, KIF1B, PLK3) () may result in resistance to cell death, a critical event that drives tumorigenesis.Citation52,Citation54,Citation265–Citation267
Apoptosis-related genes ()
Seven genes associated with apoptosis are located on chromosome 1p. Bcl-10 and Bcl2L15 are Bcl-2 family members, THAP3 is a zinc-coordinating DNA-binding protein, DNA fragmentation factor A (DFFA) and B (DFFB) are the two subunits of DFF, caspase-9 is a major initiator caspase in the apoptotic proteolytic cascade, and TNFRSF25 is a death domain-containing receptor related to TNFR-1 and CD95 (Apo-1/Fas). The deletion of 3 of these genes would have important implications for carcinogenesis through the increase in apoptosis resistance, and will be discussed in some detail.
Table 3 Apoptosis-related genes
DFF is a heterodimeric protein composed of a catalytically active 40 kD subunit, DFFB (CAD [caspase-activated DNase]), and an inhibitory 45 kD subunit, DFFA (ICAD [inhibitor of CAD]).Citation268,Citation269 When bound to DFFB, DFFA inhibits the nuclease activity of DFFB.Citation268,Citation269 During apoptosis, caspase-3 cleaves DFFA at amino acids 117 and 224 and dissociates it from DFFB, thereby releasing the inhibition of DFFB.Citation270 DFFB activity results in chromatin condensationCitation271 and the formation of the typical crescents and margination of chromatin that are characteristic of classic apoptotic cells at the ultrastructural level.Citation190,Citation266,Citation272–Citation276 Characteristic ultrastructural features of apoptotic cells treated with a ROS-generating and DNA-damaging agent are shown in . At the molecular level, the action of DFF on DNA results in the initial cleavage of DNA into 50- to 300-kb long fragments,Citation277,Citation278 representing the dismemberment of the higher order organization of chromatin into chromosomal loop domains, and the fragmentation of DNA into oligonucleosomal sized fragments that form a “ladder” on agarose gel electrophoresis.Citation279 The importance of DFF in suppressing tumorigenesisCitation280 was demonstrated by Yan et alCitation281 using DFF40-null mice. DFF-deficient cells exhibit significant increases in mutation, chromosomal instability, and survival compared with wild-type control cells.Citation281 This is probably a result of the inhibition of cell death of DNA-damaged cells resulting from the failure to undergo DNA fragmentation.Citation282,Citation283 DFF is reported to avoid chromosome instability in a p53-independent manner.Citation284 Irradiation of cells with a caspase-resistant form of DFFA led to increased clonogenic survival of cells with increased chromosomal aberrations and aneuploidy.Citation284 The ability of DFF to maintain chromsosomal stability appears to be the result of the DNA fragmentation-induced death of cells with excessive DNA damage.Citation284 Although DFFB has intrinsic DNAse activity, both DFFA and DFFB are required to generate DNase activity,Citation140,Citation269 and must be co-expressed.Citation280 DFFA has been postulated to stabilize the synthesis of DFFB,Citation270,Citation271 or mediate the correct folding and chromatin localization of DFFB.Citation271 The absence of DFF results in an increased frequency of cell transformation and enhanced susceptibility to radiation-induced carcinogenesis, indicating that DFF is a tumor suppressor.Citation280 Recently, it has been reported that the expression of DFFA protein, but not DFFA mRNA, is regulated by a specific miRNA, miR-145, suggesting a mechanism of translational regulation.Citation285 The regulation of DFFB by miRNA has not been investigated, and, so far, none of the miRNAs found on chromosome 1p () have been determined to have DFFA or DFFB as target mRNAs for translational regulation.
Figure 8 Transmission electron micrographs of HCT-116 cells reacted with 0.5 mM sodium deoxycholate for 2 hours. A) Normal cell (arrow 1) with prominent nucleolus and dispersed chromatin; arrow 2 points to a cell in early apoptosis, showing margination of chromatin, a nucleolus showing nucleolar segregation, and an increase in electron density compared with the normal cell; arrow 3 points to a cell in a late stage of apoptosis showing condensed chromatin, a marked increase in electron density compared with the cell above, and apoptotic body formation. B) Apoptotic cell in a late stage of apoptosis showing condensed chromatin (including crescent formation), an increase in electron density, and cytoplasmic vacuole (V) formation. (Uranyl acetate, lead citrate stains.)
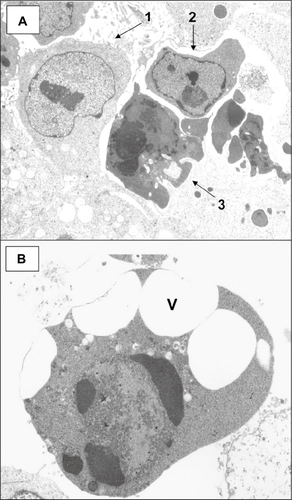
Table 4 MicroRNAs (miRNAs) and components of the miRNA processing complex
Caspase-9 is a member of the family of cysteine-aspartic acid-specific proteases (caspases), and is also referred to as Apaf-3 (apoptotic protease-activating factor 3). In the presence of cytochrome c and dATP, Apaf-1 binds to procaspase-9Citation286 via a CARD (caspase activation recruitment domain),Citation287 forming a complex referred to as the apoptosome.Citation286,Citation288,Citation289 The cellular oxidative state can affect apoptosome formation by promoting an interaction between caspase-9 and Apaf-1 via disulfide formation.Citation290 In the apoptosome, caspase-9 is activated to process other downstream caspases, including caspase-3 and caspase-2.Citation291 Caspase-9 plays an important role in apoptosis induced by genotoxic stress.Citation292,Citation293 The caspase-9-induced apoptotic pathway can result from mitochondrial membrane depolarization, formation of the apoptosome, and the activation of multiple caspases, including caspase-3 and caspase-2.Citation294 Loss of caspase-9 is therefore important to carcinogenesis, since it can result in apoptosis resistance and the propagation of DNA-damaged cells.Citation295 If caspase-9 is lost, caspase-3 cannot be activated, and thus cannot cleave many substrates including DFFA, an essential endonuclease in apoptosis (see previous page). Similarly, if caspase-9 is lost, caspase-2 may not be activated. Caspase-2 plays a specific role in genotoxic stress-induced apoptosis in some cell types.Citation296,Citation297 (However, there is another pathway for activation of caspase-2. Activation of p53 by DNA damage can result in the p53-mediated transcription of the death domain protein PIDD [p53-induced protein with a death domain], which, together with RAIDD or RIP1, can form a multiprotein complex called the PIDDosomeCitation298–Citation300 which then activates caspase-2Citation298). DNA damage can also activate caspase-2 through the activation of c-Abl.Citation301 C-Abl binds directly to caspase-9, phosphorylates it on Tyr-153, which then results in the autocleavage and activation of caspase-9 resulting in the apoptosis of excessively DNA-damaged cells.Citation301 Caspase-9 also mediates apoptosis caused by ER stress.Citation302 ER stress first activates caspase-12,Citation302 which is located on the outer membrane of the ER;Citation303 caspase 12 then activates caspase-9 through a cytochrome c-independent mechanism.Citation302 In some cells, ER stress can result in caspase-8 activation, formation of tBid, mitochondrial damage, release of cytochrome c and the activation of caspase-9 through the formation of the apoptosome.Citation304 Therefore, ER stress can activate caspase-9 through both mitochondrial-independent and -dependent mechanisms.
MiRNAs and miRNA processing ()
miRNAs are evolutionarily conserved, endogenous, small (21 to 24 nucleotides) non-coding RNAs cleaved from 70 to 100 nucleotide hairpin-shaped precursors that reduce translation and stability of target mRNAs through RISC (RNA interference effector complex)-mediated mRNA degradation and translational suppression via sequence-recognition interactions with the 3′ untranslated region of their targeted mRNAs.Citation305–Citation315 The diverse cellular functions affected by miRNAsCitation306,Citation316,Citation317 is underscored by the prediction that thousands of genes are potential miRNA targets.Citation318–Citation320 At least 800 different miRNAs predicted by computational scanning in the human genome have been documented (http://microrna.sanger.ac.uk). Individual miRNAs have the potential to downregulate large numbers of target mRNAs with seed region complementary sites in their 3′ untranslated regions.Citation321–Citation323 It has been speculated that miRNAs could regulate ∼30% of the human genome.Citation306 MiRNAs function in proliferation, cell cycle control, the prevention of replicative stress, differentiation, and apoptosis.Citation324–Citation333 More than half of the known human miRNAs are located at fragile sites, as well as at sites of LOH, amplification, and common breakpoint regions, which are particular genomic regions that are prone to alteration in cancer cells.Citation327 The overexpression or underexpression of miRNAs as a result of chromosomal additions or deletions, respectively, in individual cells can have dramatic effect on hundreds to thousands of target genes. It is, therefore, not surprising that aberrant expression of miRNAs is associated with cancerous tissues,Citation334–Citation340 and that characteristic miRNA expression profiles are features of certain human cancers.Citation341–Citation350 Impaired miRNA processing enhances cellular transformation and tumorigenesis,Citation351,Citation352 and certain miRNAs are even classified as tumor suppressors and oncogenes.Citation353–Citation355 Alterations in a series of specific miRNAs have been associated with the age of onset of colon cancer, the growth of colon cancer cells, and certain stages of colon carcinogenesis.Citation344,Citation356–Citation369 Human colon cancer profiles from 80 colon tumors and 28 samples of normal mucosa show differential miRNA expression depending on mismatch repair status and are characteristic of undifferentiated proliferative states.Citation367 Examination of the genomic regions containing differentially expressed miRNAs revealed that they were also differentially methylated in colon cancer at a far greater rate than would be expected by chance.Citation367 MiRNA profiles could accurately predict microsatellite status in a set of 39 colon cancer studied by Lanza and colleagues.Citation370 This is probably a reflection of the presence or near absence of chromosomal instabilty in the respective microsatellite stable vs unstable cancers.Citation371
There are 20 miRNAs and 3 components of the miRNA processing complex (Argonaute proteins 1,3,4) encoded on chromosome 1p (). One of the 20 miRNAs, miR-34a, is known to be regulated by p53.Citation309,Citation330,Citation372–Citation376 Tarasov et alCitation375 evaluated the differential regulation of 74 miRNAs by p53; 50 miRNAs were either positively or negatively regulated by p53, miR-34a showing the highest fold increase (33.4 fold). Although the 20 miRNAs found on chromosome 1p can have pleiotropic effects on cells, miR-34a is the most well studied for its role in cell cycle arrest and apoptosis in response to DNA damage.Citation309,Citation330,Citation374,Citation377,Citation378 The miR-34 family of miRNAs is one of only 18 mammalian miRNA familiesCitation379 that are present in flies and worms.Citation309 It is probable that links between p53 and the miRNA-34 family may have arisen early in the evolution of the stress-related p53 network.Citation309 Because of its central role in preventing carcinogenesis, miR-34a has been classified as a tumor suppressor.Citation372,Citation377 MiR-34a has numerous downstream targets, including bcl-2 (major anti-apoptotic protein), NOTCH1, Delta1 (ligand for NOTCH1), NOTCH2 (found on chromosome 1p), CDK4, CDK6, Cyclin D1, Cyclin E2, c-Met, MYCN, SIRT1 and E2F3.Citation319,Citation362,Citation374,Citation375,Citation377,Citation380–Citation384 The inhibition of NOTCH1 by miR-34a would enhance apoptosis since NOTCH1 is known to inhibit p53 activityCitation385,Citation386 and to have an anti-apoptotic roleCitation387,Citation388 in tumorigenesis. The inhibition of SIRT1 by miR-34a contributes to p53-dependent apoptosisCitation389 through deacetylating and stabilizing p53 leading to an increase in p21 and PUMA.Citation384 The E2F3 transcription factor is not known to have a role in apoptosis; however, it is a novel repressor of the ARF/p53 pathwayCitation390 and a potent transcriptional inducer of cell-cycle progression.Citation377 Therefore, the downregulation of E2F3 by miRNA-34a would have a growth inhibitory effect.Citation362,Citation374 MYCN has important roles in both cell proliferation and apoptosis, and MYCN amplification is almost always associated with the loss of chromosome 1p36.Citation382 It is probable that the effects of miR-34a on cellular molecular pathways is widespread, since enforced expression of 34A shows a dramatically altered gene expression profile with upregulation of 532 mRNA transcripts and downregulation of 681 mRNA transcripts highly enriched for those genes that regulate cell-cycle progression, apoptosis (BCL2, BIRC3 [baculoviral IAP repeat-containing 3], DcR3 [decoy receptor 3]), DNA repair, and angiogenesis.Citation330 In conclusion, although p53 is a late event in colon carcinogenesis, the deletion of a major downstream target of p53, miR-34a, as a result of chromosomal 1p deletion, could have dramatic effects on colon tumorigenesis.
MiR-101 is a miRNA that, like 34a, is pro-apoptoticCitation391 and considered to be a tumor suppressor.Citation391,Citation392 The nomenclature of miR-101-1 () and miR-101-2 is based on the fact that miR-101-1 is produced from a genomic locus on chromosome 1p31 and miR-101-2 from a genomic locus on chromosome 9p24.Citation392 Loss of heterozygosity at both 1p and 9p are known to be associated with cancer.Citation392 The mechanism by which miR-101 induces apoptosis is by targeting and decreasing the expression of the multifaceted anti-apoptotic protein Mcl-1 (myeloid cell leukemia sequence 1).Citation391 Mcl-1 undergoes rapid turnover which may serve as a convergence point for signals that affect global translation, thereby coupling translation to cell survival and the apoptotic machinery.Citation393 (The DNA damage response can also result in Mcl-1 destruction and the initiation of apoptosis.Citation394,Citation395) Mcl-1 specifically inhibits apoptosis, in part, by sequestering the pro-apoptotic Bim, Bak, tBid, and Noxa, in an inactive state. Since Mcl-1 can interact with tBid and inhibit its induction of cytochrome c release, it plays an important role in resistance to TRAIL and TNFα-induced apoptosis.Citation396,Citation397 Therefore, Mcl-1 can inhibit apoptosis induced by both the death receptor (extrinsic) and mitochondrial (intrinsic) pathways. Mcl-1 is targeted for proteasome-mediated degradation by the E3 ubiquitin ligase MULECitation398 and is rapidly degraded with a half-life of 30 minutes to 3 hours.Citation393 Its short half-life relates to the presence of a long proline-, glutamic acid-, serine-, and threonine-rich (PEST) region upstream of the Bcl-2 homology domains.Citation398 The inhibition of translation with cycloheximide can cause the rapid degradation of Mcl-1 within 30 minutes, thereby triggering the apoptotic machinery through the release of Bim and the activation of Bak and Bax.Citation393 Although full-length Mcl-1 does not interact with Bax, the caspase-mediated cleavage of Mcl-1 at Asp127 generates a fragment that induces apoptosis through direct interaction with Bax.Citation399 Phosphorylation of Mcl-1 can affect its function and degradation.Citation400 The phosphorylation of Mcl-1 is prominent in cells that accumulate in the G2/M phase of the cell cycle as a result of exposure to microtubule disrupting agents, and in synchronized cells passing through this phase.Citation401 This phosphorylation, especially at serine 64, enhances the anti-apoptotic function of Mcl-1,Citation400 thereby allowing cells to properly align their chromosomes prior to anaphase. In colorectal mucosa, the Mcl-1 protein is found in the apical cells of the crypt,Citation402,Citation403 whereas the distribution is more diffuse in the malignant cells.Citation403
In addition to the development of apoptosis resistance, the loss of miR-101 also leads to cancer progression through the overexpression of histone methytransferase EZH2 (enhancer of zeste homolog 2), a polycomb group member, with concomitant dysregulation of epigenetic pathways.Citation392,Citation404 MiR-101 also represses the expression of FOS (v-fos FBJ murine osteosarcoma viral oncogene homolog) oncogene, a key component of the AP-1 (activator protein-1) transcription factor, MYCN (a gene amplified in many tumors), and COX-2, an enzyme involved in the production of prostaglandins from the metabolism of arachidonic acid.Citation405 Enhanced expression of miRNA-101 also has an effect on the late stages of cancer, since it inhibits invasion and migration.
The p53/p63/p73 family of tumor suppressors are known to regulate the major components of the miRNA processing complex,Citation164,Citation406 which include Drosha-DGCR8, Dicer-TRBP2, and Argonaute proteins. Drosha (RNASEN) is an RNAse III endonuclease; DGCR8 is a double stranded RNA binding protein; DICER contains an RNA helicase motif required for the formation of RISC (RNA induced silencing complex); TRBP2 (trans-activation-responsive RNA binding protein 2) is a component of the miRNA loading complex (composed of DICER1, AGO2, and TRBP2) required for the formation of RISC. Argonaute proteins are endonucleases that aid in the maturation of pre-miRNAs of 60 to 70 nucleotides to mature miRNAs of 21 to 24 nucleotides; the tethering to mRNA mimics the miRNA-mediated repression of protein synthesis.Citation164,Citation407,Citation408 There are 8 members of the Argonaute family in the human genome;Citation409 4 belong to the PIWI subfamily and are expressed mainly in the testis, whereas the other 4 belong to the elF2C/AGO subfamily and are expressed in a variety of adult tissues. Ago1 and Ago2 (catalytic engine of RISC) reside in 3 complexes with distinct DICER and RNA-induced proteins involved in RNA metabolism.Citation410 Three of the 4 members of the elF2C/AGO subfamily are found in a tandem cluster of closely related Argonaute non-nucleolytic proteins,Citation411 Ago1, Ag3, and Ago4 on chromosome 1p (). Therefore, loss of chromosome 1p should have a major impact on the process of miRNA processing in the affected cells.
A family of miRNAs on chromosome 1p of particular interest to colon carcinogenesis is the miR-200 family, which includes miR-200a, -200b, and -429 (). These 3 family members are all encoded on a 7.5-kb polycistronic primary miRNA transcript and help determine the epithelial phenotype of cancer cells through the regulation of the Wnt/β-catenin signaling pathway.Citation412,Citation413 Wnt growth factors activate a cascade of intracellular events, known as the canonical Wnt pathway, which ultimately leads to a coordinated proliferation, differentiation, and sorting of the epithelial cell population that forms the colonic crypts.Citation414 In colorectal cancer, epithelial cells that acquire mutations in the Wnt/β-catenin signaling pathway gain inappropriate proliferative capabilities mimicking the effect of a permanent Wnt stimulation.Citation414 Beta-catenin is a transcription factor that translocates to the nucleus and activates target genes involved in stimulation of the cell cycle and inhibition of apoptosis. E-cadherin binds directly to β-catenin in the cytoplasm, which restricts the movement of β-catenin to the nucleus. ZEB1 and ZEB2 are proteins that repress the transcription of E-cadherin. Members of the miR-200 family were found to directly target the mRNA of ZEB1 and ZEB2,Citation412,Citation415–Citation418 upregulate E-cadherin expression in cancer cell lines, and reduce cellular motility.Citation412 Conversely, downregulation of one miR-200 family member that was tested, miR-200a, was shown to promote tumor growth by reducing E-cadherin and activating the Wnt/β-catenin signaling pathway.Citation413 Cancer progression has some similarities with embryonic development and wound healing, in which a process of epithelial-to-mesenchymal transition (EMT) occurs.Citation419 Although the EMT normally occurs as a process of stem cell differentiation, the EMT that occurs during carcinogenesis involves a change from a differentiated tumor to a more invasive dedifferentiated tumor.Citation412,Citation419,Citation420
The loss of the miR-200 family of miRNAs, coupled with the loss of 4 proteins associated with the Wnt/β-catenin signaling pathway ( below), and the loss of the pro-apoptotic miR-34a and the miRNA transcriptional protein, p73, should have a significant impact on the initiation and progression of colon cancer.
Table 5 Genes associated with the Wnt signaling pathway
Wnt/β-catenin signaling pathway ()
The Wnt signaling pathway is critical for the differentiation and sorting of the epithelial cell population necessary for the organization of the colonic crypts and for the regulation of crypt cell renewal and homeostasis.Citation414,Citation421 Wnt signaling is initiated by the binding of extracellular Wnt factors to receptors on the cell surface, which triggers a signaling cascade that leads to the accumulation of β-catenin.Citation414,Citation422 In the absence of Wnt signals, β-catenin is degraded by a multicomplex complex composed, in part, of APC (adenomatous polyposis coli), GSK3β (glycogen synthase kinase-3-beta), and the scaffold proteins Axin1 and Axin2/conductin,Citation423–Citation425 forming the β-catenin destruction box. This destruction box is responsible for the GSK3β-mediated phosphorylation of β-catenin and its subsequent degradation by the ubiquitin-proteasome pathway. The Wnt signals block this phosphorylation and degradation, resulting in the accumulation of β-catenin. Cytoplasmic β-catenin accumulation and translocation to the nucleus allows β-catenin to associate with TCF/LEF (T cell factor/lymphocyte enhancer factor) transcription factors which target genes that enhance cell survival and proliferation (ie, c-myc, cyclin D1).Citation426–Citation428 Mutations in APC, β-catenin, Axin1, or ICAT (inhibitor of beta-catenin and Tcf-interacting protein) result in the deregulated accumulation of β-catenin and the constitutive activation of Wnt signaling,Citation429–Citation431 a major cause of cancer, including colorectal cancer.Citation418,Citation424,Citation425,Citation432
There are 4 genes located on chromosome 1p that are directly involved in the Wnt signaling pathway (CTNNBIP1, DVL1, WNT2B, and WNT4) (). WNT2B and WNT4 are secreted signaling factors and Dvl1 is a cytoplasmic molecule that associates with Frat-1 to activate the Wnt signaling pathway. The loss of these positive regulators of the Wnt signaling pathway as a result of a chromosomal 1p deletion may contribute to the dysregulation of crypt organization that could initiate the carcinogenic process.Citation433 CTNNBIP1/ICAT (), on the other hand, is a negative protein regulator of the Wnt signaling pathway. ICAT disrupts β-catenin–TCF interactions,Citation434–Citation436 thereby downregulating gene expression associated with proliferation and cell survival. The crystallographic structure of ICAT indicates the mechanism by which ICAT interferes with β-catenin function. The NH2-terminal domain of ICAT binds to armadillo repeats 10–12 of β-catenin, whereas the COOH-terminal domain of ICAT binds to the groove formed by armadillo repeats 5–9.Citation435,Citation437 The armadillo repeats 5–9 are crucial for the binding of β-catenin to both TCF and E-cadherin.Citation438 The importance of ICAT in the prevention of carcinogenesis is underscored by the fact that ICAT is a multipotent inhibitor of β-cateninCitation438 by interfering with the binding of β-catenin to TCF, cadherins, and APC, with consequences for transcription, cell adhesion, and cytoskeletal function.Citation438–Citation440 The cytoplasmic and nuclear location of ICAT, using an immunohistochemical approach, is consistent with a broader role for ICAT than previously reported.Citation440
In addition to the effects on transcription and cell adhesion, ICAT can function as a pro-cell death molecule in certain situations. Overexpression of ICAT in colorectal tumor cells results in growth arrest and cell death, and serves to eliminate cells with a constitutively activated Wnt signaling pathway.Citation441 Using flow cytometry, the cell death was evidenced by a sub-G1 peak of the cell cycle, and the forced entry of cells into an illegitimate DNA synthetic phase without having undergone a prior mitosis (enhanced trypan exclusion of >4N cells).Citation441 Transgenic mice expressing ICAT also make activated T cells (dependent on β-catenin–TCF signaling for survivalCitation442,Citation443) highly susceptible to apoptosis (using annexin V staining), by reducing the expression of BclxL below a critical threshold.Citation436 The mechanism by which ICAT reduces BclxL expression is not known at the present time.
Since chromosomal instability is a major feature of colon carcinogenesis, it is appropriate to consider the role of the Wnt signaling pathway in mitotic control and aberrant Wnt signaling in the generation of chromosomal aberrations. A precedent for exploring the role of aberrant Wnt signaling in chromosomal instability are the findings that 1) multiple signaling pathways converge to orient the mitotic spindle in Caenorhabditis elegans embryos;Citation444 2) APC and EB1 (a microtubule-associated protein) have the ability to maintain proper spindle positioning in the developing nervous system of Drosophila;Citation445,Citation446 3) binding of APC protein to microtubules increases microtubule stability and is regulated by GSK3β;Citation447 4) APC has a role in chromosome segregation;Citation448 5) β-catenin is a component of the mammalian mitotic spindle and functions to ensure proper centrosome separation and subsequent establishment of a bipolar spindle;Citation449 6) GSK3β has a role in mitotic spindle dynamics and chromosome alignment,Citation450 and localizes to the centrosome and specialized cytoskeletal structures;Citation451 7) dishevelled genes are involved in mitotic progression in cooperation with polo-like kinase 1;Citation452 and 8) conductin/axin2 and Wnt signaling regulates centrosome cohesion.Citation453 It is now well established that aberrant Wnt/β-catenin signaling can induce chromosomal instability in cancer, including colon cancer.Citation454–Citation458 An understanding of the mechanisms by which specific components of the Wnt signaling pathway affect mitosis, mitotic slippage and other aspects of the cell cycle, including interaction with spindle checkpoint proteins, needs to be experimentally determined.
Tumor suppressors ()
Experiments involving somatic cell fusion and chromosome segregation established the concept that certain genes are capable of suppressing tumorigenesis.Citation459,Citation460 Tumor suppressors are genes whose miRNA or protein products reduce the formation of tumors and prevent malignant progression by decreasing proliferation, regulating the cell cycle, maintaining chromosome integrity, enhancing DNA repair, inducing apoptosis, and, by reducing angiogenesis, invasion, migration, and cell adhesion. Classic tumor suppressor genes that, when deleted or mutated, contribute to tumorigenesis in many types of tumors include p53, RB, INK4a (p16), and ARF.Citation461 In colorectal cancer, mutations and LOH of the tumor suppressor, APC, can affect both the initiation and progression of cancer, whereas the loss of p53 is a late event. Therefore, when the loss of chromosome 1p became associated with many types of cancer, including colon cancer, several groups began the quest to identify the specific tumor suppressor gene or genes located on 1p.Citation462–Citation467 Several genomic loci were identified as “hot spots” for tumor suppressor genes, which included 1p36 and 1p34. It became evident that many genes, both inside and outside of these “hot spots”, could be classified as tumor suppressors; 26 tumor suppressor genes, their genomic loci, and the function of their gene products are listed in . (Note: 11 genes classified as tumor suppressors in are not listed in other tables [– and ]).
Several tumor suppressors are haploinsufficient,Citation468 and cell cycle regulatory tumor suppressor genes seem especially dosage-sensitive.Citation469 These findings indicate that the loss of only one copy of a gene in a diploid cell could have a biologic effect.Citation469 Such a loss could contribute to cellular transformation, with the process of selection driving clonal expansion of pre-neoplastic cells.Citation8
Certain tumor suppressors play a more prominent role in tumorigenesis than others in particular tissue types. However, it is probable that the loss of numerous tumor suppressor genes as a result of a chromosomal deletion probably plays a prominent role in the initiation and progression of cancer through a “combination” of different and/or complementary adverse cellular and molecular events.Citation461,Citation467
Antioxidants ()
Four genes on chromosome 1p are associated with defense against oxidative stress (). Two of these (peroxiredoxin 1 [PRDX1] and endoplasmic reticulum protein ERP19 [TXNDC12]) utilize reducing equivalents provided through the thioredoxin system, and 2 (glutamate-cysteine ligase [modifier subunit] or GCLM and glutathione peroxidase 7 [GPX7]) utilize glutathione. One of the most important genes associated with oxidative stress is glutamate-cysteine ligase (GCL) (also called gamma-glutamylcysteine synthetase), the first rate limiting enzyme of glutathione synthesis.Citation470,Citation471 This enzyme requires coupled ATP hydrolysis to form an amide bond between the γ-carboxyl group of glutamate and the amino group of cysteine to form γ-glutamylcysteine. The enzyme consists of a heavy catalytic subunit (73 kDa) and a light (31 kDa) regulatory subunit (GCLM); the light chain or modifier subunit is found on chromosome 1p. It has been known for the past 2 decades that the ultimate formation of glutathione is required for intestinal function.Citation472 The long-term ingestion of reduced glutathione has recently been shown to suppress the accelerating effect of a beef tallow diet on colon carcinogenesis in rats.Citation473 The specific importance of GCLM to protection against oxidative stress is underscored in GCLM (−/−) knock-out mice, which are severely compromised in the oxidative stress response.Citation474
GCL can be increased by oxidative stress or glutathione depletionCitation475,Citation476 through the inhibition of SHP-1Citation477 and the activation of jun N-terminal kinase (JNK).Citation477,Citation478 The increase in GCL can protect against mitochondrial injury and numerous cellular processes that are depend on the generation of glutathione, such as cell cycle progression, inhibition of caspases (protection against apoptosis), activity of detoxification enzymes (see GSTM genes in ; discussed below), and DNA repair.Citation479–Citation482 Recent studies indicate that a reduced state of proteins in the nucleus is an important environment that induces heterochromatin formationCitation482 and the regulation of histones and PARP activities.Citation483
Defense against environmental and metabolic toxicity ()
Chromosome 1p contains 19 genes associated with protection against toxins/carcinogens derived from the environment, dietary/cooking-derived components, and metabolism (). These genes consist of 2 arylacetamide deacetylase-like enzymes, 4 members of the aldo-keto reductase family, 6 members of the cytochrome P450 family of polypeptides, all 5 members of the mu class of glutathione-S-transferases (GSTs), and 2 metal response element binding transcription factors. A compilation of the 10 most significant transcripton factors capable of targeting the 5′-upstream promoter regions of these 19 genes (GeneCards [SABiosciences’ database; UCSC Genome Browser]) indicates the possible involvement of 95 distinct transcription factors that control their expression. In addition, the Wnt/beta-catenin signaling pathway has been shown to activate various P450 family and GST mu class enzymes in mouse models.Citation484 Since transcription factors respond to different cellular demands and stresses, the presence of these genes on chromosome 1p indicates that the loss of this chromosome arm could compromise the cell’s ability to respond to a variety of environmental toxins/carcinogens that could damage DNA.
It is of interest that all 5 genes of the mu class of GSTs are located on chromosome 1p. The 5 genes are arranged in tandem in the physical order 5′-M4-M2-M1-M5-M3-3′.Citation485,Citation486 The M4-M2-M1-M5 sequence in the gene cluster is oriented in a head-to-tail orientation, whereas the M3 gene is oriented tail-to-tail with respect to the adjacent M5 gene, and is therefore transcribed in the reverse orientation relevant to the other 4 GST mu genes.Citation485 This GST mu gene cluster functions in the detoxification of electrophilic compounds by conjugating glutathione to a wide number of endogenous and exogenous toxins/carcinogens.Citation487 Genetic polymorphisms in GSTM1 increase susceptibility to gastric and colorectal adenocarcinomas.Citation488 In addition, about 70% of human loci is deleted for GSTM1 and 50% of the human population is homozygous deleted for GSTM1.Citation485 This deletion is a result of unequal crossing-over between the two 2.3 kb repeated regions in the intergenic regions that flank the GSTM1 gene. Homozygous deletion of GSTM1 results in increased baseline chromosomal aberrations in lymphocytes among smokers, indicating the role of epoxides and other reactive metabolites of polycyclic aromatic hydrocarbons in inducing genomic instability in these compromised cells.Citation489 All 5 GSTM genes have distinct promoter regions that respond to a different array of transcription factors. Therefore, the loss of chromosome 1p would compromise cellular defenses against toxins/carcinogens, especially in individuals harboring the GSTM1 deletion or other specific polymorphisms.
Development of resistance to cell death and the propagation of cells with DNA damage and chromosomal defects (summary)
We have described in this review how the combination of the persistent damage to a cell’s genome with the inability of that cell to adequately repair the damage or die in response to the excessive damage, is a dangerous situation which can result in clonal selection and the development of colon carcinogenesis. The molecular and cellular mechanisms that are associated with the death of cells are most complex, and include both caspase-dependent and caspase-independent processes. Listed in – are 27 pro-apoptotic/pro-cell death genes found on chromosome 1p, whose simultaneous loss caused by a chromosome 1p deletion could have a major impact on the development of resistance to cell death. In , we extract from those tables the specific genes whose products contribute to cell death. Caspase-9 and both subunits of DNA fragmentation factor are on the downstream execution phase of apoptosis, and the consequences of their loss are obvious. However, the loss of other gene products (eg, TP73, miR-34a) can have pleiotropic effects on cell death pathways because of multiple transcriptional or translational targets. In addition, TP73, KIF1B, and E2F2 are classified as haploinsufficient genes, with loss of function implied with the presence of only 1 allele.Citation490 Some gene products have dual DNA repair/pro-cell death functions (eg, MUTYH) and dual mitosis/pro-cell death functions (KIF1B). One can see () that, in addition to classic pro-apoptotic genes, there are dual role cell survival/pro-cell death genes, DNA damage-response genes, various tumor suppressor genes, genes associated with mitosis, miR-NAs, Wnt signaling, and protection against the generation of peroxides. The mechanism of action of these 27 genes in the control of cell fate is an active area of investigation and beyond the scope of this review. This detailed study of the implications of the loss of chromosome 1p serve as an example of how specific chromosomal deletions can have a major impact on carcinogenesis.
Table 9 Summary of pro-cell death genes on chromosome 1p
Role of dietary factors in colon carcinogenesis (Citation491–Citation538)
In this section we first address what alteration in specific dietary factors can lead to the loss of chromosome segments or entire chromosome arms in general to produce loss of heterozygosity. Second, we will consider how the consequences of the loss of genes located on chromosome 1p might be affected by pro-carcinogenic and anti-carcinogenic dietary factors. Our approach is to show how specific dietary factors may influence the molecular and cellular processes affected by chromosome 1p loss that were described in previous sections. Links of diet to any of the specific genes lost by the 1p deletion (see –) are listed in .
Table 10 Preventive effects of dietary factors on processes and signaling pathways associated with genes located on chromosome 1p
Diets high in fat,Citation473,Citation539–Citation547 but low in fiber,Citation540,Citation548–Citation551 low in vegetable intake,Citation552–Citation555 and micronutrient deficientCitation556–Citation560 induce oxidative stress and DNA damage and adversely affect many molecular pathways that prevent genomic instability and apoptosis resistance, 2 major processes that, together, enhance the development of sporadic colon cancer.
The effects of diet likely occur early in the carcinogenesis process, since an altered vegetable intake is known to affect pivotal carcinogenesis pathways in the colonic mucosa from adenoma patients and controls.Citation561 Although 2 alleles are associated with each gene, and the loss of 1 allele may be compensated for by the other, many genes are reported to be haploinsufficient, including those associated with the mitotic checkpoint.Citation562 It is relevant that TP73, KIF1B, and E2F2, found on chromosome 1p, have also been reported to be haploinsufficient,Citation490,Citation563,Citation564 and could have dramatic consequences for colon tumorigenesis if only 1 allele is expressed in colonic epithelial cells. It is possible that many other genes may be found to be haploinsufficient in the future, since a map of 1079 probable haploinsufficient genes has been compiled by systematic identification of genes unambiguously and repeatedly compromised by copy number variation among 8458 apparently healthy individuals.Citation565 Those genes with a high probability of exhibiting haploinsufficiency were enriched among genes implicated in human dominant diseases and among genes causing abnormal phenotypes in heterozygous knockout mice.Citation565 In addition, the loss of several genes on the same chromosome arm that affect a particular molecular pathway (see –) may together have a significant effect on that pathway, although the loss of a single gene may have little effect. Specific dietary factors may decrease the protein levels of certain genes through post-translational mechanisms (eg, proteasomal degradation), thereby inducing a functional pseudo-biallelic loss of a gene, one through a physical loss of the chromosomal segment harboring that gene, and the other an actual degradation of the gene product.
Although dietary factors may affect many processes associated with carcinogenesis, we will evaluate specific factors associated with oxidative stress/inflammation, since these genotoxic processes are known to have major effects on the initiation and progression of cancer, including colon cancer.Citation566–Citation578 Direct damage to DNA, assessed by immunohistochemical staining of 8-oxoG, correlates with poor survival in colorectal cancer.Citation579 ROS can cause excessive DNA double strand breaks, resulting in the loss of chromosome segments or entire arms, depending on the location of the break. In addition, several DNA repair proteins are degraded through an oxidative mechanism,Citation580,Citation581 thereby affecting DNA repair and increasing susceptibility to cancer.Citation582 Oxidative stress can affect spindle organization, induce centrosome amplification, cause proteolysis of components of the anaphase-promoting complex, and override the spindle checkpoint, thereby affecting chromosomal stability. Therefore, oxidative stress can induce a mutator phenotype in affected cells.Citation583 The big question is what dietary factors contribute directly to oxidative DNA damage and aneuploidy (alteration in the number of whole chromosomes or chromosomal segments). We now address several dietary factors that may be associated with these forms of genomic instability. Although the literature on dietary factors associated with genomic instability is substantial, we have chosen to discuss the effects of a high-fat diet, folate deficiency, and niacin deficiency, since the molecular and cellular mechanisms associated with the overabundance or deficiency of these factors have been especially well studied.
A high-fat diet derived from beef tallow or corn oil (eg, linoleic acid, palmitic acid) is one of the major causes of sporadic colon cancer. Long-chain nonesterified (“free”) fatty acids (FFA) and some of their derivatives and metabolites can modify the intracellular production of ROS, in particular superoxide anions and hydrogen peroxide, in part, through their interference with the mitochondrial electron transport chain.Citation584 FFA can also interfere with the glutathione system and stimulate the generation of superoxide anions from phagocytic NADPH oxidases.Citation584 Chronic exposure of cells to FFA (eg, palmitic acid) can also alter miRNA expression (eg, miR-34a, miR-146).Citation585
The genotoxicity associated with a high-fat diet is also caused, in part, by high concentrations of hydrophobic bile acids released into the gastrointestinal tract in response to high-fat meals where they act as detergents to aid in the digestion of fats. Our research group showed that deoxycholic acid (a major hydrophobic bile acid in the human colon) induces ROSCitation586–Citation589 in vitro, and oxidative DNA damage,Citation590 sessile adenomas,Citation591 and colon cancerCitation592 in dietary-related mouse models. In addition to the bile acid-induced formation of 8-oxoG in guanine bases of DNA and the induction of DNA strand breaks (activation of γ-H2AXCitation593 and PARPCitation594), we have shown that deoxycholic acid affects genomic instability at the chromosomal level.Citation595 Evidence indicating the induction of chromosomal damage by deoxycholic acid include the formation of micronuclei and aberrant mitoses, attenuation of activation of the nocodazole-induced spindle checkpoint, and decrease in protein expression of major spindle checkpoint proteins (eg, Mad2, BubR1, securin). The dramatic effect of deoxycholic acid on the process of mitosis is underscored by the finding that deoxycholic acid modulates 71 mitosis-related genes at the mRNA and/or protein levels in vitro and in vivo using mouse models.Citation8 The induction by hydrophobic bile acids of both DNA and chromosomal damage indicates that hydrophobic bile acids are endogenous carcinogens that, at high pathophysiologic concentrations, are capable of contributing to the initiation and progression of colon cancer.Citation8,Citation189,Citation595–Citation597 In addition to causing genomic instability, deoxycholic acid can activate survival pathways (eg, NF-κBCitation594 and autophagyCitation598), which allow for the survival and selection of cells with genomic instability.Citation8,Citation599
Coffee drinkers have a lower incidence of cancer, including that of the colon and rectum.Citation600–Citation603 One coffee compound that we found to prevent the formation of bile acid-induced proximal colon cancer in a mouse model is chlorogenic acid (CGA), the ester of caffeic acid with quinic acid.Citation592 CGA is one of the most abundant polyphenols in the human diet, with coffee, fruits (eg, blueberry, strawberry, raspberry, apple), and vegetables (eg, eggplants, potato, carrot, tomato) as its major sources.Citation493,Citation604 CGA and its metabolites are likely responsible, in part, for the lower risk of rectal cancer associated with the consumption of decaffeinated coffee in 2 large prospective cohort studies.Citation603 One possible mechanism by which polyphenols can reduce colon cancer in this model is through the reduction in deoxycholic acid levels.Citation605 In this study, Han et alCitation605 report that when rats on a high-fat diet (30% beef tallow) received dietary curcumin (component of the Indian spice turmeric) or caffeic acid (metabolite of CGA), the fecal concentration of deoxycholic acid was substantially reduced. In addition, dietary supplementation of this high-fat diet with caffeic acid, catechin (plant polyphenol), rutin (citrus flavonoid glycoside), and ellagic acid (plant polyphenol) significantly reduced the levels of fecal lithocholic acid, a second major hydrophobic bile acid and risk factor for colon cancer.Citation605
The induction of double-strand breaks is a major cause of the production of chromosomal fragments and the deletion of hundreds to thousands of genes. An important DNA repair protein in preventing large chromosomal deletions is Parp-1Citation606 (). DNA strand breakage is directly caused by ROS (which would be enhanced due to the loss of genes encoding antioxidant proteins in the chromosome 1p deletion []) or as a result of the activity of base excision repair enzymes (see ). Strand breakage activates Parp-1, which is involved with opening up chromatin and allowing DNA repair processes to occur, including base excision repair, single-strand and double-strand repair (). Shibata et alCitation606 carried out mutation analysis using Parp-1 knockout (Parp−/−) mice, and found that PARP deficiency enhanced deletion mutations, especially >1 kbp. A dietary micronutrient whose deficiency has a major effect on PARP activity is niacin (vitamin B3) obtained from meat and corn. The term niacin refers to nicotinic acid and nicotinamide, which are both used by humans to form NAD+. PARP-1 utilizes NAD+ to make poly(ADP-ribose) needed for poly(ADP-ribosyl)ation of proteins. In keeping with the protective effect of PARP, we determined that pre-treatment of cells in vitro with nicotinic acid and nicotinamide protected against bile acid-induced apoptosis,Citation607 presumably by enhancing PARP-mediated DNA repair of bile acid-induced DNA damage and replenishing the NAD+ levels in mitochondria. In addition, we showed that pre-treatment of cells with nicotinic acid and nicotinamide upregulated the mRNA levels of the glycolytic enzymes, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and glucose-6-phosphate dehydrogenase (G6PD).Citation608 GAPDH and G6PD may protect against oxidative stress, in part through the generation of the reduced pyridine nucleotides, NADH and NADPH, respectively, from NAD+.Citation608 Niacin supplementation was even reported to improve pellagra (severe niacin deficiency) in a patient with Crohn’s disease,Citation609 a pre-cancerous inflammatory conditionCitation610 associated with oxidative DNA damage.Citation611 Pellagra most probably developed in these Crohn’s disease patients through a combination of intestinal malabsorption of niacin/nicotinic acidCitation612,Citation613 and the high demand for NAD+ that accompanies DNA damage-induced PARP-1 activity (see ). Work from our laboratory indicated that CGA and its metabolites, caffeic acid, m-coumaric acid, and 3-(m-hydroxyphenyl) propionic acid, increased PARP-1 protein expression.Citation493 The modulation of PARP-1 protein levels by CGA may explain, in part, the colon cancer preventive properties of CGA when added as a supplement to the bile acid-induced colon cancer mouse model.Citation592
The mechanisms by which chromosome segments are deleted and translocated can be most complex. Deletions and translocations can arise from centromeric instability and telomeric instability.Citation7,Citation614 and have been proposed as possible mechanisms for chromosomal aberrations associated with chromosome 1.Citation615–Citation617 Centromeric instability can result from hypomethylation or acetylation of pericentromeric heterochromatin, resulting in decondensation/uncoiling/disruption of the centromereCitation618–Citation620 and loss of the affected chromosome arms. Telomeric instability is characterized by telomeric fusions, formation of anaphase bridges during mitosis, broken chromosomes upon the stress of cell division, and fusion of chromosomal fragments to chromosome ends. This cycle of chromosomal abberations is referred to as breakage–fusion–bridge cycles.Citation107–Citation114,Citation621 Six genes found on chromosome 1p (APITD1, CCDC28B, CDCA8, HDAC1, KIF2C, RCC2) are associated with centomeres (see ), and whose loss would affect centromeric instability. A deficiency of HDAC1, for example, has been reported to disrupt pericentromeric heterochromatin.Citation622 In addition to its role in the repair of interstrand cross-links,Citation623 APOLLO (aka DCLRE1B [DNA cross-link repair 1 B]) is also involved in the protection of telomeres (see ). APOLLO is stabilized when bound to the telomere-binding protein TRF2, and protects human telomeres in S phaseCitation624 (). A reduced level of APOLLO results in an increased number of telomere-induced DNA damage foci and telomeric fusions in S-phase,Citation624 suggesting that APOLLO contributes to a processing step associated with the replication of chromosome ends. Hydrophobic bile acids, probably through the generation of oxidative stress, can modulate 71 genes associated with mitosisCitation8 and decrease the protein expression of 3 major spindle checkpoint proteins (eg, Mad2, BubR1, securin).Citation8 These alterations in gene expression, coupled with direct oxidative damage to components of the mitotic apparatus, may be responsible, in part, for the observed bile acid-induced mitotic aberrations.Citation595 It is, therefore, possible that bile acids may contribute to the loss of chromosome 1p through its effects on centromere instability and telomeric fusions.
Another mechanism by which large chromosomal deletions can occur is through folic acid deficiency.Citation625,Citation626 Folic acid can attenuate the loss of heterozygosity of the DCC tumor suppressor gene in the colonic mucosa of patients with colorectal adenomas,Citation625 indicating that folic acid deficiency can affect allelic deletion and associated micronuclei formation.Citation627,Citation628 Folates are a group of water-soluble B vitamins (obtained from leafy, green vegetables, the whole grain quinoa, and lentils) whose deficiency contributes to colon cancer.Citation629–Citation633 Folates maintain DNA stability through their ability to donate one-carbon units for cellular metabolism and particularly for DNA biosynthesis, repair, and methylation.Citation629,Citation633 Methylenetetrahydrofolate reductase (MTHFR) is a key enzyme in one-carbon metabolism. MTHFR catalyzes a unidirectional reaction that determines the balance between cellular availability of 5,10-methylenetetrahydrofolate, used for thymidylate and purine synthesis, and methyltetrahydrofolate used for biological methylation.Citation629 Folate deficiency, therefore, enhances carcinogenesis by impairing normal methylation and nucleotide synthesis, and creates an imbalance between the partitioning of cellular folates into these two pathways. Inhibition of folate metabolism results in excessive uracil misincorporation into DNACitation633,Citation634 with approximately 4 million uracil bases/cell.Citation559 The repair of 2 adjacent uracil residues on opposite strands of DNA can result in a double-strand break, leading to chromosomal breakage and aneuploidy.Citation558,Citation629,Citation634 Folate deficiency also induces hypomethylation and inhibits DNA excision repair in immortalized normal human colon epithelial cellsCitation633 and in the rat colon.Citation635
Recent studies have implicated folate deficiency in the modulation of miRNA expression.Citation497,Citation636 Using microarrays of 385 known human miRNAs, it was determined that folate deficiency in vitro in cultured cells induced a statistically significant fold-change in 24 miRNAs.Citation636 One of these miRNAs was miR-34a, which is found on chromosome 1p and involved in p53-mediated signaling (see and the section on MiRNA and MiRNA Processing). MiRNAs were also determined to be altered in patients on a folate-deficient diet.Citation636 In addition to folate deficiency, polymorphisms of MTHFR and altered folate levels are associated with colon cancer risk.Citation637–Citation640 The fact that MTHFR is located on chromosome 1p at 1p36.22 indicates that the loss of this chromosome arm, coupled with folate deficiency, can have major effects on genomic instability.
In this section we have considered how dietary factors such as niacin, folic acid, and a low-fat diet associated with low bile acid levels, together with antioxidants that protect against oxidative DNA damage (), might affect the processes relevant to carcinogenesis that are altered by chromosome 1p loss. In addition to a deficiency in dietary factors that prevent oxidative DNA damage, a deficiency of certain dietary factors that modulate DNA repair proteins, miRNA expression, antioxidant enzymes, defenses against environmental toxicity, and the Wnt signaling pathway () can exacerbate the effects of the loss of chromosome 1p. An understanding of the complex molecular and cellular pathways that are affected by dietary factors is an enormous undertaking, but one that has become a focus of colon cancer prevention.
Acknowledgements
This work was supported in part by NIH 5 R01 CA119087, Arizona Biomedical Research Commission Grant #0803, VA Merit Review Grant 0142 of the Southern Arizona Veterans Affairs Health Care System and Biomedical Diagnostics and Research, Inc., Tucson, Arizona.
Disclosure
The authors declare no conflicts of interest.
References
- SteinbeckRGChromosome division figures reveal genomic instability in tumorigenesis of human colon mucosaBr J Cancer199877102710339569034
- HermsenMPostmaCBaakJColorectal adenoma to carcinoma progression follows multiple pathways of chromosomal instabilityGastroenterology20021231109111912360473
- RibasMMasramonLAizaGCapellaGMiroRPeinadoMAThe structural nature of chromosomal instability in colon cancer cellsFASEB J20031728929112475895
- RichterHSlezakPWalchADistinct chromosomal imbalances in nonpolypoid and polypoid colorectal adenomas indicate different genetic pathways in the development of colorectal neoplasmsAm J Pathol200316328729412819033
- RajagopalanHNowakMAVogelsteinBLengauerCThe significance of unstable chromosomes in colorectal cancerNat Rev Cancer2003369570112951588
- PostmaCHermsenMACoffaJChromosomal instability in flat adenomas and carcinomas of the colonJ Pathol200520551452115685687
- SteweniusYGorunovaLJonsonTStructural and numerical chromosome changes in colon cancer develop through telomere-mediated anaphase bridges, not through mitotic multipolarityProc Natl Acad Sci U S A20051025541554615809428
- PayneCMBernsteinCDvorakKBernsteinHHydrophobic bile acids, genomic instability, Darwinian selection, and colon carcinogenesisClin Exp Gastroenterol20081194721677822
- AshktorabHSchafferAADaremipouranMSmootDTLeeEBrimHDistinct genetic alterations in colorectal cancerPloS One20105e887920126641
- PinoMSChungDCThe chromosomal instability pathway in colon cancerGastroenterology20101382059207220420946
- BacolodMDBaranyFGene dysregulations driven by somatic copy number aberrations-biological and clinical implications in colon tumors: A paper from the 2009 William Beaumont Hospital Symposium on Molecular PathologyJ Mol Diagn1255256120709793
- ReichmannAMartinPLevinBChromosomes in human large bowel tumors. A study of chromosome 1Cancer Genet Cytogenet1984122953016744225
- MulerisMSalmonRJDutrillauxBCharacteristic chromosomal imbalances in 18 near-diploid colorectal tumorsCancer Genet Cytogenet1987292893013479234
- MulerisMSalmonRJDutrillauxBExistence of two distinct processes of chromosomal evolution in near-diploid colorectal tumorsCancer Genet Cytogenet19883243503162707
- LeisterIWeithABruderleinSHuman colorectal cancer: High frequency of deletions at chromosome 1p35Cancer Res199050723272351977517
- BravardALuccioniCMulerisMLefrancoisDDutrillauxBRelationships between UMPK and PGD activities and deletions of chromosome 1p in colorectal cancersCancer Genet Cytogenet19915645561660788
- Couturier-TurpinMHEsnousCLouvelAPoirierYCouturierDChromosome 1 in human colorectal tumors. Cytogenetic research on structural changes and their significanceHum Genet1992884314381740320
- BardiGJohanssonBPandisNCytogenetic aberrations in colorectal adenocarcinomas and their correlation with clinicopathologic featuresCancer1993713063148422622
- BardiGPandisNFengerCKronborgOBommeLHeimSDeletion of 1p36 as a primary chromosomal aberration in intestinal tumorigenesisCancer Res19935318951898467511
- BardiGJohanssonBPandisNCytogenetic analysis of 52 colorectal carcinomas – non-random aberration pattern and correlation with pathologic parametersInt J Cancer1993554224288375927
- BommeLBardiGPandisNFengerCKronborgOHeimSClonal karyotypic abnormalities in colorectal adenomas: Clues to the early genetic events in the adenoma-carcinoma sequenceGenes Chromosomes Cancer1994101901967522042
- BardiGSukhikhTPandisNFengerCKronborgOHeimSKaryotypic characterization of colorectal adenocarcinomasGenes Chromosomes Cancer199512971097535093
- GerdesHChenQElahiAHRecurrent deletions involving chromosomes 1, 5, 17, and 18 in colorectal carcinoma: Possible role in biological and clinical behavior of tumorsAnticancer Res19951513247733622
- LotheRAAndersenSNHofstadBDeletion of 1p loci and microsatellite instability in colorectal polypsGenes Chromosomes Cancer1995141821888589034
- PramlCFinkeLHHerfarthCSchlagPSchwabMAmlerLDeletion mapping defines different regions in 1p34.2-pter that may harbor genetic information related to human colorectal cancerOncogene199511135713627478557
- Di VinciAInfusiniEPeveriCRisioMRossiniFPGiarettiWDeletions at chromosome 1p by fluorescence in situ hybridization are an early event in human colorectal tumorigenesisGastroenterol1996111102107
- BommeLBardiGPandisNFengerCKronborgOHeimSChromosome abnormalities in colorectal adenomas: Two cytogenetic subgroups characterized by deletion of 1p and numerical aberrationsHum Pathol199627119211978912830
- BardiGParadaLABommeLCytogenetic findings in metastases from colorectal cancerInt J Cancer1997726046079259398
- OgunbiyiOAGoodfellowPJGagliardiGPrognostic value of chromosome 1p allelic loss in colon cancerGastroenterology19971137617669287966
- Di VinciAInfusiniEPeveriCCorrelation between 1p deletions and aneusomy in human colorectal adenomasInt J Cancer19987545509426689
- Di VinciAInfusiniENigroSMonacoRGiarettiWIntratumor distribution of 1p deletions in human colorectal adenocarcinoma is commonly homogeneous. Indirect evidence of early involvement in colorectal tumorigenesisCancer1998834154229690532
- TomlinsonIIlyasMJohnsonVA comparison of the genetic pathways involved in the pathogenesis of three types of colorectal cancerJ Pathol19981841481529602705
- BommeLHeimSBardiGAllelic imbalance and cytogenetic deletion of 1p in colorectal adenomas: A target region identified between D1S199 and D1S234Genes Chromosomes Cancer1998211851949523193
- Di VinciAInfusiniEPeveriCIntratumor heterogeneity of chromosome 1, 7, 17, and 18 aneusomies obtained by FISH and association with flow cytometric DNA index in human colorectal adenocarcinomasCytometry19993536937510213203
- ParadaLAMaranonAHallenMCytogenetic analyses of secondary liver tumors reveal significant differences in genomic imbalances between primary and metastatic colon carcinomasClin Exp Metastasis19991747147910763912
- RagnarssonGEiriksdottirGJohannsdottirJTJonassonJGEgilssonVIngvarssonSLoss of heterozygosity at chromosome 1p in different solid human tumours: association with survivalBr J Cancer1999791468147410188892
- RashidAHoulihanPSBookerSPetersenGMGiardielloFMHamiltonSRPhenotypic and molecular characteristics of hyperplastic polyposisGastroenterology200011932333210930367
- ThorstensenLQvistHHeimSEvaluation of 1p losses in primary carcinomas, local recurrences and peripheral metastases from colorectal cancer patientsNeoplasia2000251452211228544
- Couterier-TurpinMHBertrandVCouturierDDistal deletion of 1p in colorectal tumors: An initial event and/or a step in carcinogenesis? Study by fluorescence in situ hybridization interphase cytogeneticsCancer Genet Cytogenet2001124475511165322
- ThiagalingamSLakenSWillsonJKMechanisms underlying losses of heterozygosity in human colorectal cancersProc Natl Acad Sci U S A2001982698270211226302
- ShihIMZhouWGoodmanSNLengauerCKinzlerKWVogelsteinBEvidence that genetic instability occurs at an early stage of colorectal tumorigenesisCancer Res20016181882211221861
- NowakMAKomarovaNLSenguptaAThe role of chromosomal instability in tumor initiationProc Natl Acad Sci U S A200299162261623112446840
- HoglundMGisselssonmDHansenGBSallTMitelmanFNilbertMDissecting karyotypic patterns in colorectal tumors: Two distinct but overlapping pathways in the adenoma-carcinoma transitionCancer Res2002625939594612384560
- CianciulliACosimelliMMarzanoRGenetic and pathologic significance of 1p, 17p, and 18q aneusomy and the ERBB2 gene in colorectal cancer and related normal colonic mucosaCancer Genet Cytogenet2004151525915120910
- GiarettiWMolinuSCeccarelliJPrevostoCChromosomal instability, aneuploidy, and gene mutations in human sporadic colorectal adenomasCell Oncol20042630130515623940
- ZhouCZQiuGQZhangFHeLPengZHLoss of heterozygosity on chromosome 1 in sporadic colorectal carcinomaWorld J Gastroenterol2004101431143515133848
- TsafrirDBacolodMSelvanayagamZRelationship of gene expression and chromosomal abnormalities in colorectal cancerCancer Res2006662129213716489013
- FijnemanRJCarvalhoBPostmaCMongeraSvan HinsberghVWMeijerGALoss of 1p36, gain of 8q24, and loss of 9q34 are associated with stroma percentage of colorectal cancerCancer Lett200725822322917977645
- BrosensRPHaanJCCarvalhoBCandidate driver genes in focal chromosomal aberrations of stage II colon cancerJ Pathol201022141142420593488
- SandforthFWitzelLBalzerTGutschmidtSJanickeIRieckenEOIdentification of patients at high risk for colorectal carcinoma from biopsy studies of the apparently normal colorectal mucosa. A multivariate analysisEur J Clin Invest1991212953021909633
- ChhatwalVJNgoiSSChanSTChiaYWMoochhalaSMAberrant expression of nitric oxide synthase in human polyps, neoplastic colonic mucosa and surrounding peritumoral normal mucosaCarcinogenesis199415208120857525094
- BernsteinCBernsteinHGarewalHA bile acid-induced apoptosis assay for colon cancer risk, and associated quality control studiesCancer Res1999592353235710344743
- BernsteinCBernsteinHPayneCMGarewalHField defects in progression to adenocarcinoma of the colon and esophagusElectronic J Biotechnol20003117 Available on the Web: http://www.ejb.org/content/vol3/issue3/full/1.
- BernsteinHHolubecHWarnekeJAPatchy field defects of apoptosis resistance and dedifferentiation in flat mucosa of colon resections from colon cancer patientsAnn Surg Oncol2002950551712052764
- SuterCMMartinDIWardRLHypomethylation of L1 retrotransposons in colorectal cancer and adjacent normal tissueInt J Colorectal Dis2004199510114534800
- RoyHKLiuYWaliRKFour-dimensional elastic light-scattering fingerprints as preneoplastic markers in the rat model of colon carcinogenesisGastroenterology20041261071108115057746
- RoyHKKimYLLiuYRisk stratification of colon carcinogenesis through enhanced backscattering spectroscopy analysis of the uninvolved colonic mucosaClin Cancer Res20061296196816467111
- HaoCYMooreDHWongPBenningtonJLLeeNMChenLCAlteration of gene expression in macroscopically normal colonic mucosa from individuals with a family history of sporadic colon cancerClin Cancer Res2005111400140715746039
- PayneCMHolubecHBernsteinCCrypt-restricted loss and decreased protein expression of cytochrome c oxidase subunit I as potential hypothesis-driven biomarkers of colon cancer riskCancer Epidemiol Biomarkers Prev2005142066207516172211
- BadvieSHanna-MorrisAAnreyevHJCohenPSainiSAllen-MershTGA “field change” of inhibited apoptosis occurs in colorectal mucosa adjacent to colorectal adenocarcinomaJ Clin Pathol20065994294616679352
- BernsteinHPrasadAHolubecHReduced Pms2 in non-neoplastic flat mucosa from patients with colon cancer correlates with reduced apoptosis competenceAppl Immunohistochem Mol Morphol20061416617216785784
- KawakamiKRuszkiewiczABennettGDNA hypermethylation in the normal colonic mucosa of patients with colon cancerBr J Cancer20069459359816421593
- AlbertsDSEinspahrJGKrouseRSKaryometry of the colonic mucosaCancer Epidemiol Biomarkers Prev2007162704271618086777
- PayneCMBernsteinCBernsteinHField change of apoptosis resistance in colonic mucosa of patients with colorectal carcinomaJ Clin Path2007 [electronic letter published February 5, 2007].
- BernsteinCBernsteinHPayneCMDvorakKGarewalHField defects in progression to gastrointestinal tract cancersCancer Lett200826011018164807
- BelshawNJElliottGOFoxallRJProfiling CpG island field methylation in both morphologically normal and neoplastic human colonic mucosaBr J Cancer20089913614218542073
- ChaoHBrownREField effect in cancer – an updateAnn Clin Lab Sci20093933133719880759
- DanielCRBostickRMFlandersWDTGF-α expression as a potential biomarker of risk within the normal-appearing colorectal mucosa of patients with and without incident sporadic adenomaCancer Epidemiol Biomarkers Prev200918657319124482
- BelshawNJPalNTappHSPatterns of DNA methylation in individual colonic crypts reveal aging and cancer-related field defects in the morphologically normal mucosaCarcinogenesis2010311158116320395289
- NguyenHLoustaunauCFacistaADeficient Pms2, ERCC1, Ku86, CcOI in field defects during progression to colon cancerJ Vis Exp201072841 Pii:193110.3791/1931
- ParaskevaCBuckleBGSheerDWigleyCBThe isolation and characterization of colorectal epithelial cell lines at different stages in malignant transformation from familial polyposis coli patientsInt J Cancer19843449566746117
- ParaskevaCFinertySPowellSImmortalization of a human colorectal adenoma cell line by continuous in vitro passage: Possible involvement of chromosome 1 in tumour progressionInt J Cancer1988419089123372063
- ParaskevaCFinertySMountfordRAPowellSCSpecific cytogenetic abnormalities in two new human colorectal adenoma-derived epithelial cell linesCancer Res198949128212862917357
- ParaskevaCHarveyAFinertySPowellSPossible involvement of chromosome 1 in in vitro immortalization: Evidence from progression of a human adenoma-derived cell line in vitroInt J Cancer1989b437437462539335
- WilliamsACHarperSJParaskevaCNeoplastic transformation of a human colonic epithelial cell line: In vitro evidence for the adenoma to carcinoma sequenceCancer Res199050472447302369746
- TanakaKYanoshitaRKonishiMSuppression of tumourigenicity in human colon carcinoma cells by introduction of normal chromosome 1p36 regionOncogene19938225322588101648
- RoschkeAVGlebovOKLababidiSGehlhausKSWeinsteinJNKirschIRChromosomal instability is associated with higher expression expression of genes implicated in epithelial-mesenchymal transition, cancer invasiveness, and metastasis and with lower expression of genes involved in cell cycle checkpoints, DNA repair, and chromatin maintenanceNeoplasia2008101222123018953431
- NegriniSGorgoulisVGHalazonetisTDGenetic instability – an evolving hallmark of cancerNat Rev Mol Cell Biol20101122022820177397
- SawyerJRHusainMLukacsJLStangebyCBinzRLAl-MeftyOTelomeric fusion as a mechanism for the loss of 1p in meningiomaCancer Genetics Cytogenet20031453848
- GregorySGBarlowKFMcLayKEThe DNA sequence and biological annotation of human chromosome 1Nature20064431532116710414
- BartekJBartkovaJLukasJDNA damage signalling guards against activated oncogenes and tumour progressionOncogene2007267773777918066090
- HarperJWElledgeThe DNA damage response: Ten years afterMol Cell20072873974518082599
- JacksonSPBartekThe DNA-damage response in human biology and diseaseNature20094611071107819847258
- CicciaAElledgeSJThe DNA damage response: Making it safe to play with knivesMol Cell20104017920420965415
- NiggEAMitotic kinases as regulators of cell division and its checkpointsNat Rev Mol Cell Biol20012213211413462
- NybergKAMichelsonRJPutnamCWWeinertTAToward maintaining the genome: DNA damage and replication checkpointsAnnu Rev Genet20023661765612429704
- SancarALindsey-BoltzLAUnsal-KacmazKLinnSMolecular mechanisms of mammalian DNA repair and the DNA damage checkpointsAnnu Rev Biochem200473398515189136
- SuTTCellular responses to DNA damage: One signal, multiple choicesAnnu Rev Genet20064018720816805666
- HakemRDNA-damage repair; the good, the bad, and the uglyEMBO J20082758960518285820
- WoodJLChenJDNA-damage checkpoints: Location, location, locationTrends Cell Biol20081845145518760607
- ReinhardtHCYaffeMBKinases that control the cell cycle in response to DNA damage: Chk1, Chk2, and MK2Curr Opin Cell Biol20092124525519230643
- DecordierICundariEKirsch-VoldersMMitotic checkpoints and the maintenance of the chromosome karyotypeMutat Res200865131318242118
- Gimenez-AbianJFDiaz-MartinezLAWirthKGAndrewsCAGimenez-MartinGClarkeDJRegulated separation of sister centromeres depends on the spindle assembly checkpoint but not on the anaphase promoting complex/cyclosomeCell Cycle200541561157516205119
- KopsGJPLWeaverBAAClevelandDWOn the road to cancer: Aneuploidy and the mitotic checkpointNat Rev Cancer2005577378516195750
- MayKMHardwickKGThe spindle checkpointJ Cell Sci20061194139414217038540
- MusacchioASalmonEDThe spindle-assembly checkpoint in space and timeNat Rev Mol Cell Biol2007837939317426725
- SuijkerbuijkSJEKopsGJPLPreventing aneuploidy: The contribution of mitotic checkpoint proteinsBiochim Biophys Acta20081786243118472014
- ThirthagiriERobinsonCMHuntleySSpindle assembly checkpoint and centrosome abnormalities in oral cancerCancer Lett200725827628517959302
- MikhailovAColeRWRiederCLDNA damage during mitosis in human cells delays the metaphase/anaphase transition via the spindle-assembly checkpointCurr Biol2002121797180612419179
- D’Adda di FagagnaFReaperPMClay-FarraceA DNA damage checkpoint response in telomere-initiated senescenceNature200342619419814608368
- LongheseMPDNA damage response at functional and dysfunctional telomeresGenes Develop20082212514018198332
- MaserRSDePinhoRATelomeres and the DNA damage response: Why the fox is guarding the henhouseDNA Repair2004397998815279784
- MeierAFieglerHMunozPSpreading of mammalian DNA-damage response factors studied by ChIP-chip at damaged telomeresEMBO J2007262707271817491589
- PanticMZimmermannSEl DalyHTelomere dysfunction and loss of p53 cooperate in defective mitotic segregation of chromosomes in cancer cellsOncogene2006254413442016547498
- TakaiHSmogorzewskaAde LangeTDNA damage foci at dysfunctional telomeresCurr Biol2003131549155612956959
- ViscardiVClericiMCartagena-LirolaHLongheseMPTelomeres and DNA damage checkpointsBiochimie20058761362415989978
- GisselssonDPetterssonLHoglundMChromosomal breakage-fusion-bridge events cause genetic intratumor heterogeneityProc Natl Acad Sci U S A2000975357536210805796
- HoffelderDRLuoLBurkeNAWatkinsSCGollinSMSaundersWSResolution of anaphase bridges in cancer cellsChromosoma200411238939715156327
- KitadaKYamasakiTThe complicated copy number alterations in chromosome 7 of a lung cancer cell line is explained by a model based on repeated breakage-fusion-bridge cyclesCancer Genet Cytogenet2008185111918656688
- LoAWISabatierLFouladiBPottierGRicoulMMurnaneJPDNA amplification by breakage/fusion/bridge cycles initiated by spontaneous telomere loss in a human cancer cell lineNeoplasia2002453153812407447
- McClintockBThe behavior in successive nuclear divisions of a chromosome broken at meiosisProc Natl Acad Sci U S A19392540541616577924
- McClintockBThe fusion of broken ends of chromosomes following nuclear fusionProc Natl Acad Sci U S A19422845846316578057
- SelvarajahSYoshimotoMParkPCThe breakage-fusion-bridge (BFB) cycle as a mechanism for generating genetic heterogeneity in osteosarcomaChromosoma200611545946716897100
- ShimizuNShingakiKKaneko-SasaguriYHashizumeTKandaTWhen, where and how the bridge breaks: Anaphase bridge breakage plays a crucial role in gene amplification and HSR generationExp Cell Res200530223324315561104
- BreeRTNearyCSamaliALowndesNFThe switch from survival responses to apoptosis after chromosomal breaksDNA Repair2004398999515279785
- BrodskyMHWeinertBTTsangGDrosophila melanogaster MNK/Chk2 and p53 regulate multiple DNA repair and apoptotic pathways following DNA damageMol Cell Biol2004241219123114729967
- KastanMBDNA damage responses: Mechanisms and roles in human diseaseMol Cancer Res2008651752418403632
- KohnKWPommierYMolecular interaction map of the p53 and Mdm2 logic elements, which control the Off-On switch of p53 in response to DNA damageBiochem Biophys Res Comm200533181682715865937
- LeeMWKimW-JBeardsleyDIBrownKDN-Methyl-N’-Nitro-N-Nitrosoguanidine activates multiple cell death mechanisms in human fibroblastsDNA Cell Biol20072668369417678437
- LiontosMNiforouKVelimeziGModulation of the E2F1-driven cancer cell fate by the DNA damage response machinery and potential novel E2F1 targets in osteosarcomasAm J Pathol200917537639119541929
- MichalakEVillungerAErlacherMStrasserADeath squads enlisted by the tumour suppressor p53Biochem Biophys Res Comm200533178679815865934
- MorrisonCRiederCLChromosome damage and progression into and through mitosis in vertebratesDNA Repair200431133113915279802
- KopsGJPLFoltzDRClevelandDWLethality to human cancer cells through massive chromosome loss by inhibition of the mitotic checkpointProc Natl Acad Sci U S A200410168704
- BekierMEFischbachRLeeJTaylorWRLength of mitotic arrest induced by microtubule-stabilizing drugs determines cell death after mitotic exitMol Cancer Ther200981646165419509263
- NittaMKobayashiOHondaSSpindle checkpoint function is required for mitotic catastrophe induced by DNA-damaging agentsOncogene2004236548655815221012
- RiederCLMaiatoHStuck in division or passing through: What happens when cells cannot satisfy the spindle assembly checkpointDev Cell2004763765115525526
- WeaverBAAClevelandDWDecoding the links between mitosis, cancer, and chemotherapy: The mitotic checkpoint, adaptation, and cell deathCancer Cell2005871216023594
- AhmadKGolicKGTelomere loss in somatic cells of Drosophila causes cell cycle arrest and apoptosisGenetics19991511041105110049921
- AokiHIwadoEEllerMSTelomere 3’ overhang-specific DNA oligonucleotides induce autophagy in malignant glioma cellsFASEB J2007212918293017449721
- ArkusNA mathematical model of cellular apoptosis and senescence through the dynamics of telomere lossJ Theoret Biol2005235133215833310
- ArtandiSEAttardiLDPathways connecting telomeres and p53 in senescence, apoptosis, and cancerBiochem Biophys Res Comm200533188189015865944
- EllerMSPuriNHadshiewIMVennaSSGilchrestBAInduction of apoptosis by telomere 3- overhang-specific DNAExp Cell Res200227618519312027448
- EspejelSFrancoSRodriquez-PeralesBoufflerSDCigudosaJCBlascoMAMammalian Ku86 mediates chromosomal fusions and apoptosis caused by critically short telomeresEMBO J2002212207221911980718
- HemannMTStrongMAHaoL-YGreiderCWThe shortest telomere, not average telomere length, is critical for cell viability and chromosomal stabilityCell2001107677711595186
- HerbertB-SPittsAEBakerSIInhibition of human telomerase in immortal human cell cells leads to progressive telomere shortening and cell deathProc Natl Acad Sci U S A199996142761428110588696
- KaminkerPGKimS-HTaylorRDTANK2, a new TRF1-associated poly(ADP-ribose) polymerase, causes rapid induction of cell death upon overexpressionJ Biol Chem2001276358913589911454873
- KarlsederJBroccoliDDaiYHardySde LangeTp53- and ATM-dependent apoptosis induced by telomeres lacking TRF2Science19992831321132510037601
- RamirezRCarracedoJJimenezRCanelaAHerreraEMassive telomere loss is an early event of DNA damage-induced apoptosisJ Biol Chem200327883684212409303
- TitenSWAGolicKGTelomere loss provokes multiple pathways to apoptosis and produces genomic instability in Drosophila melanogasterGenetics20081801821183218845846
- ZhangXMarVZhouWHarringtonLRobinsonMOTelomere shortening and apoptosis in telomerase-inhibited human tumor cellsGenes Dev1999132388239910500096
- PlescaDMazumderSAlmasanADNA damage response and apoptosisMethods Enzymol200844610712218603118
- RoosWPKainaBDNA damage-induced cell death by apoptosisTrends Mol Med20061244045016899408
- YuJZhangLThe transcriptional targets of p53 in apoptosis controlBiochem Biophys Res Comm200533185185815865941
- KitagawaKNiikuraYCaspase-independent mitotic death (CIMD)Cell Cycle200871001100518414023
- CastedoMPerfettiniJ-LRoumierTMitotic catastrophe constitutes a special case of apoptosis whose suppression entails aneuploidyOncogene2004234362437015048075
- CastedoMPerfettiniJLRoumierTAndreauKMedemaRKroemerGCell death by mitotic catastrophe: A molecular definitionOncogene232825283715077146
- MansillaSPriebeWPortugalJMitotic catastrophe results in cell death by caspase-dependent and caspase-independent mechanismsCell Cycle20065536016319532
- VakifahmetogluHOlssonMZhivotovskyBDeath through a tragedy: mitotic catastropheCell Death Differ2008151153116218404154
- BorgesHLLindenRWangJYJDNA damage-induced cell death: Lessons from the central nervous systemCell Res200818172618087290
- WangJYChoSKCoordination of repair, checkpoint and cell death responses to DNA damageAdv Protein Chem20046910113515588841
- FearonERVogelsteinBA genetic model for colorectal tumorigenesisCell1990617597672188735
- Murray-ZmijewskiFLaneDPBourdonJCp53/p63/p73 isoforms: an orchestra of isoforms to harmonise cell differentiation and response to stressCell Death Differ20061396297216601753
- BourdonJ-Cp53 and its isoforms in cancerBr J Cancer20079727728217637683
- MarabeseMVikhanskayaFBrogginiMp73: a chiaroscuro gene in cancerEur J Cancer2007431361137217428654
- OswaldCStieweTIn good times and bad: p73 in cancerCell Cycle200871726173118583938
- ScianMJCarchmanEHMohanrajLWild-type p53 and p73 negatively regulate expression of proliferation related genesOncogene2008272583259317982488
- TomkovaKTomkaMZajacVContributions of p53, p63, and p73 to the developmental diseases and cancerNeoplasma20085517718118348649
- VilgelmAEWashingtonMKWeiJChenHPrassolovVSZaikaAIInteractions of the p53 protein family in cellular stress response in gastrointestinal tumorsMol Cancer Ther2010969370520197393
- HsiehSCLoPKWangFFMouse DDA3 gene is a direct transcriptional target of p53 and p73Oncogene2002213050305712082536
- JangCYWongJCoppingerJASekiAYatesJR3rdFangGDDA3 recruits microtubule depolymerase Kif2a to spindle poles and controls spindle dynamics and mitotic chromosome movementJ Cell Biol200818125526718411309
- MerloPFulcoMCostanzoAA role of p73 in mitotic exitJ Biol Chem2893035430360
- TomasiniRMakTWMelinoGThe impact of p53 and p73 on aneuploidy and cancerTrends Cell Biol20081824425218406616
- BoominathanLSome facts and thoughts: p73 as a tumor suppressor gene in the network of tumor suppressorsMol Cancer200762717407586
- BoominathanLThe tumor suppressors p53, p63, and p73 are regulators of microRNA processing complexPloS One20105e1061520485546
- RosenbluthJMPietenpolJAThe jury is in: p73 is a tumor suppressor after allGenes Dev2008222591259518832062
- CollavinLLunardiADel SalGp53-family proteins and their regulators: Hubs and spokes in tumor suppressionCell Death Differ20101790191120379196
- Zawacka-PankauJKosteckaASznarkowskaAHedstromEKawiakAp73 tumor suppressor protein. A close relative of p53 not only in structure but also in anti-cancer approach?Cell Cycle2010972072820160513
- SimonelliVNarcisoLDogliottiEFortiniPBase excision repair intermediates are mutagenic in mammalian cellsNucleic Acids Res2005334404441116077026
- NeeleyWLEssigmannJMMechanisms of formation, genotoxicity, and mutation of guanine oxidation productsChem Res Toxicol20061949150516608160
- DavidSSO’SheaVLKunduSBase excision repair of oxidative DNA damageNature200744794195017581577
- LindahlTWoodRDQuality control by DNA repairScience19992861897190510583946
- KairupanCScottRJBase excision repair and the role of MUTYHHered Cancer Clin Pract2007519920919725997
- TenesaACampbellHBarnetsonRPorteousMDunlopMFarringtonSMAssociation of MUTYH and colorectal cancerBr J Cancer20069523924216804517
- SieberOMHowarthKMThirlwellCMyh deficiency enhances intestinal tumorogenesis in multiple intestinal neoplasia (ApcMin/+) miceCancer Res2004648876888115604247
- HiranoSTominagaYIchinoeAMutator phenotype of MUTYH-null mouse embryonic stem cellsJ Biol Chem2003278381213812412917422
- SlupskaMMBaikalovCLutherWMChiangJHWeiYFMillerJHCloning and sequencing a human homolog (hMYH) of the Escherichia coli mutY gene whose function is required for the repair of oxidative DNA damageJ Bacteriol1996178388538928682794
- ShinmuraKYamaguchiSSaitohTAdenine excisional repair function of MYH protein on the adenine: 8-hydroxyguanine base pair in double-stranded DNANucleic Acids Res2000284912491811121482
- BolgoghIMilliganDLeeMSBassettHLloydSMcCulloughAKhMYH cell cycle-dependent expression, subcellular localization and association with replication foci: evidence suggesting replication-coupled repair of adenine:8-oxoguanine mispairsNucleic Acids Res2001292802280911433026
- ScharerODJiricnyJRecent progress in the biology, chemistry and structural biology of DNA glycosylasesBioessays20012327028111223884
- JiricnyJManaGDNA repair defects in colon cancerCurr Opin Genet Develop2003136169
- De MurciaGde MurciaJMPoly(ADP-ribose) polymerase: A molecular nick-sensorTrends Biol Sci199419172176
- MortusewiczOAmeJ-CSchreiberVLeonhardtHFeedback-regulated poly(ADP-ribosyl)ation by PARP-1 is required for rapid response to DNA damage in living cellsNucleic Acids Res2007357665767517982172
- YoshiharaKTanaigawaYBurzioLKoideSSEvidence for adenosine diphosphate ribosylation of Ca2+, Mg2+– dependent endo-nucleaseProc Natl Acad Sci U S A197572289293235125
- WielckensKSchmidtAGeorgeEBredehorstRHilzHDNA fragmentation and NAD depletion. Their relationship to the turnover of endogenous mon(ADP-ribosyl) and poly(ADP-ribosyl) proteinsJ Biol Chem198225712872128776813330
- HaHCSnyderSHPoly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletionProc Natl Acad Sci U S A199996139781398210570184
- ZongWXDitsworthDBauerDEWangZQThompsonCBAlkylating DNA damage stimulates a regulated form of necrotic cell deathGenes Dev2004181272128215145826
- CiprianiGRapizziEVannacciARizzutoRMoroniFChiarugiANuclear poly(ADP-ribose) polymerase-1 rapidly triggers mitochondrial dysfunctionJ Biol Chem2005280172271723415750180
- LiuHKnabbJRSpikeBTMacleodKFElevated poly-(ADP-ribose)-polymerase activity sensitizes retinoblastoma-deficient cells to DNA damage-induced necrosisMol Cancer Res200971099110919584263
- BernsteinHBernsteinCPayneCMDvorakovaKGarewalHBile acids as carcinogens in human gastrointestinal cancersMutat Res2005589476515652226
- PayneCMBernsteinCBernsteinHApoptosis overview emphasizing oxidative stress, DNA damage and signal-transduction pathwaysLeuk Lymphoma19951943938574171
- OkaSOhnoMTsuchimotoDSakumiKFuruichiMNakabeppuYTwo distinct pathways of cell death triggered by oxidative damage to nuclear and mitochondrial DNAsEMBO J20082742143218188152
- OkaSOhnoMNakabeppuYConstruction and characterization of a cell line deficient in repair of mitochondrial, but not nuclear, oxidative DNA damageMethods Mol Biol200955425126519513679
- IchikawaJTsuchimotoDOkaSOxidation of mitochondrial deoxynucleotide pools by exposure to sodium nitroprusside induces cell deathDNA Repair2008741843018155646
- KagedalKJohanssonACJohanssonULysosomal membrane permeabilization during apoptosis-involvement of Bax?Int J Exp Pathol20058630932116191103
- WangHYuSWKohDWApoptosis-inducing factor substitutes for caspase executioners in NMDA-triggered excitotoxic neuronal deathJ Neurosci200424109631097315574746
- YuSWWangHPoitrasMFMediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factorScience200229725926312114629
- AndrabiSAKimNSYuS-WPoly(ADP-ribose) (PAR) polymer is a death signalProc Natl Acad Sci U S A2006103183081831317116882
- WangYDawsonVLDawsonTMPoly(ADP-ribose) signals to mitochondrial AIF: A key event in parthanatosExp Neurol200921819320219332058
- YuS-WAndrabiSAWangHApoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell deathProc Natl Acad Sci U S A2006103183141831917116881
- LiptonSABossy-WetzelEDueling activities of AIF in cell death versus survival: DNA binding and redox activityCell200211114715012408857
- YeHCandeCStephanouNCDNA binding is required for the apoptogenic action of apoptosis inducing factorNat Struct Biol2002968068412198487
- VahsenNCandeCDupaignePPhysical interaction of apoptosis-inducing factor with DNA and RNAOncogene2006251763177416278674
- SusinSALorenzoHKZamzamiNMolecular characterization of mitochondrial apoptosis-inducing factorNature19993974414469989411
- ModjtahediNGiordanettoFMadeoFKroemerG(2006) Apoptosis-inducing factor: Vital and lethalTrends Cell Biol20051626427216621561
- HongSJDawsonTMDawsonVLNuclear and mitochondrial conversations in cell death: PARP-1 and AIF signalingTrends Pharmacol Sci20042525926415120492
- Van WijkSJHagemanGJPoly(ADP-ribose) polymerase-1 mediated caspase-independent cell death after ischemia/reperfusionFree Radic Biol Med200539819015925280
- BoujradHGubkinaORobertNKranticSSusinSAAIF-mediated programmed necrosis: A highly regulated way to dieCell Cycle2007626111618
- KranticSMechawarNReixSQuirionRApoptosis-inducing factor: A matter of neuron life and deathProg Neurobiol20078117919617267093
- LiGYOsborneNNOxidative-induced apoptosis to an immortalized ganglion cell line is caspase independent but involves the activation of poly(ADP-ribose) polymerase and apoptosis-inducing factorBrain Res20081188354318053973
- LorenzoHKSusinSATherapeutic potential of AIF-mediated caspase-independent programmed cell deathDrug Resist Updates200710235255
- MoubarakRSYusteVJArtusCSequential activation of poly(ADP-ribose) polymerase 1, calpains, and Bax is essential in apoptosis-inducing factor-mediated programmed necrosisMol Cell Biol2007274844486217470554
- SonY-OJangY-SHeoJ-SChungW-TChoiK-CLeeJ-CApoptosis-inducing factor plays a critical role in caspase-independent, pyknotic cell death in hydrogen peroxide-exposed cellsApoptosis20091479680819418225
- SonYOKookSHJangYSShiXLeeJCCritical role of poly(ADP-ribose) polymerase-1 in modulating the mode of cell death caused by continuous oxidative stressJ Cell Biochem200910898999719711368
- NorbergEOrreniusSZhivotovskyBMitochondrial regulation of cell death: Processing of apoptosis-inducing factorBiochem Biophys Res Comm20103969510020494118
- OteraHOhsakayaSNagauraZIshiharaNMiharaKExport of mitochondrial AIF in response to proapoptotic stimuli depends on processing at the intermembrane spaceEMBO J2005241375138615775970
- UrenRTDewsonGBonzonCLithgowTNewmeyerDDKluckRMMitochondrial release of pro-apoptotic proteins: Electrostatic interactions can hold cytochrome c but not Smac/DIABLO to mitochondrial membranesJ Biol Chem20052802266227415537572
- PolsterBMBasanezGEtxebarriaAHardwickJMNichollsDGCalpain I induces cleavage and release of apoptosis-inducing factor from isolated mitochondriaJ Biol Chem20052806447645415590628
- YusteVJMoubarakRSDelettreCCysteine protease inhibition prevents mitochondrial apoptosis-inducing factor (AIF) releaseCell Death Differ2005121445144815933737
- NorbergEGogvadzeVVakifahmetogluHOrreniusSZhivotovskyBOxidative modification sensitizes mitochondrial apoptosis-inducing factor to calpain-mediated processingFree Radic Biol Med20104879179720043986
- YacoubAParkMAHannaDOSU-03012 promotes caspase-independent but PERK-, cathepsin B-, BID-, and AIF-dependent killing of transformed cellsMol Pharmacol20067058960316622074
- ChaitanyaGVBabuPPMultiple apoptogenic proteins are involved in the nuclear translocation of apoptosis inducing factor during transient focal cerebral ischemia in ratBrain Res2008124617819018938146
- NorbergEGogvadzeVOttMAn increase in intracellular Ca2+ is required for the activation of mitochondrial calpain to release AIF during cell deathCell Death Differ2008151857186418806756
- VoslerPSSunDWangSCalcium dysregulation induces apoptosis-inducing factor release: Cross-talk between PARP-1 and calpain-signaling pathwaysExp Neurol200921821322019427306
- GagneJPHunterJMLabrecqueBChabotBPoirierGGA proteomic approach to the identification of heterogeneous nuclear ribonucleoproteins as a new family of poly(ADP-ribose)-binding proteinsBiochem J200337133134012517304
- GagneJPHendzelMJDroitAPoirierGGThe expanding role of poly(ADP-ribose) metabolism: Current challenges and new perspectivesCurr Opin Cell Biol20061814515116516457
- GagneJPIsabelleMLoKSProteome-wide identification of poly(ADP-ribose) binding proteins and poly(ADP-ribose)-associated protein complexesNucleic Acids Res2008366959697618981049
- ChenMHeHZhanSKrajewskiSReedJCGottliebRABid is cleaved by calpain to an active fragment in vitro and during myocardial ischemia/reperfusionJ Biol Chem2001276307243072811404357
- DengYRenXYangLLinYWuXA JNK-dependent pathway is required for TNFalpha-induced apoptosisCell2003115617014532003
- CulmseeCZhuCLandshamerSApoptosis-inducing factor triggered by poly(ADP-ribose) polymerase and bid mediates neuronal cell death after oxygen-glucose deprivation and focal cerebral ischemiaJ Neurosci200525102621027216267234
- LetaiABassikMCWalenskyLDSorcinelliMDWeilerSKorsmeyerSJDistinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeuticsCancer Cell2002218319212242151
- ChenDZhouQCaspase cleavage of BimEL triggers a psoitive feedback amplification of apoptotic signalingProc Natl Acad Sci U S A20041011235124014732682
- LiouAKZhouZPeiWLimTMYinXMChenJBimEL up-regulation potentiates AIF translocation and cell death in response to MPTPFASEB J2005191350135215941767
- AlanoCCSwansonRAPlayers in the PARP-1 cell-death pathway: JNK1 joins the castTrends Biochem Sci20063130931116679020
- XuYHuangSLiuZ-GHanJPoly(ADP-ribose) polymerase-1 signaling to mitochondria in necrotic cell death requires RIP1/TRAF2-mediated JNK activationJ Biol Chem20062818788879516446354
- SongZFJiXPLiXXWangSJWangSHZhangYInhibition of the activity of poly(ADP-ribose) polymerase reduces heart ischaemia/reperfusion injury via suppressing JNK-mediated AIF translocationJ Cell Mol Med2008121220122818782186
- LeiKDavisRJJNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosisProc Natl Acad Sci U S A20031002432243712591950
- HeeresJTHergenrotherPJPoly(ADP-ribose) makes a date with deathCurr Opin Chem Biol20071164465317936669
- BurtonTREisenstatDDGibsonSPBNIP3 (Bcl-2 interacting protein) acts as transcriptional repressor of apoptosis-inducing factor expression preventing cell death in human malignant gliomasJ Neurosci2009294189419919339613
- PietschECSykesSMMcMahonSBMurphyMEThe p53 family and programmed cell deathOncogene2008276507652118955976
- YuanZMShioyaHIshikoTp73 is regulated by tyrosine kinase c-Abl in the apoptotic response to DNA damageNature199939981481710391251
- StieweTPutzerBMRole of the p53-homologue p73 in E2F1-induced apoptosisNat Genet20002646446911101847
- TohWHSiddiqueMMBoominathanLLinKWSabapathyKc-Jun regulates the stability and activity of the p53 homologue, p73J Biol Chem2004279447134472215302867
- MelinoGBernassolaFRanalliMp73 induces apoptosis via PUMA transactivation and Bax mitochondrial translocationJ Biol Chem20042798076808314634023
- RossiMSayanAETerrinoniAMelinoGKnightRAMechanism of induction of apoptosis by p73 and its relevance to neuroblastoma biologyAnn N Y Acad Sci2004102814314915650240
- RamadanSTerrinoniACataniMVp73 induces apoptosis by different mechanismsBiochem Biophys Res Commun200533171371715865927
- CarterSLEklundACKohaneISHarrisLNSzallasiZA signature of chromosomal instability inferred from gene expression profiles predicts clinical outcome in multiple human cancersNat Genet2006381043104816921376
- Perez de CastroIde CarcerGMarcosMA census of mitotic cancer genes: New insights into tumor cell biology and cancer therapyCarcinogenesis20072889991217259655
- TarinJJVendrellFJTenJBlanesRvan BleromJCanoAThe oxidizing agent tertiary butyl hydroperoxide induces disturbances in spindle organization, c-meiosis, and aneuploidy in mouse oocytesMol Human Reproduct19962895901
- ChoiWJBanerjeeJFalconeTBenaJAgarwalASharmaRKOxidative stress and tumor necrosis factor-α-induced alterations in metaphase II mouse oocyte spindle structureFertl Steril2007884 Suppl12201231
- D’AngiolellaVSantarpiaCGriecoDOxidative stress overrides the spindle checkpointCell Cycle2007657657917351333
- ChangT-SJeongWLeeD-YChoC-SRheeSGThe ring-H2-finger protein APC11 as a target of hydrogen peroxideFree Rad Biol Med20043752153015256223
- BlagosklonnyMVMitotic arrest and cell fate: Why and how mitotic inhibition of transcription drives mutually exclusive eventsCell Cycle20076707417245109
- DelcuveGPHeSDavieJRMitotic partitioning of transcription factorsJ Cell Biochem20081051818459122
- GascoigneKETaylorSSCancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugsCancer Cell20081411112218656424
- ElhajoujiACunhaMKirsch-VoldersMSpindle poisons induce polyploidy by mitotic slippage and micronucleate mononucleates in the cytokinesis-block assayMutagenesis1998131931989568594
- DaiWWangQLiuTSlippage of mitotic arrest and enhanced tumor development in mice with BubR1 haploinsufficiencyCancer Res20046444044514744753
- TaoWSouthVJZhangYInduction of apoptosis by an inhibitor of the mitotic kinesin KSP requires both activation of the spindle assembly checkpoint and mitotic slippageCancer Cell20058495916023598
- BritoDARiederCLMitotic checkpoint slippage in humans occurs via cyclin B destruction in the presence of an active checkpointCurr Biol2006161194120016782009
- StevensFEBeamishHWarrenerRGabrielliBHistone deacetylase inhibitors induce mitotic slippageOncogene2008271345135417828304
- BritoDAYangZRiederCLMicrotubules do not promote mitotic slippage when the spindle assembly checkpoint cannot be satisfiedJ Cell Biol200818262362918710927
- RiffellJLZimmermanCKhongAMcHardyLMRobergeMEffects of chemical manipulation of mitotic arrest and slippage on cancer cell survival and proliferationCell Cycle2009830293042
- LeeJKimJAMargolisRLFotedarRSubstrate degradation by the anaphase promoting complex occurs during mitotic slippageCell Cycle201091792180120436289
- XuFLRbaibiYKiselyovKLazoJSWipfPSaundersWSMitotic slippage in non-cancer cells induced by a microtubule disruptor, disorazole C1Chem Biol2010101
- DodsonHBourkeEJeffersLJCentrosome amplification induced by DNA damage occurs during a prolonged G2 phase and involves ATMEMBO J2004233864387315359281
- ButlerLMHewettPJFitridgeRACowledPADeregulation of apoptosis in colorectal carcinoma: Theoretical and therapeutic implicationsAust N Z J Surg199969889410030808
- PayneCMBernsteinHBernsteinCGarewalHThe role of apoptosis in biology and pathology: Resistance to apoptosis in colon carcinogenesisUltrastruct Pathol1995a192212487571081
- NelsonDATanTTRabsonABAndersonDDegenhardtKWhiteEHypoxia and defective apoptosis drive genomic instability and tumorigenesisGenes Develop2008182095210715314031
- EnariMSakahiraHYokoyamaHOkawaKIwamatsuANagataSA caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICADNature199839143509422506
- HalenbeckRMacDonaldHRoulstonAChenTTConroyLWilliamsLTCPAN, a human nuclease regulated by the caspase-sensitive inhibitor DFF45Curr Biol199885375409560346
- LiuXZouHSlaughterCWangXDFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosisCell1997891751849108473
- LiuXLiPWidlakPThe 40-kDa subunit of DNA fragmentation factor induces DNA fragmentation and chromatin condensation during apoptosisProc Natl Acad Sci U S A199895846184669671700
- KerrJFRWyllieAHCurrieARApoptosis: A basic biological phenomenon with wide-ranging implications in tissue kineticsBr J Cancer1972262392574561027
- WyllieAHKerrJFCurrieARCell death: The significance of apoptosisInt Rev Cytol1980682513057014501
- SearleJKerrJFRBishopCJNecrosis and apoptosis: Distinct modes of cell death with fundamentally different significancePathol Annu1982172292597182752
- WyllieAHMorrisRGSmithALDunlopDChromatin cleavage in apoptosis: Association with condensed chromatin morphology and dependence on macromolecular synthesisJ Pathol198414267776422024
- PayneCMBjoreCGJrSchultzDAChange in the frequency of apoptosis after low- and high-dose X-irradiation of human lymphocytesJ Leukocyte Biol1992524334401402391
- OberhammerFWilsonJWDiveCApoptotic death in epithelial cells: Cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentationEMBO J199312367936848253089
- WidlakPDFF40/CAD hypersensitive sites are potentially involved in high molecular weight DNA fragmentation during apoptosisCell Mol Biol Lett20005373379
- McllroyDSakahiraHTalanianRVNagataSInvolvement of caspase 3-activated DNase in internucleosomal DNA cleavage induced by diverse apoptotic stimuliOncogene1999184401440810442630
- WidlakPGerrardWTRoles of the major apoptotic nuclease-DNA fragmentation factor in biology and diseaseCell Mol Life Sci20096626327418810317
- YanBWangHPengYA unique role of the DNA fragmentation factor in maintaining genomic stabilityProc Natl Acad Sci U S A2006a1031504150916432220
- ZhangJLiuXSchererDCvan KaerLWangXXuMResistance to DNA fragmentation and chromatin condensation in mice lacking the DNA fragmentation factor 45Proc Natl Acad Sci U S A19989512480124859770511
- ZhangJWangXBoveKEXuMDNA fragmentation factor 45-deficient cells are more resistant to apoptosis and exhibit different dying morphology than wild-type control cellsJ Biol Chem1999274374503745410601318
- YanBWangHWangHApoptotic DNA fragmentation factor maintains chromosome stability in a p53-independent mannerOncogene2006b255370537616619042
- ZhangJGuoHQianGMiR-145, a new regulator of the DNA fragmentation factor-45 (DFF45)-mediated apoptotic networkMol Cancer2010921120687965
- LiPNijhawanDBudihardjoICytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascadeCell1997914794899390557
- HofmannKBucherPTschoppJThe CARD domain: A new apoptotic signaling motifTrends Biochem Sci1997221551569175472
- LiuXKimCNYangJJemmersonRWangXInduction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome cCell1996861471578689682
- ZouHLiYLiuXWangXAn APAF-1 cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9J Biol Chem1999274115491155610206961
- ZuoYXiangBYangJOxidative modification of caspase-9 facilitates its activation via disulfide-mediated interaction with Apaf-1Cell Res20091944945719238172
- SleeEAHarteMTKluckRMOrdering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent mannerJ Cell Biol19991442812929922454
- ZhivotovskyBOrreniusSCaspase-2 function in response to DNA damageBiochem Biophys Res Comm200533185986715865942
- SamrajAKSohnDSchulze-OsthoffKSchmitzILoss of caspase-9 reveals its essential role for caspase-2 activation and mitochondrial membrane depolarizationMol Biol Cell200718849317079734
- GuerreroADChenMWangJDelineation of the caspase-9 signaling cascadeApoptosis20081317718617899380
- KuidaKHaydarTFKuanCYReduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9Cell1998943253379708735
- RobertsonJDGogvadzeVZhivotovskyBOrreniusSDistinct pathways for stimulation of cytochrome c release by etoposideJ Biol Chem2000275324383244310961984
- LassusPOpitz-ArayaXLazebnikYRequirement for caspase-2 in stress-induced apoptosis before mitochondrial permeabilizationScience20022971352135412193789
- TinelATschoppJThe PIDDosome, a protein complex implicated in activation of caspase-2 in response to genotoxic stressScience200430484384615073321
- JangTHBaeJYParkOKIdentification and analysis of dominant negative mutants of RAIDD and PIDDBiochim Biophys Acta201018041557156320406701
- MacePDRiedlSJMolecular cell death platforms and assembliesCurr Opin Cell Biol20102282883620817427
- RainaDPandeyPAhmadRc-Abl tyrosine kinase regulates caspase-9 autocleavage in the apoptotic response to DNA damageJ Biol Chem2005280111471115115657060
- MorishimaNNakanishiKTakenouchiHShibataTYasuhikoYAn endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12J Biol Chem2002277342873429412097332
- JeongWLeeD-YParkSRheeSGERp16, and endoplasmic reticulum-resident thiol-disulfide oxidoreductase. Biochemical properties and role in apoptosis induced by endoplasmic reticulum stressJ Biol Chem2008283255572556618628206
- JimboAFujitaEKourokuYER stress induces caspase-8 activation, stimulating cytochrome c release and caspase-9 activationExp Cell Res200328315616612581736
- Lagos-QuintanaMRauhutRLendeckelWTuschiTIdentification of novel genes coding for small expressed RNAsScience200129485385811679670
- BartelDPMicroRNAs: Genomics, biogenesis, mechanism, and functionCell200411628129714744438
- BartelDPChenCZMicromanagers of gene expression: The potentially widespread influence of metazoan microRNAsNat Rev Genet2004539640015143321
- HeLHannonGJMicroRNAs: Small RNAs with a big role in gene regulationMol Cell200426457522007
- HeLHeXLimLPA microRNA component of the p53 tumour suppressor networkNature20074471130113417554337
- KimVNMicroRNA biogenesis: Coordinated cropping and dicingNat Rev Mol Cell Biol2005637638515852042
- FarhKKGrimsonAJanCThe widespread impact of mammalian microRNAs on mRNA repression and evolutionScience20053101817182116308420
- CarthewRWGene regulation by microRNAsCurr Opin Genet Dev20061620320816503132
- Valenci-SanchezMALiuJHannonGJParkerRControl of translation and mRNA degradation by miRNAs ans siRNAsGenes Dev20062051552416510870
- TsangJZhuJvan OudenaardenAMicroRNA-mediated feedback and feedforward loops are recurrent network motifs in mammalsMol Cell20072675376717560377
- KimVNHanJSiomiMCBiogenesis of small RNAs in animalsNat Rev Mol Cell Biol20091012613919165215
- PillaiRSMicroRNA function: Multiple mechanisms for a tiny RNA?RNA2005111753176116314451
- KloostermanWPPlasterkRHThe diverse functions of microRNAs in animal development and diseaseDev Cell20061144145017011485
- KrekAGrunDPoyMNCombinatorial microRNA target predictionsNat Genet20053749550015806104
- LewisPrediction of mammalian microRNA targetsCell200311578779814697198
- LewisBPBurgeCBBartelDPConserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targetsCell2005120152015652477
- LimLPLauNCGarrett-EngelePMicroarray analysis shows that some microRNAs downregulate large numbers of target mRNAsNature200543376977315685193
- SelbachMSchwanhausserBThierfelderNFangZKhaninRRajewskyNWidespread changes in protein synthesis induced by microRNAsNature2008455586318668040
- BaekDVillenJShinCCamargoFDGygiSPBartelDPThe impact of microRNAs on protein outputNature2008455647118668037
- CimminoACalinGAFabbriMmiR-15 and miR-16 induce apoptosis by targeting BCL2Proc Natl Acad Sci U S A200510336873692
- HayashitaYOsadaHTatematsuYA polycistronic microRNA cluster, mir-17–92, is overexpressed in human lung cancers and enhances cell proliferationCancer Res2005659628963216266980
- MiskaEAHow microRNAs control cell division, differentiation and deathCurr Opin Genet Dev20051556356816099643
- CalinGASevignaniCDumitruCDHuman microRNA genes are frequently located at fragile sites and genomic regions involved in cancersProc Natl Acad Sci U S A20041012999300414973191
- ChenJFMandelEMThomsonJMThe role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiationNat Genet20063822823316380711
- CarletonMClearyMALinsleyPSMicroRNAs and cell cycle regulationCell Cycle200762127213217786041
- ChangTCWentzelEAKentOATransactivation of mir-34a by p53 broadly influences gene expression and promotes apoptosisMol Cell20072674575217540599
- YiRPoyMNStoffelMFuchsEA skin microRNA promotes differentiation by repressing “stemness”Nature200845222522918311128
- LalANavarroFMaherCAmiR-24 inhibits cell proliferation by targeting E2F2, MYC, and other cell-cycle genes via binding to “seedless” 3’UTR microRNA recognition elementsMol Cell20093561062519748357
- BuenoMJGomez de CedronMLaresgoitiUFernandez-PiquerasJZubiagaAMMalumbresMMultiple E2F-induced microRNAs prevent replicative stress in response to mitogenic signalingMol Cell Biol2010302983299520404092
- MeltzerPSCancer genomics: Small RNAs with big impactsNature200543574574615944682
- DalmayTEdwardsDRMicroRNAs and the hallmarks of cancerOncogene2006256170617517028596
- Esquela-KerscherASlackFJOncomirs – microRNAs with a role in cancerNat Rev Cancer2006625926916557279
- BandresEAgirreXRamirezNZarateEGarcia-FoncillasJMicroRNAs as cancer players: Potential clinical and biological effectsDNA Cell Biol20072627328217504023
- ChoWCSOncomiRs: The discovery and progress of microRNAs in cancersMol Cancer20076 60;10.1186/1476-4598-6-60
- VenturaAJacksTMicroRNAs and cancer. Short RNAs go a long wayCell200913658659119239879
- NicolosoMSSpizzoRShimizuMRossiSCalinGAMicroRNAs – the micro steering wheel of tumour metastasesNat Rev Cancer2009929330219262572
- CalinGALiuCGSevignaniCMicroRNA profiling reveals distinct signatures in B cell chronic lymphocytic leukemiasProc Natl Acad Sci U S A2004b101117551176015284443
- CalinGACroceCMMicroRNA signatures in human cancersNat Rev Cancer2006685786617060945
- LuJGetzGMiskaEAMicroRNA expression profiles classify human cancersNature200543583483815944708
- CumminsJMHeYLearyRJThe colorectal microRNAomeProc Natl Acad Sci U S A20061033687369216505370
- VoliniaSCalinGALiuCGA microRNA expression signature of human solid tumors defines cancer gene targetsProc Natl Acad Sci U S A20061032257226116461460
- LeeEJGusevYJiangJExpression profiling identifies microRNA signature in pancreatic cancerInt J Cancer20071201046105417149698
- SubramanianSLuiWOLeeCHMicroRNA expression signature of human sarcomasOncogene2008272015202617922033
- RosenfeldNAharonovRMeiriEMicroRNAs accurately identify cancer tissue originNat Biotechnol20082646246918362881
- VandenboomTGIILiYPhilipPASarkarFHMicroRNA and cancer: tiny molecules with major implicationsCurr Genomics200899710919440450
- HuXSchwartzJKLewisJSJrA microRNA expression signature for cervical cancer prognosisCancer Res2010701441144820124485
- KumarMSLuJMercerKLGolubTRJacksTImpaired microRNA processing enhances cellular transformation and tumorigenesisNat Genet20073967367717401365
- ChangTCYuDLeeYSWidespread microRNA repression by Myc contributes to tumorigenesisNat Genet200840435018066065
- ChenCZMicroRNAs as oncogenes and tumor suppressorsN Eng J Med200535317681771
- KentOAMendellJTA small piece in the cancer puzzle: MicroRNAs as tumor suppressors and oncogenesOncogene2006256188619617028598
- ZhangBPanXCobbGPAndersonTAMicroRNAs as oncogenes and suppressorsDev Biol200730211216989803
- MichaelMZO’ConnorSMvan Holst PellekaanNGYoungGPJamesRJReduced accumulation of specific microRNAs in colorectal neoplasiaMol Cancer Res2003188289114573789
- AkaoYNakagawaYNaoeTlet-7 microRNA functions as a potential growth suppressor in human colon cancer cellsBiol Pharm Bull20062990390616651716
- BandresECubedoEAgirreXIdentification by real-time PCR of 13 mature microRNAs differentially expressed in colorectal cancer and non-tumoral tissuesMol Cancer200652916854228
- XiYShalgiRFodstadOPilpelYJuJDifferentially regulated micro-RNAs and actively translated messenger RNA transcripts by tumor suppressor p53 in colon cancerClin Cancer Res2006122014202416609010
- ShiBSepp-LorenzinoLPriscoMLinsleyPdeAngelisTBasergaRMicro RNA 145 targets the insulin receptor substrate-1 and inhibits the growth of colon cancer cellsJ Biol Chem2007282325823259017827156
- SlabyOSvobodaMFabianPAltered expression of miR-21. MiR-31, miR-143 and miR-145 is related to clinicopathologic features of colorectal cancerOncology20077239740218196926
- TazawaHTsuchiyaNIzumiyaMNakagamaHTumor-suppressive miR-34a induces senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cellsProc Natl Acad Sci U S A2007104154721547717875987
- SchepelerTReinertJTOstenfeldMSDiagnostic and prognostic microRNAs in stage II colon cancerCancer Res2008686416642418676867
- SchetterAJLeungSYSohnJJMicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinomaJAMA200829942543618230780
- MotoyamaKInoueHTakatsunoYOver- and under-expressed microRNAs in human colorectal cancerInt J Oncol2009341069107519287964
- NgEKTsangWPNgSSMicroRNA-143 targets DNA methyltransferases 3A in colorectal cancerBr J Cancer200910169970619638978
- SarverAlFrenchAJBorralhoPMHuman colon cancer profiles show differential microRNA expression depending on mismatch repair status and are characteristic of undifferentiated proliferative statesBMC Cancer2009940119922656
- YantissRKGoodarziMZhouXKClinical, pathologic, and molecular features of early-onset colorectal carcinomaAm J Surg Pathol20093357258219047896
- WangYXZhangXYZhangBFYangCQChenXMGaoHJInitial study of microRNA expression profiles of colonic cancer without lymph node metastasisJ Dig Dis201011505420132431
- LanzaGFerracinMGafaRmRNA/microRNA gene expression profile in microsatellite unstable colorectal cellsMol Cancer200765417716371
- AtkinNBMicrosatellite instabilityCytogenet Cell Genet20019217718111435683
- BommerGTGerinIFengYp53-mediated activation of miRNA34 candidate tumor-suppressor genesCurr Biol2007171298130717656095
- HermekingHp53 enters the microRNA worldCancer Cell20071241441817996645
- Raver-ShapiraNMarcianoEMeiriETranscriptional activation of miR-34a contributes to p53-mediated apoptosisMol Cell20072673174317540598
- TarasovVJungPVerdoodtBDifferential regulation of microRNAs by p53 revealed by massively parallel sequencing: miR-34a is a p53 target that induces apoptosis and G1-arrestCell Cycle200761586159317554199
- DijkstraMKvan LomKTielemansD17p13/TP53 deletion in B-CLL patients is associated with micoRNA-34a downregulationLeukemia20092362562718818704
- WelchCChenYStallingsRLMicroRNA-34a functions as a potential tumor suppressor by inducing apoptosis in neuroblastomaOncogene2007265017502217297439
- RokhlinOWScheinkerVSTaghiyevAFBumcrotDGloverRACohenMBMicroRNA-34 mediates AR-dependent p53-induced apoptosis in prostate cancerCancer Biol Ther200871288129618497571
- RubyJGJanCPlayerCLarge-scale sequencing reveals 21U-RNAs and additional microRNAs and endogenous siRNAs in C. elegansCell20061271193120717174894
- ColeKAAttiyehEFMosseYPA functional screen identifies miR-34a as a candidate neuroblastoma tumor suppressor geneMol Cancer Res2008673574218505919
- SunFFuHLiuQDownregulation of CCND1 and CDK6y miR-34a induces cell cycle arrestFEBS Lett20085821564156818406353
- WeiJSSongYKDurinckSThe MYCN oncogene is a direct target of miR-34aOncogene2009275204521318504438
- LiYGuessousFZhangYMicroRNA-34a inhibits glioblastoma growth by targeting multiple oncogenesCancer Res2009697569757619773441
- YamakuchiMLowensteinCJMiR-34, SIRT1 and p53: The feedback loopCell Cycle2009871271519221490
- BeverlyLJFelsherDWCapobiancoAJSuppression of p53 by Notch in lymphomagenesis: Implications for initiation and regressionCancer Res657159716816103066
- MungamuriSKYangXThorADSomasundaramKSurvival signaling by Notch1: Mammalian target of rapamycin (mTOR)-dependent inhibition of p53Cancer Res2006664715472416651424
- LeongKGKarsanARecent insights into the role of Notch signaling in tumorigenesisBlood20061072223223316291593
- MieleLGoldeTOsborneBNotch signaling in cancerCurr Mol Med2006690591817168741
- YamakuchiMFerlitoMLowensteinCJmiR-34a repression of SIRT1 regulates apoptosisProc Natl Acad Sc U S A2008105134211342618755897
- GinsbergDE2F3-a novel repressor of the ARF/p53 pathwayDev Cell2004674274315177020
- SuHYangJRXuTMicroRNA-101, down-regulated in hepatocellular carcinoma, promotes apoptosis and suppresses tumorigenicityCancer Res2009691135114219155302
- FriedmanJMLiangGJonesPAThe tumor suppressor microRNA-101 becomes an epigenetic player by targeting the Polycomb group protein EZH2 in cancerCell Cycle200982313231419625769
- AdamsKWCooperGMRapid turnover of MCL-1 couples translation to cell survival and apoptosisJ Biol Chem20072826192620017200126
- CuconatiAMukherjeeCPerezDWhiteEDNA damage response and Mcl-1 destruction initiate apoptosis in adenovirus-infected cellsGenes Dev2003172922293214633975
- NijhawanDFangMTraerEElimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiationGenes Dev2003171475149612783855
- ClohessyJGZhuangJde BoerJGil-GomezGBradyHJMMcl-1 interacts with truncated Bid and inhibits its induction of cytochrome c release and its role in receptor-mediated apoptosisJ Biol Chem20062815750575916380381
- HanJGoldsteinLAGastmanBRRabinowichHInterrelated roles for Mcl-1 and BIM in regulation of TRAIL-mediated mitochondrial apoptosisJ Biol Chem2006281101531016316478725
- ZhongQGaoWDuFWangXMule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosisCell20051211085109515989957
- MenoretEGomez-BougiePSurgetSMcl-1(127–350) fragment induces apoptosis through direct interaction with BaxFEBS Lett201058448749219968986
- KobayashiSLeeSHMengXWSerine 64 phosphorylation enhances the antiapoptotic function of Mcl-1J Biol Chem2007282184071841717463001
- DominaAMSmithJHCraigRWMyeloid cell leukemia 1 is phosphorylated through two distinct pathways, one associated with extracellular signal-regulated kinase activation and the other with G2/M accumulation or protein phosphatase 1/2A inhibitionJ Biol Chem2000275216882169410777489
- KrajewskiSBodrugSKrajewskaMImmunohistochemical analysis of Mcl-1 protein in human tissues. Differential regulation of Mcl-1 and Bcl-2 protein production suggests a unique role for Mcl-1 in control of programmed cell death in vivoAm J Pathol1995146130913197778670
- BackusHHVan GroeningenCJVosWDifferential expression of cell cycle and apoptosis related proteins in colorectal mucosa, primary colon tumours, and liver metastasesJ Clin Pathol20025520621111896073
- VaramballySCaoQManiRSGenomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancerScience20083221695169919008416
- LiSFuHWangYMicroRNA-101 regulates expression of the v-fos FBJ murine osteosarcoma viral omcogene homolog (FOS) oncogene in human hepatocellular carcinomaHepatol20094911941202
- SuzuckiHIYamagataKSugimotoKIwamotoTKatoSMiyazonoKModulation of microRNA processing by p53Nature200946052953319626115
- CarmellMAXuanZZhangMQHannonGJThe argoanute family: Tentacles that reach into RNAi, developmental control, stem cell maintenance, and tumorigenesisGenes Develop2002162733274212414724
- PillaiRSArtusCGFilipowiczWTethering of human Ago proteins to mRNA mimics the miRNA-mediated repression of protein synthesisRNA2004101518152515337849
- SasakiTShiohamaAMinoshimaSShimizuNIdentification of eight members of the Argonaute family in the human genome small star, filledGenomics20038232333012906857
- HockJWeinmannLEnderCProteomic and functional analysis of Argonaute-containing mRNA-protein complexes in human cellsEMBO Rep200781052106017932509
- WuLFanJBelascoJGImportance of translation and nonnucleolytic ago proteins for on-target RNA interferenceCurr Biol2008181327133218771919
- ParkSMGaurABLengyelEPeterMEThe miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2Genes Develop20082289490718381893
- SaydamOShenYWurdingerTDownregulated microRNA-200a in meningiomas promotes tumor growth by reducing E-cadherin and activating the Wnt/β-catenin signaling pathwayMol Cell Biol2009295923594019703993
- SchneikertJBehrensJThe canonical WNT signaling pathway and its APC partner in colon cancer developmentGut20075641742516840506
- KorpalMLeeESHuGKangYThe miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2J Biol Chem2008149101491418411277
- BendoraiteAKnoufECGargKSRegulation of miR-200 family microRNAs and ZEB transcription factors in ovarian cancer: Evidence supporting a mesothelial-to-epithelial transitionGynecol Oncol201011712519854497
- BrabletzSBrabletzTThe ZEB/miR-200 feedback loop – a motor of cellular plasticity in development and cancerEMBO Rep201067067720706219
- HuangKZhangJ-XHanLMicroRNA roles in beta-catenin pathwayMol Cancer2010925220858269
- SavagnerPLeaving the neighborhood: Molecular mechanisms involved during epithelial-mesenchymal transitionBioessays20012391292311598958
- FuchsIBLichteneggerWBuehlerHThe prognostic significance of epithelial-mesenchymal transition in breast cancerAnticancer Res2002223415341912530097
- CleversHWnt/beta-catenin signaling in development and diseaseCell200612746948017081971
- HuangHHeXiWnt/-catenin signaling: New (and old) players and new insightsCurr Opin Cell Biol20082011912518339531
- AkiyamaTWnt/β-catenin signalingCytokine Growth Factor Rev200027328210959075
- BienzMCleversHLinking colorectal cancer to Wnt signalingCell200010331132011057903
- PeiferMPolakisPWnt signaling in oncogenesis and embryogenesis –a look outside the nucleusScience20002871606160910733430
- HeTCSparksABRagoCIdentification of c-MYC as a target of the APC pathwayScience1998281150915129727977
- ShtutmanMZhurinskyJSimchaIThe cyclin D1 gene is a target of the β-catenin/LEF-1 pathwayProc Natl acad Sci U S A1999965522552710318916
- TetsuOMcCormickFβ-catenin regulates expression of cyclin D1 in colon carcinoma cellsNature199939842242610201372
- MorinPJSparksABKorinekVActivation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APCScience1997275178717909065402
- SatohSDaigoYFurukawaYAXIN1 mutations in hepatocellular carcinomas and growth suppression in cancer cells by virus-mediated transfer of AXIN1Nat Genet20002424525010700176
- KoyamaTTagoK-INakamuraTMutation and expression of the β-catenin-interacting protein ICAT in human colorectal tumorsJpn J Clin Oncol20023235836212417602
- GilesRHvan EsJHCleversHCaught up in a Wnt storm: Wnt signaling in cancerBiochim Biophys Acta2003165312412781368
- HumphriesAWrightNAColonic crypt organization and tumorigenesisNat Rev Cancer2008841542418480839
- TagoKNakamuraTNishitaMInhibition of Wnt signaling by ICAT, a novel β-catenin-interacting proteinGenes Dev2000141741174910898789
- DanielsDLWeisWIICAT inhibits beta-catenin binding to Tcf/Lef-family transcription factors and the general coactivator p300 using independent structural modulesMol Cell20021057358412408825
- HossainMZYuQXuMSenJMICAT expression disrupts β-catenin-TCF interactions and impairs survival of thymocytes and activated mature T cellsInt Immunol20082092593518511409
- GrahamTAClementsWKKimelmanDXuWThe crystal structure of the β-catenin/ICAT complex reveals the inhibitory mechanism of ICATMol Cell20021016741687
- StowJLICAT is a multipotent inhibitor of β-catenin. Focus on “Role for ICAT in β-catenin-dependent nuclear signaling and cadherin functions”Am J Physiol Cell Physiol2004286C745C74615001424
- HulskenJBirchmeierWBehrensJE-cadherin and APC compete for the interaction with β-catenin and the cytoskeletonJ Cell Biol1994206120697806582
- GottardiCJGumbinerBMRole for ICAT in β-catenin-dependent nuclear signaling and cadherin functionsAm J Physiol Cell Physiol2004286C747C75614613891
- SekiyaTNakamuraTKazukiYOverexpression of Icat induces G2 arrest and cell death in tumor cell mutants for adenomatous polyposis coli, β-catenin, or axinCancer Res2002623322332612036951
- IoannidisVBeermannFCleversHHeldWThe beta-catenin-TCF-1 pathway ensures CD4(+)CD8(+) thymocyte survivalNat Immunol2001269169711477404
- XieHHuangZSadimMSSunZStabilized beta-catenin extends thymocyte survival by up-regulating Bcl-xLJ Immunol20051757981798816339534
- WalstonTTuskeyCEdgarLMultiple Wnt signaling pathways converge to orient the mitotic spindle in early C. elegans embryosDev Cell2004783184115572126
- McCartneyBMMcEwenDGGrevengoedEMaddoxPBejsovecAPeiferMDrosophila APC2 and Armadillo participate in tethering mitotic spindles to cortical actinNat Cell Biol2001393393811584277
- RogersSLRogersGCSharpDJValeRDDrosophila EB1 is important for proper assembly, dynamics, and positioning of the mitotic spindleJ Cell Biol200215887388412213835
- ZumbrunnJKinoshitaKHymanAANathkeISBinding of the adenomatous polyposis coli protein to microtubules increases microtubule stability and is regulated by GSK3 beta phosphorylationCurr Biol200111444911166179
- KaplanKBBurdsAASwedlowJRBekirSSSorgerPKNathkeISA role for the Adenomatous Polyposis Coli protein in chromosome segregationNat Cell Biol2001342943211283619
- KaplanDDMeigsTEKellyPCaseyPJIdentification of a role for β-catenin in the establishment of a bipolar mitotic spindleJ Biol Chem2004279108291083214744872
- WakefieldJGStephensDJTavareJMA role for glycogen synthase kinase-3 in mitotic spindle dynamics and chromosome alignmentJ Cell Sci200311663764612538764
- BobinnecYMorinXDebecAShaggy/GSK-3β kinase localizes to the centrosome and to specialized cytoskeletal structures in DrosophilaCell Motil Cytoskel200663313320
- KikuchiKNiikuraYKitagawaKKikuchiADishevelled, a Wnt signalling component, is involved in mitotic progression in cooperation with Plk1EMBO J2010293470348320823832
- HadjihannasMVBrucknerMBehrensJConductin/axin2 and Wnt signalling regulates centrosome cohesionEMBO Rep20101131732420300119
- FoddeRKuiperJRosenbergCMutations in the APC tumour suppressor gene cause chromosomal instabilityNat Cell Biol2001343343811283620
- GreenRAKaplanKBChromosome instability in colorectal tumor cells is associated with defects in microtubule plus-end attachments caused by a dominant mutation in APC attachments caused by a dominant mutation in APCJ Cell Biol200316394996114662741
- HadjihannasMVBrucknerMJerchowBBirchmeierWDietmaierWBehrensJAberrant Wnt/β-catenin signaling can induce chromosomal instability in colon cancerProc Natl Acad Sci U S A2006103107471075216815967
- HadjihannasMVBehrensJCIN by WNT. Growth pathways, mitotic control and chromosomal instability in cancerCell Cycle200652077208116969101
- AokiKAokiMSugaiMChromosomal instability by β-catenin/TCF transcription in APC or β-catenin mutant cellsOncogene2007263511352017160019
- HarrisHMillerOJKleinGWorstPTachibanaTSuppression of malignancy by cell fusionNature19692233633685387828
- StanbridgeEJSuppression of malignancy in human cellsNature197626017201264187
- SherrCJPrinciples of tumor suppressionCell200411623524697614744434
- CarlingTImanishiYGazRDArnoldAAnalysis of the RAD54 gene on chromosome 1p as a potential tumor-suppressive gene in parathyroid adenomasInt J Cancer199983808210449612
- SulmanEPWhitPSBrodeurGMGenomic annotation of the meningioma tumor suppressor locus on chromosome p34Oncogene200421014102014749765
- BagchiAPapazogluCWuCHD5 is a tumor suppressor at human 1p36Cell200712845947517289567
- FugitaTIgarashiJOkawaERCDH5, a tumor suppressor gene deleted from 1p36.31 in neuroblastomasJ Natl Cancer Inst200810094094918577749
- OkawaERGotohTManneJExpression and sequence analysis of candidates for the 1p36.31 tumor suppressor gene deleted in neuroblastomasOncogene20082780381017667943
- BagchiAMillsAAThe quest for the 1p36 tumor suppressorCancer Res2008682551255618413720
- CookWDMcCawBJAccommodating haploinsufficient tumor suppressor genes in Knudson’s modelOncogene2000193434343810918600
- SantarosaMAshworthAHaploinsufficiency for tumour suppressor genes: When you don’t need to go all the wayBiochim Biophys Acta2004165410512215172699
- FormanHJZhangHRinnaAGlutathione: Overview of its protective roles, measurement, and biosynthesisMol Aspects Med20093011218796312
- FranklinCCBackosDSMoharIWhiteCCFormanHJKavanaghTJStructure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligaseMol Aspects Med200930869818812186
- MartenssonJJainAMeisterAGlutathione is required for intestinal functionProc Natl Acad Sci U S A199087171517192308931
- ShiraishiRFujiseTKurokiTLong-term ingestion of reduced glutathione suppressed an accelerating effect of beef tallow diet on colon carcinogenesis in ratsJ Gastroenterol2009441026103519568688
- YangYDieterMZChenYShertzerHGNebertDWDaltonTPInitial characterization of the glutamate-cysteine ligase modifier subunit Gclm (−/−) knockout mouse. Novel model system for a severely compromised oxidative stress responseJ Biol Chem2002277494464945212384496
- OchiTHydrogen peroxide increases the activity of γ-glutamylcysteine synthetase in cultured Chinese hamster V79 cellsArch Toxicol199570961038773181
- TianLShiMMFormanHJIncreased transcription of the regulatory subunit of γ-glutamylcysteine synthetase in rat lung epithelial L2 cells exposed to oxidative stress or glutathione depletionArch Biochem Biophys19973421261339185621
- RinnaAFormanHJSHP-1 inhibition by 4-hydroxynonenol activates jun N-terminal kinase and glutamate cysteine ligaseAm J Respir Cell Mol Biol2008399710418276794
- DickinsonDAIiesKEWatanabeN4-hydroxynonenal induces glutamate cysteine ligase through JNK in HBE1 cellsFree Radic Biol Med20023397498712361807
- SiitonenTAlaruikkaPMantymaaPProtection of acute myeloblastic leukemia cells against apoptotic cell death by high glutathione and G-glutamylcysteine synthetase levels during etoposide-induced oxidative stressAnn Oncol1999101361136710631466
- BottaDFranklinCCWhiteCCGlutamate-cysteine ligase attenuates TNF-induced mitochondrial injury and apoptosisFree Radic Biol Med20043763264215288121
- LuSCRegulation of glutathione synthesisMol Aspects Med200930425918601945
- MarkovicJGarcia-GimenezJLGimenoAVinaJPallardoFVRole of glutathione in cell nucleusFree Radic Res20104472173320528574
- Diaz VivancosPWolffTMarkovicJPallardoFVFoyerCHA nuclear glutathione cycle within the cell cycleBiochem J201043116917820874710
- GieraSBraeuningAKohleCWnt/beta-catenin signaling activates and determines hepatic zonal expression of glutathione S-transferases in mouse liverToxicol Sci2010115223320118494
- PatskovskyYVHuangMQTakayamaTListowskyIPearsonWRDistinctive structure of the human GSTM3 gene – inverted orientation relative to the mu class glutathione transferase gene clusterArch Biochem Biophys199936185939882431
- YuKDFanLDiGHGenetic variants in GSTM3 gene within GSTM4-GSTM2-GSTM1-GSTM5-GSTM3 cluster influence breast cancer susceptibility depending on GSTM1Breast Cancer Res Treat201012148549619856098
- Pool-ZobelBVeeriahSBohmerF-DModulation of xenobiotic metabolising enzymes by anticarcinogens-focus on glutathione S-transferases and their role as targets of dietary chemoprevention in colorectal carcinogenesisMutat Res2005591749216083918
- KatohTNagataNKurodaYGlutathione S-transferase M1 (GSTM1) and T1 (GSTT1) genetic polymorphism and susceptibility to gastric and colorectal adenocarcinomaCarcinogenesis199617185518598824506
- ScarpatoNHirvonenAMiglioreLFalckGNorppaHInfluence of GSTM1 and GSTT1 polymorphisms on the frequency of chromosome aberrations in lymphocytes of smokers and pesticide-exposed greenhouse workersMutat Res19973892272359093388
- GriesmannHSchlerethKKrauseMSamansBStieweTp53 and p73 in suppression of Myc-driven lymphomagenesisInt J Cancer200912450250618942718
- GaoKHenningSMNiuYThe citrus flavonoid naringenin stimulates DNA repair in prostate cancer cellsJ Nutr Biochem200617899516111881
- MorelIAbaleaVCillardPCillardJRepair of oxidized DNA by the flavonoid myricetinMethods Enzymol200133530831611400380
- BernsteinHCrowley-SkillicornCBernsteinCPayneCMDvorakKGarewalHLanseerBRDietary compounds that enhance DNA repair and their relevance to cancer and agingNew Research on DNA RepairHauppauge, NYNova Science Publishers, Inc200799113
- Della RagioneFCucciollaVCrinitiVIndacoSBorrielloAZappiaVAntioxidants induce different phenotypes by a distinct modulation of signal transductionFEBS Lett200253228929412482581
- YoshidaTMaedaAHorinakaMQuercetin induces gadd45 expression through a p53-independent pathwayOncol Rep2005141299130316211300
- CataniMVCostanzoASaviniIAscorbate up-regulates MLH1 (Mut L homologue-1) and p73: Implications for the cellular response to DNA damageBiochem J200236444144712023887
- DavisCDRossSAEvidence for dietary regulation of microRNA expression in cancer cellsNutr Rev20086647748218667010
- PogribnyIPTryndyakVPRossSABelandFADifferential expression of microRNAs during hepatocarcinogenesis induced by methyl deficiency in ratsNutr Rev200866Suppl 1S33S3518673486
- GarzonRPichiorriFPalumboTMicroRNA gene expression during retinoic acid-induced differentiation of human acute promyelocytic leukemiaOncogene2007264148415717260024
- SunMEstrovZJiYCoombesKRHarrisDHKurzrockRCurcumin (diferuloylmethane) alters the expression profiles of microRNAs in human pancreatic cancer cellsMol Cancer Ther2008746447318347134
- Boesch-SaadatmandiCLobodaAWagnerAEEffect of quercetin and its metabolites isorhamnetin and quercetin-3-glucuronide on inflammatory gene expression: Role of miR-155J Nutr Biochem2010623. [Epub ahead of print].
- DavidsonLAWangNShahMSn-3 polyunsaturated fatty acids modulate carcinogen-directed non-coding microRNA signatures in rat colonCarcinogenesis2009302077208419825969
- GaedickeSZhangXSchmelzerCVitamin E dependent microRNA regulation in rat liverFEBS Lett20085823542354618817776
- PaulSDeCastroAJLeeHJDietary intake of pterostilbene, a constituent of blueberries, inhibits the β-catenin/p65 downstream signaling 1 pathway and colon carcinogenesis in ratsCarcinogenesis2010311272127820061362
- SarkarFHLiYWangZKongDCellular signaling perturbation by natural productsCell Signal2009211541154719298854
- SarkarFHLiYWangZKongDThe role of neutroceuticals in the regulation of Wnt and Hedgehog signaling in cancerCancer Metastasis Rev20102938339420711635
- RyuMJChoMSongJYNatural derivatives of curcumin attenuate the Wnt/beta-catenin pathway through down-regulation of the transcriptional coactivator p300Biochem Biophys Res Commun20083771304130819000900
- JaiswalASMarlowBPGuptaNNarayanSBeta-catenin-mediated transactivation and cell-cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cellsOncogene20028414842712466962
- YanCJamaluddinMSAggarwalBMyersJBoydDDGene expression profiling identifies activating transcription factor 3 as a novel contributor to the proapoptotic effect of curcuminMol Cancer Ther2005423324115713895
- TaraporeRSSiddiquiIASaleemMAdhamiVMSpiegelmanVSMukhtarHSpecific targeting of Wnt/β-catenin signaling in human melanoma cells by a dietary triterpene lupeolCarcinogenesis2010311844185320732907
- MasellaRDi BenedettoRVariRFilesiCGiovanniniCNovel mechanisms of natural antioxidant compounds in biological systems: Involvement of glutathione and glutathione-related enzymesJ Nutr Biochem20051657758616111877
- SuzuckiKKoikeHMatsuiHGenistein, a soy isoflavone, induces glutathione peroxidase in the human prostate cancer cell lines LNCAP and PC-3Int J Cancer20029984685212115487
- LuceriCCaderniGSannaADolaraPRed wine and black tea polyphenols modulate the expression of cycloxygenase-2, inducible nitric oxide synthase and glutathione-related enzymes in azoxymethane-induced F344 rat colon tumorsJ Nutr20021321376137912042461
- MyhrstadMCCarlsenHNordstromOBlomhoffRMoskaugJOFlavonoids increases the intracellular glutathione level by transactivation of the γ-glutamylcysteine synthetase catalytic subunit promoterFree Radic Biol Med20023238639311864778
- ScharfGPrustomerskySKnasmullerSSchulte-HermannRHuberWWEnhancement of glutathione and γ-glutamylcysteine synthetase, the rate limiting enzyme of glutathione synthesis, by chemopreventive plant-derived food and beverage components in the human hepatoma cell line HepG2Nutr Cancer200345748312791507
- MoskaugJOCarlsenHMyhrstadMCBlomhoffRPolyphenols and glutathione synthesis regulationAm J Clin Nutr2005811 Suppl277S283S15640491
- NaHKSurhYJModulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCGFood Chem Toxicol2008461271127818082923
- DickinsonDAIiesKEZhangHBlankVFormanHJCurcumin alters EpRE and AP-1 binding complexes and elevates glutamate-cysteine ligase gene expressionThe FASEB J200317473475
- HuberWWScharfGRossmanithWThe coffee components Kahweol and Cafestol induce γ-glutamylcysteine synthetase, the rate limiting enzyme of chemoprotective glutathione synthesis, in several organs of the ratArch Toxicol2002b7568569411876501
- FianderHSchneiderHDietary orto-phenols that induce glutathione S-transferase and increase the resistance of cells to hydrogen peroxide are potential cancer chemopreventives that act by two mechanisms: The alleviation of oxidative stress and the detoxification of mutagenic xenobioticsCancer Lett200015611712410880760
- HuberWWParzefallWModification of N-acetyltransferases and glutathione S-transferases by coffee components: Possible relevance for cancer riskMethods Enzymol200540130734116399395
- GuglielmiFLuceriCGiovannelliLDolaraPLodoviciMEffect of 4-coumaric acid and 3,4-dihydroxybenzoic acid on oxidative DNA damage in rat colonic mucosaBr J Nutr20038958158712720578
- MundayRMundayCMLow soses of diallyl disulfide, a compound derived from garlic, increase tissue activities of quinone reductase and glutathione transferase in the gastrointestinal tract of the ratNutr Cancer199934424810453440
- EbertMNKlinderAPetersWHExpression of glutathione S-transferases (GST) in human colon cells and inducibility of GSTM2 by butyrateCarcinogenesis2003241637164412896903
- HuberWWTeitelCHColesBFPotential chemoprotective effects of the coffee components kahweol and cafestol palmitates via modification of hepatic N-acetyltransferase and glutathione S-transferase activitiesEnviron Mol Mutagen20044426527615468054
- Rice-EvansCPlant polyphenols: Free radical scavengers or chain-breaking antioxidants?Biochem Soc Symp1995611031168660388
- KasaiHFukadaSYamaizumiZSugieSMoriHAction of chlorogenic acid in vegetables and fruits as an inhibitor of 8-hydroxydeoxyguanosine formation in vitro and in a rat carcinogenesis modelFood Chem Toxicol20003846747110762733
- SchaeferSBaumMEisenbrandGDietrichHWillFJanzowskiCPolyphenolic apple juice extracts and their major constituents reduce oxidative damage in human colon cell linesMol Nutr Food Res200650243316317784
- FabianiRRosignoliPDe BartolomeoAOxidative DNA damage is prevented by extracts of olive oil, hydroxytyrosol, and other olive phenolic compounds in human blood mononuclear cells and HL60 cellsJ Nutr20081381411141618641183
- ShiYWangWHuangCJiaZYaoSZhengRFast repair of oxidative DNA damage by phenylpropanoid glycosides and their analoguesMutagenesis200823192618003628
- LodoviciMCasaliniCDe FilippoCInhibition of 1,2-dimethylhydrazine-induced oxidative DNA damage in rat colon mucosa by black tea complex polyphenolsFood Chem Toxicol2000381085108811033196
- BancroftLKLuptonJRDavidsonLADietary fish oil reduces oxidative DNA damage in rat colonocytesFree Radic Biol Med20033514915912853071
- MachowetzAPoulsenHEGruendelSEffect of olive oils on biomarkers of oxidative DNA stress in Northern and Southern EuropeansFASEB J200721455217110467
- QuilesJLOchoaJJRamirez-TortosaCDietary fat type (virgin olive vs sunflower oils) affects age-related changes in DNA double-strand-breaks, antioxidant capacity and blood lipids in ratsExp Gerontol2004391189119815288693
- RosignoliPFabianiRDe BartolomeoAProtective activity of butyrate on hydrogen peroxide-induced DNA damage in isolated human colonocytes and HT29 tumour cellsCarcinogenesis2001221675168011577008
- EbertMNBeyer-SehlmeyerGLiegibelUMKautenburgerTBeckerTWPool-ZobelBLButyrate induces glutathione S-transferase in human colon cells and protects from genetic damage by 4-hydroxynonenalNutr Cancer20014115616412094619
- BelloirCSinghVDauratCSiessMHLe Bon AM. Protective effects of garlic sulfur compounds against DNA damage induced by direct- and indirect-acting genotoxic agents in HepG2 cellsFood Chem Toxicol20064482783416595265
- FragaCGMotchnikPAShigenagaMKHelbockHJJacobRAAmersBNAscorbic acid protects against endogenous oxidative DNA damage in human spermProc Natl Acad Sci U S A19918811003110061763015
- GiovannucciEGoldinBThe role of fat, fatty acids, and total energy intake in the etiology of human colon cancerAm J Clin Nutr1997661564S1571S9394716
- RiegerMAParlesakAPool-ZobelBLRechkemmerGBodeCA diet high in fat and meat but low in dietary fibre increases the genotoxic potential of ‘faecal water’Carcinogenesis1999202311231610590225
- FujiseTIwakiriRKakimotoTLong-term feeding of various fat diets modulates azoxymethane-induced colon carcinogenesis through Wnt/beta-catenin signaling in ratsAm J Physiol Gastrointest Liver Physiol2007292G1150G115617194898
- EndoHHosonoKFujisawaTInvolvement of JNK pathway in the promotion of the early stage of colorectal carcinogenesis under high-fat dietary conditionsGut2009581637164319570763
- PearsonJRGillCIRRowlandIRDiet, fecal water, and colon cancer – development of a biomarkerNutr Rev20096750952619703259
- XichunZLong-term exposure to various types of fat modulates acrylamide-induced preneoplastic lesions of colon mucosa through Wnt/beta-catenin signaling in ratsToxicol Mech Methods20091928529119778218
- LarssonSCRafterJHolmbergLBergkvistLWolkARed meat consumption and risk of cancers of the proximal colon, distal colon, and rectum: the Swedish Mammography CohortInt J Cancer200511382983415499619
- GleiMLatunde-DadaGOKlinderAIron-overload induces oxidative DNA damage in the human colon carcinoma cell line HT29 clone 19AMutat Res200251915116112160900
- IlsleyJNMBelinskyGSGudaKDietary iron promotes azoxymethane-induced colon tumors in miceNutr Cancer20044916216915489209
- BinghamSADayNELubenREuropean Prospective Investigation into Cancer and NutritionDietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): An observational studyLancet20033611496150112737858
- YoungGPHuYLe LeuRKNyskohusLDietary fibre and colorectal cancer: A model for environment – gene interactionsMol Nutr Food Res20054957158415864783
- TodenSBirdARToppingDLConlonMAHigh red meat diets induce greater numbers of colonic DNA double-strand breaks than white meat in rats: Attenuation by high-amylose maize starchCarcinogenesis2007282355236217916911
- DahmCCKeoghRHSpencerEADietary fiber and colorectal cancer risk: A nested case-control study using food diariesJ Natl Cancer Inst201010261462620407088
- HertogMGBueno-de-MesquitaHBFehilyAMSweetnamPMElwoodPCKromhoutDFruit and vegetable consumption and cancer mortality in the Caerphilly studyCancer Epidemiol Biomarkers Prev199656736778877056
- MillenAESubarAFGraubardBIFruit and vegetable intake and prevalance of colorectal adenoma in a cancer screening trialAm J Clin Nutr2007861754176418065596
- RimandoAMSuhNBiological/chemopreventive activity of stilbenes and their effect on colon cancerPlanta Med2008741635164318843589
- Van DuijnhovenFJBueno-De-MesquitaHBFerrariPFruit, vegetables, and colorectal cancer risk: The European Prospective Investigation into Cancer and NutritionAm J Clin Nutr2009891441145219339391
- TsengMMurraySEKupperLLSandlerRSMicronutrients and the risk of colorectal adenomas.Am J Epidemiol1996144100510148942430
- AmesBNMicronutrient deficiencies. A major cause of DNA damageAnn N Y Acad Sci19998898710610668486
- AmesBNDNA damage from micronutrient deficiencies is likely to be a major cause of cancerMutat Res200147572011295149
- FenechMMicronutrients and genomic stability: A new paradigm for recommended dietary allowances (RDAs)Food Chem Toxicol2002401113111712067572
- AmesBNPrevention of mutation, cancer, and other age-associated diseases by optimizing micronutrient intakeJ Nucleic Acids2010 pii: 725071.
- Van BredaSGvan AgenEEngelsLGAltered vegetable intake affects pivotal carcinogenesis pathways in colon mucosa from adenoma patients and controlsCarcinogenesis2004252207221615271855
- RickeRMvan ReeJHvan DeursenJMWhole chromosome instability and cancer: A complex relationshipTrends Genet20082445746618675487
- MunirajanAKAndoKMukaiAKIF1Bbeta functions as a haploinsufficient tumor suppressor gene mapped to chromosome 1p36.2 by inducing apoptotic cell deathJ Biol Chem2008283244262443418614535
- OpavskyRTsaiSYGuimondMSpecific tumor suppressor function for E2F2 in Myc-induced T cell lymphomagenesisProc Natl Acad Sci U S A2007104154001540517881568
- HuangNLeeIMarcotteEMHurlesMECharacterising and predicting haploinsufficiency in the human genomePLoS Genet20106e100115420976243
- EmeritIKeckMLevyAFeingoldJMichelsonAMActivated oxygen species as the origin of chromosome breakage and sister chromatid exchangeMutat Res19821031651726276742
- BlakeboroughMHOwenRWBiltonRFFree radical generating mechanisms in the colon: Their role in the induction and promotion of colorectal cancer?Free Radic Res Commun198963593672792847
- BabbsCFFree radicals and the etiology of colon cancerFree Radic Biol Med199081912002185144
- EmeritIReactive oxygen species, chromosome mutation and cancer. Possible role of clastogenic factors in carcinogenesisFree Radic Biol Med199416991098300000
- PoulsenHEPriemeHLoftSRole of oxidative DNA damage in cancer initiation and promotionEur J Cancer19987916
- BalkwillFMantovaniAInflammation and cancer: Back to Virchow?Lancet200135753954511229684
- JacksonALLoebAAThe contribution of endogenous sources of DNA damage to the multiple mutations in cancerMutat Res200147772111376682
- GackowskiDBanaszkiewiczZRozalskiRJawienAOlinskiRPersistent oxidative stress in colorectal carcinoma patientsInt J Cancer200210139539712209966
- KlaunigJEKamendulisLMThe role of oxidative stress in carcinogenesisAnn Rev Pharmacol Toxicol20044423926714744246
- ValkoMIzakovicMMazurMRhodesCJTelserJRole of oxygen radicals in DNA damage and cancer incidenceMol Cell Biochem2004266375615646026
- StorzPReactive oxygen species in tumor progressionFront Biosci2005101881189615769673
- KunduJKSurhYJInflammation: Gearing the journey to cancerMutat Res2008659153018485806
- ColottaFAllavenaPSicaAGarlandaCMantovaniACancer-related inflammation, the seventh hallmark of cancer: Links to genetic instabilityCarcinogenesis2009301073108119468060
- SheridanJWangLMTosettoMNuclear oxidative damage correlates with poor survival in colorectal cancerBr J Cancer200910038138819066606
- ChangCLMarraGChauhanDPOxidative stress inactivates the human DNA mismatch repair systemAm J Physiol Cell Physiol2002283C148C15412055083
- SongJYLimJWKimHMorioTKimKHOxidative stress induces nuclear loss of DNA repair proteins Ku70 and Ku80 and apoptosis in pancreatic acinar AR42J cellsJ Biol Chem2003278366763668712867423
- BerwickMVineisPMarkers of DNA repair and susceptibility to cancer in humans: An epidemiologic reviewJ Natl Cancer Inst20009287489710841823
- LoebKRLoebLAGenetic instability and the mutator phenotype. Studies in ulcerative colitisAm J Pathol19991541621162610362784
- SchonfeldPWojtczakLFatty acids as modulators of the cellular production of reactive speciesFree Radic Biol Med20084523124118482593
- LovisPRoggliELaybuttDRAlterations in microRNA expression contribute to fatty acid-induced pancreatic β-cell dysfunctionDiabetes2008572728273618633110
- Washo-StultzDCrowley-WeberCLDvorakovaKRole of mitochondrial complexes I and II, reactive oxygen species and arachidonic acid metabolism in deoxycholate-induced apoptosisCancer Lett200217712914411825660
- PayneCMWeberCCrowley-SkillicornCDeoxycholate induces mitochondrial oxidative stress and activates NF-kB through multiple mechanisms in HCT-116 colon epithelial cellsCarcinogenesis20072821522216887864
- Washo-StultzDHoglenNBernsteinHBernsteinCPayneCMRole of nitric oxide and peroxynitrite in bile salt-induced apoptosisNutr Cancer19993518018810693173
- Dall’AgnolMBernsteinCBernsteinHGarewalHPayneCMIdentification of S-nitrosylated proteins after chronic exposure of colonic epithelial cells to deoxycholateProteomics200661654166216404723
- BernsteinHHolubecHBernsteinCUnique dietary-related mouse model of colitisInflamm Bowel Dis20061227829316633050
- PayneCMHolubecHBhattacharyyaAKBernsteinCBernsteinHExposure of mouse colon to dietary bile acid supplement induces sessile adenomasInflamm Bowel Dis20091672973020135625
- BernsteinCHolubecHBhattacharyyaAKCarcinogenicity of deoxycholate, a secondary bile acidArch Toxicol201110.1007/s00204-011-0648–7
- HolubecHPayneCMBernsteinHAssessment of apoptosis by immunohistochemical markers compared to cellular morphology in ex vivo-stressed colonic mucosaJ Histochem Cytochem20055322923515684335
- PayneCMCrowleyCWasho-StultzDThe stress-response proteins poly(ADP-ribose) polymerase and NF-κB protect against bile salt-induced apoptosisCell Death Differ1998562363610200517
- PayneCMCrowley-SkillicornCBernsteinCHolubecHMoyerMPBernsteinHHydrophobic bile acid-induced micronuclei formation, mitotic perturbations, and decreases in spindle checkpoint proteins: Relevance to genomic instability in colon carcinogenesisNutr Cancer20106282584020661832
- KniselyASStrautnieksSSMeierYHepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiencyHepatol200644478486
- JansenPLMEndogenous bile acids as carcinogensRev Hepatol200747434435
- PayneCMCrowley-SkillicornCHolubecHDeoxycholate, an endogenous cytotoxin/genotoxin, induces the autophagic stress-survival pathway: Implications for colon carcinogenesisJ Toxicol2009 article ID 785907:11410.1155/2009/785907
- Crowley-WeberCLPayneCMGleason-GuzmanMDevelopment and molecular characterization of colon cell lines resistant to the tumor promoter and multiple stress-inducer, deoxycholateCarcinogenesis2002232063208012507930
- GiovannucciEMeta-analysis of coffee consumption and risk of colorectal cancerAm J Epidemiol1998147104310529620048
- TavaniALa VecchiaCCoffee and cancer: A review of epidemiological studies, 1990–1999Eur J Cancer Prev2000924125610958327
- TavaniALa VecchiaCCoffee, decaffeinated coffee, tea and cancer of the colon and rectum: A review of epidemiological studies, 1990–2003Cancer Causes Control20041574375715456988
- MichelsKBWillettWCFuchsCSGiovannucciECoffee, tea, and caffeine consumption and incidence of colon and rectal cancerJ Natl Cancer Inst20059728229215713963
- MattilaPKumpulainenJDetermination of free and total phenolic acids in plant-derived foods by HPLC with diode-array detectionJ Agric Food Chem2002503660366712059140
- HanYHaraguchiTIwanagaSConsumption of some polyphenols reduces fecal deoxycholic acid and lithocholic acid, the secondary bile acids of risk factors of colon cancerJ Agric Food Chem2009578587859019711910
- ShibataAKamadaNMasumuraKParp-1 deficiency causes an increase of deletion mutations and inserions/rearrangements in vivo after treatment with an alkylating agentOncogene2005241328133715608683
- CrowleyCPayneCMBernsteinHBernsteinCRoeDThe NAD+ precursors, nicotinic acid and nicotinamide protect cells against apoptosis induced by a multiple stress inducer, deoxycholateCell Death Differ2000731432610745276
- YanQBriehlMCrowleyCLPayneCMBernsteinHBernsteinCThe NAD+ precursors, nicotinic acid and nicotinamide upregulate glyveraldehyde-3-phosphate dehydrogenase and glucose-6-phosphate dehydrogenase mRNA in Jurkat cellsBiochem Biophys Res Comm199925513313610082668
- ZakiIMillardLPellagra complicating Crohn’s diseasePostgrad Med J1995714964977567761
- KiranRPKhouryWChurchJMLaveryICFazioVWRemziFHColorectal cancer complicating inflammatory bowel disease: Similarities and differences between Crohn’s and ulcerative colitis based on three decades of experiemceAnn Surg201025233033520622662
- D’OdoricoABortolanSCardinRReduced plasma antioxidant concentrations and increased oxidative DNA damage in inflammatory bowel diseaseScand J Gastroenterol2001361289129411761019
- PollackSEnatRHaimSZinderOBarzilaiDPellagra as the presenting manifestation of Crohn’s diseaseGastroenterol1982825 Pt 1948952
- Abu-QurshinRNaschitzJEZuckermannENashEEldarSYeshurunDCrohn’s disease associated with pellagra and increased excretion of 5-hydroxyindolacetic acidAm J Med Sci19973131111139030678
- Martinez-ACvan WelyKHAre aneuploidy and chromosome breakage caused by a CINgle mechanism?Cell Cycle201092275228020519949
- SawyerJRSwansonCMKollerMANorthPERossSWCentromeric instability of chromosome 1 resulting in multi-branched chromosomes, telomeric fusion, and “jumping translocations” of 1q in a human immunodeficiency virus-related non-Hodgkin’s lymphomaCancer199576123812448630904
- SawyerJRHusainMPravdenkovaSKrishtAAl-MeftyOA role for telomeric and centromeric instability in the progression of chromosome aberrations in meningioma patientsCancer20008844045310640979
- RaimondiSCRagsdaleSTBehmFRiveraGWilliamsDLMultiple telomeric associations of a trisomic whole q arm of chromosome 1 in a child with acute lymphoblastic leukemiaCancer Genet Cytogenet19874787933466672
- AlmeidaAKokalj-VokacNLefrancoisDHypomethylation of classical satellite DNA and chromosomal instability in lymphoblastoid cell linesHum Genet1993915385468340107
- Kokalj-VokacNAlmeidaAViegas-PequignotEJeanpierreMMalfoyBDutrillauxBSpecific induction of uncoiling and recombination by azacytidine in classical satellite-containing constitutive heterochromatinCytogenet Cell Genet19936311157680606
- SawyerJRTricotGMattoxSJagannathSBarlogieBJumping translocations of chromosome 1q in multiple myeloma: Evidence for a mechanism involving decondensation of pericentromeric heterochromatinBlood199891173217419473240
- VukovicBBeheshtiBParkPCorrelating breakage-fusion-bridge events with the overall chromosomal instability and in vitro karyotype evolution in prostate cancerCytogenet Genome Res200711611117268171
- RobbinsARJablonskiSAYenTJInhibitors of histone deacetylases alter kinetochore assembly by disrupting pericentromeric heterochromatinCell Cycle2005471772615846093
- DemuthIDigweedMConcannonPHuman SNM1B is required for normal cellular response to both DNA interstrand crosslink-inducing agents and ionizing radiationOncogene2004238611861815467758
- YeJLenainCBauwensSTRF2 and Apollo cooperate with topoisomerase 2α to protect human telomeres from replicative damageCell201014223024220655466
- NagothuKKJaszewskiRMoragodaLFolic acid mediated attenuation of loss of heterozygosity of DCC tumor suppressor gene in the colonic mucosa of patients with colorectal adenomasCancer Detect Prevent20032729730412893078
- WangXThomasPXueJFenechMFolate deficiency induces aneuploidy in human lymphocytes in vitro-evidence using cytokinesis-blocked cells and probes specific for chromosomes 17 and 21Mutat Res200455116718015225591
- FenechMCrottJWMicronuclei, nucleoplasmic bridges and nuclear buds induced in folic acid deficient human lymphocytes – evidence for breakage-fusion-bridge cycles in the cytokinesis-block micronucleus assayMutat Res200250413113612106653
- LindbergHKWangXJarventausHFalckGCNorppaHFenechMOrigin of nuclear buds and micronuclei in normal and folate-deprived human lymphocytesMutat Res2007617334517250856
- ChoiS-WMasonJBFolate status: Effects on pathways of colorectal carcinogenesisJ Nutr20021322413S2418S12163703
- KimYIFolate and colorectal cancer: An evidence-based critical reviewMol Nutr Food Res20075126729217295418
- MajumdarAPKodaliUJaszewskiRChemopreventive role of folic acid in colorectal cancerFront Biosci200492725273215353309
- KimJKimDHLeeBHFolate intake and the risk of colorectal cancer in a Korean populationEur J Clin Nutr2009631057106419550429
- DuthieSJFolate and cancer: How DNA damage, repair and methylation impact on colon carcinogenesisJ Inherit Metab Dis20113410110920544289
- BlountBCMackMMWehrCMFolate deficiency causes uracil misincorporation into human DNA and chromosome breakage: Implications for cancer and neuronal damageProc Natl Acad Sci U S A199794329032959096386
- ChoiSWKimYIWeitzelJNMasonJBFolate depletion impairs DNA excision repair in the colon of the ratGut19984393999771411
- MarsitCJEddyKKelseyKTMicroRNA responses to cellular stressesCancer Res200666108431084817108120
- LevineAJSiegmundKDErvinCMThe methylenetetrahydrofolate reductase 677C→T polymorphism and distal colorectal adenoma riskCancer Epidemiol Biomark Prev20009657663
- KawakamiKOmuraKKanehiraEWatanabeGMethylenetet-rahydrofolate reductase polymorphism is associated with folate pool in gastrointestinal cancer tissueAnticancer Res20012128528911299748
- LittleJSharpLDuthieSNarayananSColon cancer and genetic variation in folate metabolism: the clinical bottom lineJ Nutr20031333758S3766S14608111
- ChangSCLinPCLinJKYangSHWangHSLiAFRole of MTHFR polymorphisms and folate levels in different phenotypes of sporadic colorectal cancersInt J Colorectal Dis20072248348916941173