Abstract
Background:
To evaluate the efficacy of rifaximin for reduction of gastrointestinal symptoms in patients with irritable bowel syndrome (IBS).
Methods:
Medical records were identified for consecutive patients diagnosed with IBS according to Rome III criteria, who had abnormal lactulose breath test results and had received rifaximin 1200 mg/day for 10 days. The efficacy of rifaximin for reducing gastrointestinal symptoms and for eradicating small intestinal bacterial overgrowth was ascertained in these patients. In addition, these endpoints were examined in patients who were initially unresponsive to rifaximin 1200 mg/day and received subsequent rifaximin 2400 mg/day.
Results:
Patients who received rifaximin 1200 mg/day (n = 162) experienced a mean improvement of 52% in global IBS symptoms at the end of rifaximin treatment. Similarly, initially unresponsive patients who received additional rifaximin 2400 mg/day (n = 81) experienced a 53% mean improvement in global IBS symptoms. Forty-nine percent of patients who received initial rifaximin and 47% of patients who received high-dose rifaximin achieved ≥50% global symptom improvement during at least one follow-up visit. Normalization of lactulose breath test results was only apparent in some patients who received high-dose rifaximin. Rifaximin was well tolerated.
Conclusion:
Rifaximin 1200 mg/day for 10 days reduced gastrointestinal symptoms in patients with IBS. Patients with incomplete symptom resolution may respond to increased doses of rifaximin.
Introduction
Irritable bowel syndrome (IBS) is a disease of altered gastrointestinal motility, in which patients often experience predominant symptoms of constipation or diarrhea.Citation1 It is prevalent in the US, affecting up to 20% of individuals,Citation2 but the etiology of the disease remains elusive.Citation3 Several psychological and organic causes have been postulated to contribute to the development of IBS, including psychosocial factors (depression, anxiety),Citation3,Citation4 alterations in gastrointestinal neurotransmission (serotonin levels),Citation3,Citation4 and small intestinal bacterial overgrowth.Citation3–Citation6 Small intestinal bacterial overgrowth, in particular, has come into the spotlight because of data suggesting that patients with IBS may have altered intestinal microflora, with decreased concentrations of coliforms, lactobacilli, and bifidobacteria, as well as increased numbers of anaerobes, Escherichia coli, and bacteroides.Citation3,Citation7,Citation8 These recent advances in understanding the etiology of IBS have provided a new avenue for potential therapies, such as nonsystemic antibiotics, that may target disease pathology.Citation9–Citation12
Typical treatments for IBS, such as antispasmodics, antidepressants, C-2 chloride channel activators, and 5-HT3 receptor antagonists, focus on symptom relief,Citation1,Citation13 and do not address the underlying cause of the disorder. Current guidelines from the American College of Gastroenterology provide a strong recommendation (ie, 1B) for antidepressants, C-2 chloride channel activators for females with constipation-predominant IBS, and 5-HT3 receptor antagonists for females with severe diarrhea-predominant IBS. However, limited availability, tolerability, and efficacy for all IBS subtypes emphasize the necessity for other treatment options.Citation14 The American College of Gastroenterology guidelines have strongly recommended the use of nonsystemic antibiotics, such as rifaximin, for IBS based on moderate evidence. Rifaximin is approved for treatment of travelers’ diarrhea and the reduction of hepatic encephalopathy recurrence, and has been shown to alleviate symptoms in patients with IBS.Citation11,Citation15–Citation19 Increased dosage of rifaximin has been shown to normalize glucose breath test results in patients with small intestinal bacterial overgrowth,Citation20 and given the connection between small intestinal bacterial overgrowth and IBS, treatment with a higher dose of rifaximin may provide symptom relief in patients with recurrent IBS.
This retrospective chart review examined the efficacy of rifaximin 1200 mg/day in alleviating global symptoms in patients with IBS and the ability of high-dose rifaximin (2400 mg/day) to improve IBS symptoms in patients initially unresponsive to rifaximin. The study also evaluated the ability of rifaximin to eradicate small intestinal bacterial overgrowth in patients with IBS.
Materials and methods
Patient population
Medical records from a single center were identified for consecutive patients diagnosed with IBS according to Rome III criteria, who were aged ≥16 years, had an abnormal lactulose breath test result between October 2006 and July 2007, and had received rifaximin 1200 mg/day (Xifaxan®, Salix Pharmaceuticals, Inc, Morrisville, NC) for 10 days at any time. Before the lactulose breath test, patients were given dietary restrictions (eg, no beans or heavy meat 24 hours before testing), could not chew gum or participate in strenuous physical exercise nine hours before testing, and were not allowed to smoke two hours before the lactulose breath test. For this test, patients ingested 10 g of lactulose. An abnormal lactulose breath test result was defined as a ≥10 ppm increase in baseline H2 or CH4 excretion within 90 minutes. Patients were excluded if they had used an oral antibiotic within three weeks before rifaximin treatment or if they had a history of inflammatory bowel disease, bowel resection surgery, or a colonic mucosal biopsy compatible with inflammatory bowel disease or microscopic colitis. Furthermore, patients were excluded if they displayed any of the following at baseline: a positive sprue screen (antigliadin antibody IgA, tissue transglutaminase IgA, total IgA), a positive stool antigen test for Giardia or Cryptosporidium, positivity for fecal leukocytes, positivity for Clostridium difficile toxins A or B, or levels of C-reactive protein >8 mg/L.
Data extraction
Data for baseline IBS symptoms, rifaximin dosing regimen, concomitant medication usage, and IBS symptom improvement were extracted from patient charts. Only charts of patients who received initial treatment with rifaximin 1200 mg/day and were retreated with rifaximin 2400 mg/day if symptoms continued were included in the analyses. Collection of lactulose breath test results obtained initially and 4–14 days following rifaximin completion was also performed. Note was made of hydrogen-positive, methane-positive, hydrogen- and methane-positive, flatline, negative, distal-positive, high-baseline, and inconclusive lactulose breath test results.
Response to initial rifaximin (1200 mg/day) and high-dose rifaximin (2400 mg/day) was based on global symptom improvement from baseline and was assessed by the patients using a visualized scale ranging from 0% to 100%. Patients were also asked to rate the percentage improvement from baseline of specific IBS symptoms (abdominal pain or discomfort, bloating, constipation, diarrhea, gas, or other symptoms) at follow-up visits after both treatment regimens. Symptom improvement was categorized as complete improvement (≥91%), partial improvement (1%–90%), or no improvement (0%). Diagnosis of IBS was noted to be constipation-predominant IBS, diarrhea-predominant IBS, or mixed-symptom IBS when reported. Completion of rifaximin therapy was ascertained from all charts, and when withdrawal from the study was noted, the reason was recorded as adverse event related or not related to rifaximin. Specific treatment-related adverse events were also collected from all charts.
Data analysis
The average percent global improvement of baseline IBS symptoms at the end of rifaximin therapy (both initial rifaximin 1200 mg/day and retreatment with rifaximin 2400 mg/day) was analyzed without regard to lactulose breath test measurements. Percentages of patients with improvement in global and IBS-related symptoms (eg, abdominal pain and discomfort, bloating, constipation, diarrhea, and gas) were recorded for each patient at all visits. The percentage of patients who achieved ≥50% global IBS symptom improvement during at least one follow-up visit (≥50% best global improvement) was also ascertained. Normalization of lactulose breath test results within 4–14 days after rifaximin cessation and the percentage of patients with normal lactulose breath test results and global symptom improvement at the end of rifaximin therapy were evaluated. Subanalyses were also conducted for improvement of global and specific IBS symptoms and lactulose breath test results by IBS subclassification (ie, diarrhea-predominant IBS, constipation-predominant IBS, or mixed-symptom IBS).
Results
Patient population
A total of 236 patient charts were reviewed, and 162 were selected for further analysis after application of inclusion/exclusion parameters. All 162 patients received initial treatment with rifaximin 1200 mg/day for 10 days. Among these individuals, 45 (28%) were classified as having diarrhea-predominant IBS, 33 (20%) were classified as having constipation-predominant IBS, and 24 (15%) were classified as having mixed-symptom IBS. Lactulose breath test results indicated that 91 patients (56%) were hydrogen positive, 32 (20%) were methane positive, and 9 (6%) were positive for both hydrogen and methane (). In addition, 81 of the initial 162 patients (50%) received subsequent treatment with rifaximin 2400 mg/day for 10 days because of incomplete symptom resolution ().
Table 1 Patient demographics and characteristics
Response to initial treatment with rifaximin
Patients who received rifaximin 1200 mg/day experienced a mean improvement of 52% in global IBS symptoms at the end of rifaximin treatment (). In addition, after initial rifaximin 1200 mg/day for 10 days, 49% of patients (79 of 162) reported ≥50% best global improvement in IBS symptoms (). This improvement was IBS-subtype specific, with 56% of patients (25 of 45) diagnosed with diarrhea-predominant IBS experiencing ≥50% best global improvement compared with 45% of individuals (15 of 33) with constipation-predominant IBS (). Complete symptom relief (≥91% improvement) was achieved in 19 of 162 patients (12%) and was not dependent on IBS subcategory (). Eight percent of patients (13 of 162) achieved partial relief (1%–90%) of overall symptoms, with individuals having constipation-predominant IBS receiving the greatest benefit. The predominant symptom (diarrhea or constipation) was completely relieved in 13% of patients with diarrhea-predominant IBS and in 12% of patients with constipation-predominant IBS (). Similarly, 17% of patients with mixed-symptom IBS displayed complete relief of diarrhea, and 13% had complete relief of constipation (). Only 2% of patients treated with initial rifaximin displayed a normal lactulose breath test result post-treatment.
Figure 1 Mean percent improvement in global IBS symptoms at the end of rifaximin treatment. The mean percent improvement in global IBS symptoms was similar in patients with IBS who received rifaximin 1200 mg/day for 10 days (52%) and in patients with IBS who received subsequent high-dose rifaximin (2400 mg/day) for 10 days (53%).
Abbreviation: IBS, irritable bowel syndrome.
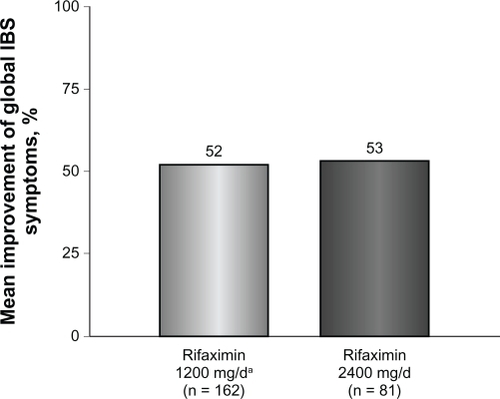
Figure 2 Percentage of patients obtaining ≥50% best global improvement following rifaximin treatment. Global symptoms improved in 79 of 162 patients (49%) who received rifaximin 1200 mg/day for 10 days compared with 38 of 81 patients (47%) who received high-dose rifaximin (2400 mg/day) for 10 days.
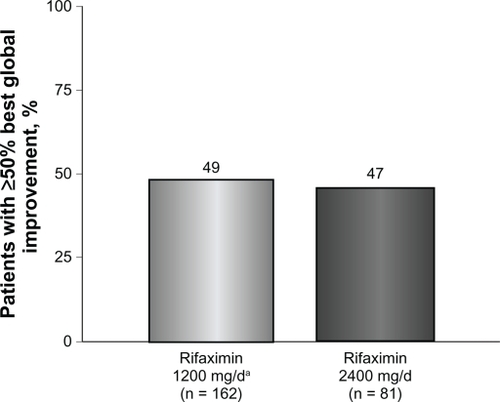
Figure 3 Percentage of patients with IBS-D or IBS-C who obtained ≥50% best global improvement following rifaximin treatment. Rifaximin effectively reduced irritable bowel syndrome symptoms in patients with either subtype. Interestingly, rifaximin 2400 mg/day elicited ≥50% symptom improvement in patients with either subtype who initially lacked response to rifaximin 1200 mg/day.
Abbreviations: IBS-C, constipation-predominant irritable bowel syndrome; IBS-D, diarrhea-predominant irritable bowel syndrome.
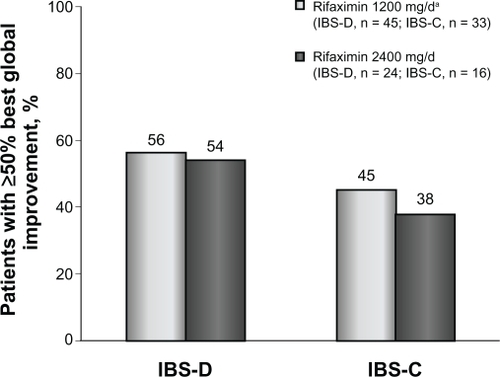
Table 2 Complete relief of IBS symptoms following rifaximin treatment
Response to further treatment with high-dose rifaximin
Patients who did not respond to initial rifaximin were administered additional rifaximin (2400 mg/day for 10 days, n = 81). Patients who received high-dose rifaximin (2400 mg/day) experienced a mean improvement in global IBS symptoms of 53% at the termination of rifaximin therapy, which was similar to the percent improvement experienced in patients responsive to rifaximin 1200 mg/day (). In addition, treatment with high-dose rifaximin elicited ≥50% best global symptom improvement in patients initially lacking a response to lower-dose rifaximin. Among the 81 patients receiving further rifaximin treatment, 38 (47%) exhibited ≥50% best improvement in overall global symptoms, which was similar to that reported during initial rifaximin 1200 mg/day for 10 days (). Patients with mixed-symptom IBS received the greatest benefit from high-dose rifaximin, with six of 11 patients (55%) reporting best global improvement ≥50%. More patients with diarrhea-predominant IBS experienced best global improvement ≥50% compared with individuals with constipation-predominant IBS, with 13 of 24 patients with diarrhea-predominant IBS (54%) displaying best global improvement ≥50% compared with only six of 16 patients with constipation-predominant IBS (38%, ). Complete symptom reduction (≥91%) was reported in nine of 81 patients (11%) and was not subtype dependent. However, complete reduction of the predominant symptom (diarrhea or constipation) was greater in patients with diarrhea-predominant IBS (17%) than in patients experiencing constipation-predominant IBS (6%, ). Interestingly, patients with mixed-symptom IBS reported larger remission of diarrheal symptoms (18%) than constipation symptoms (0%, ). Partial relief (1%–90%) of global IBS symptoms was achieved in 10% of initially unresponsive patients after high-dose rifaximin, and of these, 8%, 13%, and 18% of patients with diarrhea-predominant IBS, constipation-predominant IBS, and mixed-symptom IBS, respectively, reported partial relief. In contrast with initial rifaximin therapy, high-dose rifaximin successfully normalized lactulose breath test results in nine of 81 patients (11%).
Adverse events
Of the 162 patients receiving initial rifaximin 1200 mg/day for 10 days, only 13 (8%) reported adverse events. Similarly, only five of 81 patients (6%) administered additional rifaximin 2400 mg/day for 10 days reported adverse events. Most of these adverse events occurred in the first 3–4 days of treatment and consisted of flu-like illness with diarrhea and nausea. These symptoms resolved by day 5 and were thought to be secondary to “bacterial kill”.
Discussion
This retrospective chart review demonstrated that rifaximin 1200 mg/day for 10 days improved global and IBS-related symptoms. This finding is consistent with data from two Phase III clinical trials showing that rifaximin 550 mg three times daily for 14 days adequately relieved global IBS symptoms and IBS-related bloating for at least two of the first four weeks of treatment, and improved daily assessments of IBS symptoms, bloating, abdominal pain, and stool consistency compared with placebo.Citation19 However, the initial dose of rifaximin examined in the current work (ie, rifaximin 1200 mg/day) was lower than that used in the Phase III trials (ie, 1650 mg/day). Several prospective studies have shown that rifaximin 800 mg/day or 1200 mg/day for 10 days improved IBS symptoms and eradicated small intestinal bacterial overgrowth,Citation15,Citation16 but because of differences in study design and inclusion criteria, it is not possible to compare these studies with the Phase III trials. Thus, the optimum dose of rifaximin for IBS remains unknown. Results from the current study, however, imply that patients who are initially unresponsive to rifaximin therapy may benefit from an escalation in rifaximin dose.
Few studies have examined the efficacy of rifaximin for specific subpopulations of IBS (ie, diarrhea-predominant IBS, constipation-predominant IBS, and mixed-symptom IBS). Phase III clinical trials of rifaximin have limited patient inclusion to “non-constipation IBS” (ie, diarrhea-predominant IBS or mixed-symptom IBS);Citation19 therefore, possible differences in the efficacy of rifaximin among patients with different IBS subtypes remains unknown. In the current study, rifaximin was effective in all subtypes of IBS. Although a greater percentage of patients with diarrhea-predominant IBS had an initial response, patients with mixed-symptom IBS were more likely to respond to high-dose rifaximin. In addition, symptoms of diarrhea and constipation were equally relieved in patients with mixed-symptom IBS.
Rifaximin is a generally well-tolerated nonsystemic antibiotic that elicits few adverse events. Studies of rifaximin 800 mg/day or 1200 mg/day in patients with IBS demonstrated a similar occurrence of adverse events in the placebo and rifaximin groups.Citation5,Citation15,Citation16 Although one might expect a larger number of adverse events with an increased medication dose, rifaximin 1600 mg/dayCitation19 or 2400 mg/day is well tolerated.
These results are limited by the retrospective nature of the study and the lack of a placebo comparison group, but they provide evidence that rifaximin 1200 mg/day may be a safe and beneficial alterative for patients with IBS and that studies designed to examine the efficacy of rifaximin in patients with diverse IBS symptoms are warranted. Furthermore, higher doses of rifaximin (ie, 2400 mg/day) may increase the percentage of responsive patients.
Disclosure
The author has no competing interests or financial support for research to disclose.
References
- SchoenfeldPEfficacy of current drug therapies in irritable bowel syndrome: What works and does not workGastroenterol Clin North Am200534231933515862938
- OldenKWDiagnosis of irritable bowel syndromeGastroenterology200212261701170412016433
- BarbaraGDe GiorgioRStanghelliniVCremonCSalvioliBCorinaldesiRNew pathophysiological mechanisms in irritable bowel syndromeAliment Pharmacol Ther200420Suppl 21915335408
- TalleyNJIrritable bowel syndromeIntern Med J2006361172472817040359
- MajewskiMMcCallumRWResults of small intestinal bacterial overgrowth testing in irritable bowel syndrome patients: Clinical profiles and effects of antibiotic trialAdv Med Sci20075213914218217406
- PosserudIStotzerPOBjörnssonESAbrahamssonHSimrénMSmall intestinal bacterial overgrowth in patients with irritable bowel syndromeGut200756680280817148502
- Krogius-KurikkaLLyraAMalinenEMicrobial community analysis reveals high level phylogenetic alterations in the overall gastrointestinal microbiota of diarrhoea-predominant irritable bowel syndrome sufferersBMC Gastroenterol200999520015409
- TanaCUmesakiYImaokaAHandaTKanazawaMFukudoSAltered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndromeNeurogastroenterol Motil201022551251919903265
- PimentelMChatterjeeSChowEJParkSKongYNeomycin improves constipation-predominant irritable bowel syndrome in a fashion that is dependent on the presence of methane gas: Subanalysis of a double-blind randomized controlled studyDig Dis Sci20065181297130116832617
- PimentelMChowEJLinHCNormalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome: A double-blind, randomized, placebo-controlled studyAm J Gastroenterol200398241241912591062
- YangJLeeHRLowKChatterjeeSPimentelMRifaximin versus other antibiotics in the primary treatment and retreatment of bacterial overgrowth in IBSDig Dis Sci200853116917417520365
- PimentelMChowEJLinHCEradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndromeAm J Gastroenterol200095123503350611151884
- FrissoraCLCashBDThe role of antibiotics vs conventional pharmacotherapy in treating symptoms of irritable bowel syndromeAliment Pharmacol Ther200725111271128117509095
- BrandtLJCheyWDFoxx-OrensteinAEAn evidence-based position statement on the management of irritable bowel syndromeAm J Gastroenterol2009104Suppl 1S1S3519521341
- PimentelMParkSMirochaJKaneSVKongYThe effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndromeAnn Intern Med2006145855756317043337
- ShararaAIAounEAbdul-BakiHMounzerRSidaniSElHajjIA randomized double-blind placebo-controlled trial of rifaximin in patients with abdominal bloating and flatulenceAm J Gastroenterol2006101232633316454838
- PeraltaSCottoneCDoveriTAlmasioPLCraxiASmall intestine bacterial overgrowth and irritable bowel syndrome-related symptoms: Experience with rifaximinWorld J Gastroenterol200915212628263119496193
- LowKHwangLHuaJZhuAMoralesWPimentelMA combination of rifaximin and neomycin is most effective in treating irritable bowel syndrome patients with methane on lactulose breath testJ Clin Gastroenterol201044854755019996983
- PimentelMLemboACheyWDfor the TARGET Study GroupRifaximin therapy for patients with irritable bowel syndrome without constipationN Engl J Med20113641223221208106
- ScarpelliniEGabrielliMLauritanoCEHigh dosage rifaximin for the treatment of small intestinal bacterial overgrowthAliment Pharmacol Ther200725778178617373916